Abstract
Galectin-3 plays an important role in fibroblast activation and fibrosis in animal models. Elevated galectin-3 levels are associated with poor long-term survival in heart failure (HF). We examined the relation between plasma galectin-3 levels and myocardial indices of systolic HF. We measured plasma galectin-3 in 133 chronic HF and 45 advanced decompensated HF subjects with echocardiographic and hemodynamic evaluation. In our chronic HF cohort, median plasma galectin-3 level was 13.9ng/mL [interquartile range: 12.1–16.9ng/mL]. Higher galectin-3 was associated with more advanced age (r=0.22, p=0.010) and poor renal function (estimated glomerular filtration rate [eGFR]: r= −0.24, p=0.007; cystatin C: r= 0.38, p<0.0001), and predicted all-cause mortality (Hazard ratio [HR] 1.86 [95% confidence interval: 1.36–2.54], p<0.001). In multivariate analysis, galectin-3 remained an independent predictor of all-cause mortality after adjusting for age, eGFR, left ventricular (LV) ejection fraction (EF), and mitral E/septal Ea (HR 1.94 [1.30–2.91], p=0.001). However, galectin-3 did not predict the combined endpoint of all-cause mortality, cardiac transplantation, or HF hospitalization (p>0.05). Furthermore, there were no relations between galectin-3 and LV end-diastolic volume index (r= −0.05, p=0.61), LVEF (r= 0.10, p=0.25), or LV diastolic function (mitral E/septal Ea: r= 0.06, p=0.52; left atrial volume index: r= 0.08, p=0.41). In our advanced decompensated HF cohort, we did not observe any relation between galectin-3 and echocardiographic or hemodynamic indices. In conclusion, high plasma galectin-3 levels were associated with renal insufficiency and poorer survival in patients with chronic systolic HF. However, we did not observe a relation between galectin-3 and echocardiographic or hemodynamic indices.
Keywords: Heart failure, galectin-3, renal function, prognosis
Introduction
Increasing evidence has implicated galectin-3 in the pathogenesis and progression of heart failure (HF). In animal models, galectin-3 has been demonstrated to be robustly expressed in the failing heart and linked to myocardial fibrosis and remodeling1–4 in addition to renal fibrosis5. Intra-pericardial infusion of recombinant galectin-3 in healthy rats triggers inflammation involving macrophage and mast cell infiltration, cardiac interstitial and perivascular fibrosis, hypertrophy, and left ventricular systolic and diastolic dysfunction1,4. In chronic stable and acute decompensated HF patients, elevated plasma galectin-3 levels have been linked to renal dysfunction and increased all-cause mortality risk6–8, and within the chronic HF setting, serum galectin-3 levels have been demonstrated to correlate with serum markers of cardiac extracellular matrix turnover9. Hence there is direct implication that galectin-3 is a mediator in the development of cardiac hypertrophy and fibrosis, and a potential biomarker for HF. Herein, we examine the relation between galectin-3 levels and echocardiographic indices of cardiac structure and function in patients with chronic stable HF, as well as echocardiographic and hemodynamic indices of cardiac structure and function in patients with advanced decompensated systolic HF.
Methods
We investigated the role of galectin-3 in human systolic HF in two separate cohorts. This study was approved by the Cleveland Clinic Institutional Review Board, and all subjects gave informed consent. In our chronic systolic HF cohort, we studied 133 patients enrolled in the Assessment of Doppler Echocardiography Prognosis and Therapy study, a single-center, prospective cohort study examining the natural history of stable but symptomatic chronic systolic HF patients with careful echocardiographic and biochemical phenotyping. Subjects enrolled were 18 to 75 years of age, had a diagnosis of HF for at least 3 months, a left ventricular (LV) ejection fraction (EF) ≤35% at the time of enrollment, New York Heart Association (NYHA) functional class I–IV symptoms, and were free of significant renal, hepatic, and valvular diseases10. Estimated glomerular filtration rate (eGFR) was calculated using the standard 4-variable Modification of Diet in Renal Disease equation11. Subjects were followed prospectively by telephone follow-up and chart review for heart failure hospitalizations and adverse events, with all-cause mortality data confirmed by Social Security Death Index up to 5 years of follow-up. In our advanced decompensated HF cohort, we measured plasma galectin-3 levels in 45 patients with advanced HF admitted to the HF intensive care unit with hemodynamic assessment and monitoring at the Cleveland Clinic for intensive medical therapy including vasoactive drugs. Subjects were 18 years of age or older, had an LVEF ≤35% for at least 6 months, elevated filling pressures defined by a pulmonary capillary wedge pressure >18 mmHg and/or a central venous pressure >8 mmHg. Exclusion criteria included mechanical ventilation, renal replacement therapy, post-cardiac transplantation and post tricuspid valve surgery.
In both studies, patients underwent comprehensive echocardiographic evaluation of cardiac structure as well as systolic and diastolic performance by an experienced research sonographer at the time of blood sample collection. Comprehensive transthoracic echocardiography was performed using commercially available HDI 5000 (Phillips Medical Systems, N.A., Bothell, Washington) and Acuson Sequoia (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania) machines for our chronic systolic HF cohort and commercially available Vingmed, System Seven (General Electric Healthcare, USA) machine for our advanced decompensated systolic HF cohort. Two-dimensional and color Doppler imaging was performed in standard parasternal and apical views. Diastolic indices (including pulse-wave Doppler, color M-mode, and tissue Doppler imaging) were acquired over ten consecutive beats using sweep speeds of 50 and 100 cm/s using previously described techniques and diastolic staging10,12. The LVEF and cardiac volumes were measured using Simpson's biplane method. LV mass was calculated according to previously published recommendations13. All ventricular volume and mass measurements were indexed to body surface area. Measurements were averaged over three cycles (five cycles for atrial fibrillation).
All samples were collected into ethylenediaminetetraacetic acid plasma vacuum collecting tubes on ice simultaneously at the time of echocardiography and hemodynamic evaluation by delegated research personnel. Plasma was immediately separated, processed, and preserved in aliquots at −80°C until analysis. Plasma galectin-3 levels were determined by a commercially available sandwich enzyme-linked immunosorbent assay kit (Cat. No. BMS279/2, Bender MedSystems GmbH, Vienna, Austria) according to the manufacturer's protocol. A 2-fold dilution was utilized to bring concentrations within the measuring range of the assay 0.39–25ng/mL. Intra-assay and inter-assay coefficients of variation were <5%. Plasma cystatin C levels were determined by the N Latex cystatin C assay (Dade-Behring, Deerfield IL), a latex-enhanced nephelmetric immunoassay using rabbit polyclonal antibodies. The measuring range was 0.25–7.90 mg/L. Intra-assay and inter-assay coefficients of variation were < 1.8%. Recovery was 92.4–101.3% and the values were comparable between serum and plasma14. Plasma amino-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were assayed using a commercially available assay (Roche Elecsys® proBNP assay, Roche Diagnostics, Indianapolis IN). All laboratory analyses were performed by investigators blinded to clinical outcomes.
Continuous variables were summarized as mean ± standard deviation if normally distributed, and as median and interquartile range if non-normally distributed. Normality was assessed by the Shapiro-Wilk W test. Spearman's rank correlation method was used as a nonparametric measure of association between continuous plasma galectin-3 levels and clinical and echocardiographic indices. The Wilcoxon rank-sum or Kruskal-Wallis test were used to compare differences in continuous plasma galectin-3 levels across categorical clinical or echocardiographic indices. The Cox proportional hazards model was used to assess the clinical risk associated with increasing continuous standardized increments of natural logarithm-transformed plasma galectin-3 levels. The optimal receiver operating characteristic curve cut-off value for prediction of adverse clinical events was chosen as the value maximizing sensitivity plus specificity. The proportional hazards assumption was verified with log (time) vs. log[-log(survival)] plots. Kaplan-Meier survival plots were calculated from baseline to time of all-cause mortality. All p-values reported are from two-sided tests and a p-value <0.05 was considered statistically significant. Statistical analyses were performed using JMP 8.0.2 and SAS 9.0 (SAS Institute, Cary, NC).
Results
In our chronic HF cohort, the mean and median plasma galectin-3 levels were 14.8 ± 4.0ng/mL and 13.9 [interquartile range (IQR) 12.1–16.9]ng/mL, respectively (Table 1). Higher plasma galectin-3 levels were associated with advanced age (Spearman's r=0.22, p=0.010), poor renal function (eGFR: r= −0.24, p=0.007; cystatin C: r=0.38, p<0.0001) (Table 2), and higher NYHA functional class (rank sums p=0.026). Plasma galectin-3 levels were lower with beta-blocker use (13.4 [IQR 11.9–16.3] versus 14.9 [IQR 12.6–17.6] ng/mL, p=0.024) and spironolactone use (13.1 [IQR 11.0–15.3] versus 14.3 [IQR 12.3–17.2] ng/mL, p=0.043), but did not differ according to gender (p=0.92), ethnicity (p=0.48), diabetic status (p=0.58), or ischemic etiology (p=0.24). In our advanced decompensated HF cohort, the mean and median plasma galectin-3 levels were 14.7 ± 4.0ng/mL and 14.7 [IQR 12.1–17.0]ng/mL, respectively (Table 1). Again, higher plasma galectin-3 levels demonstrated even stronger association with poor renal function (eGFR: r= −0.39, p=0.024; cystatin C: r= 0.40, p=0.006) (Table 2) and with ischemic etiology (p=0.013), but were not associated with age (p=0.65), NYHA functional class (p=0.54), gender (p=0.64), diabetic status (p=0.07), or beta-blocker or spironolactone use (p>0.10 for both).
Table 1.
Baseline Subject Characteristics for Chronic Heart Failure (n=133) and Advanced Decompensated Heart Failure (n=45) Cohorts
| Variable | Chronic Heart Failure (n=133) | Advanced Decompensated Heart Failure (n=45) |
|---|---|---|
| Age (years) | 57 ± 13 | 55 ± 14 |
| Body mass index (kg/m2) | 28 ± 5 | 28 ± 6 |
| Men | 99 (74%) | 39 (87%) |
| Ischemic heart failure etiology | 56 (42%) | 21 (47%) |
| NYHA class III or IV | 46 (35%) | 35 (95%) |
| Hypertension | 75 (58%) | 15 (33%) |
| Diabetes mellitus | 36 (27%) | 12 (27%) |
| Echocardiographic indices: | ||
| Left ventricular mass index (g/m2) | 156 ± 46 | 186 ± 64 |
| Indexed left ventricular end-diastolic volume (mL/m2) | 109 ± 34 | 111 ± 52 |
| Left ventricular ejection fraction (%-unit) | 26 ± 6 | 28 ± 8 |
| Diastolic stage III | 44 (37%) | 41 (91%) |
| Medications: | ||
| Angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers | 122 (94%) | 12 (29%) |
| Other vasodilators | 15 (11%) | 25 (56%) |
| Beta-blockers | 84 (64%) | 14 (33%) |
| Spironolactone | 35 (28%) | 13 (31%) |
| Digoxin | 74 (60%) | 15 (36%) |
| Laboratory Data: | ||
| Aminoterminal pro-B-type natriuretic peptide (pg/mL) | 1,240 [529 – 3407] | 4,582 [2286 – 7537] |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 70 ± 23 | 78 ± 47 |
| Cystatin C (ng/mL) | 1.23 [1.03 – 1.62] | 1.63 [1.19 – 2.67] |
| Galectin-3 (ng/mL) | 13.9 [12.1 – 16.9] | 14.7 [12.1 – 17.0] |
Table 2.
Univariate Correlations between Plasma Galectin-3 Levels and Clinical and Echocardiographic Characteristics for Chronic Heart Failure (n=133) and Advanced Decompensated Heart Failure (n=45) Cohorts
| Variable | Chronic Heart Failure (n=133) | Advanced Decompensated Heart Failure (n=45) | ||
|---|---|---|---|---|
| r | p | r | p | |
| Age (years) | 0.22 | 0.010 | 0.07 | 0.647 |
| Body mass index (kg/m2) | 0.01 | 0.913 | 0.00 | 0.974 |
| Echocardiographic indices: | ||||
| Left ventricular structure: | ||||
| Left ventricular mass index (g/m2) | 0.04 | 0.688 | −0.12 | 0.460 |
| Left ventricular end-systolic volume index (mL/m2) | −0.07 | 0.482 | −0.08 | 0.607 |
| Left ventricular end-diastolic volume index (mL/m2) | −0.05 | 0.608 | −0.07 | 0.650 |
| Left ventricular systolic function: | ||||
| Left ventricular ejection fraction (%) | 0.10 | 0.253 | 0.04 | 0.825 |
| LV Diastolic Function: | ||||
| Mitral ratio of peak early to late diastolic filling velocity | 0.05 | 0.596 | 0.18 | 0.357 |
| Mitral deceleration time (ms) | 0.02 | 0.867 | 0.07 | 0.631 |
| Early diastolic myocardial relaxation velocity at septal mitral annulus (cm/s) | 0.00 | 0.974 | −0.01 | 0.945 |
| Early diastolic myocardial relaxation velocity at lateral mitral annulus (cm/s) | 0.07 | 0.426 | 0.04 | 0.822 |
| Averaged early diastolic myocardial relaxation velocity at mitral annulus (cm/s) | 0.08 | 0.354 | 0.02 | 0.925 |
| Ratio of the mitral inflow early wave to the early diastolic myocardial relaxation velocity at septal mitral annulus | 0.06 | 0.516 | 0.10 | 0.539 |
| Ratio of the mitral inflow early wave to the early diastolic myocardial relaxation velocity at lateral mitral annulus | 0.07 | 0.434 | 0.10 | 0.511 |
| Ratio of the mitral inflow early wave to the averaged early diastolic myocardial relaxation velocity at mitral annulus | 0.09 | 0.321 | 0.15 | 0.361 |
| Pulmonary vein systolic/diastolic ratio | −0.05 | 0.599 | −0.19 | 0.323 |
| Left atrial volume index (mL/m2) | 0.08 | 0.413 | −0.08 | 0.596 |
| Right ventricular systolic function: | ||||
| Right ventricular systolic wave | −0.10 | 0.273 | 0.10 | 0.529 |
| Right ventricular diastolic function: | ||||
| Ratio of the tricuspid inflow early wave to the early diastolic myocardial relaxation velocity at tricuspid annulus | 0.05 | 0.603 | 0.02 | 0.900 |
| Hepatic vein systolic/diastolic ratio | 0.08 | 0.454 | −0.13 | 0.668 |
| Right atrial volume index (mL/m2) | 0.02 | 0.842 | −0.30 | 0.052 |
| Estimated Right-sided Pressures: | ||||
| Tricuspid regurgitation jet velocity (cm/s) | 0.12 | 0.182 | 0.21 | 0.240 |
| Right ventricular systolic pressure (mmHg) | 0.13 | 0.209 | 0.14 | 0.384 |
| Laboratory Data: | ||||
| Estimated glomerular filtration rate (mL/min/1.73m2) | −0.24 | 0.007 | −0.39 | 0.024 |
| Cystatin C (mg/L) | 0.38 | <0.0001 | 0.40 | 0.006 |
| Aminoterminal-proB-type natriuretic peptide (pg/mL) | 0.18 | 0.053 | 0.04 | 0.793 |
In our chronic HF cohort, there was no correlation between plasma galectin-3 levels and indices of LV structure, including indexed LV mass or LV end-systolic or end-diastolic volume (p>0.48 for both), indices of left or right ventricular systolic function, including LVEF or right ventricular systolic wave (p>0.25 for both), or indices of LV diastolic function, including ratio of the mitral inflow early wave to the early diastolic myocardial relaxation velocity at septal mitral annulus, pulmonary vein systolic/diastolic ratio, or left atrial volume index (p>0.41 for all, Table 2). Furthermore, plasma galectin-3 levels did not correlate indices of right ventricular function including semi-quantitative right ventricular systolic function severity. There was weak correlation between galectin-3 and NT pro-BNP that reached marginal significance (r=0.18, p=0.053) (Table 2). In our advanced decompensated HF cohort, plasma galectin-3 levels were not associated with indices of LV structure (p>0.46 for all), LVEF or indices of LV diastolic dysfunction or right ventricular function (p>0.32 for all, Table 2). Also, plasma galectin-3 was not associated with any hemodynamic measures measured by pulmonary artery catheter, including pulmonary capillary wedge pressure (p=0.45) and cardiac index (p=0.70), as well as pulmonary artery mean pressure (p=0.99) and central venous pressure (p=0.76).
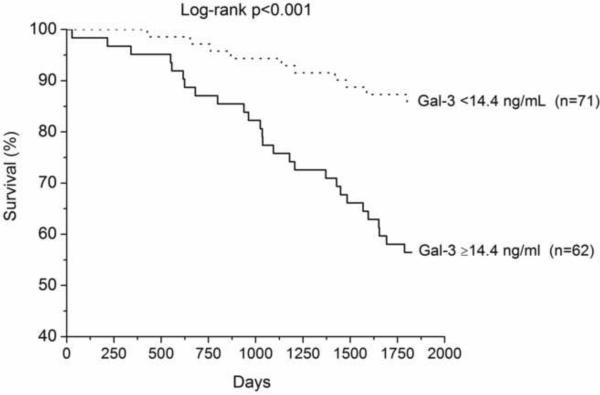
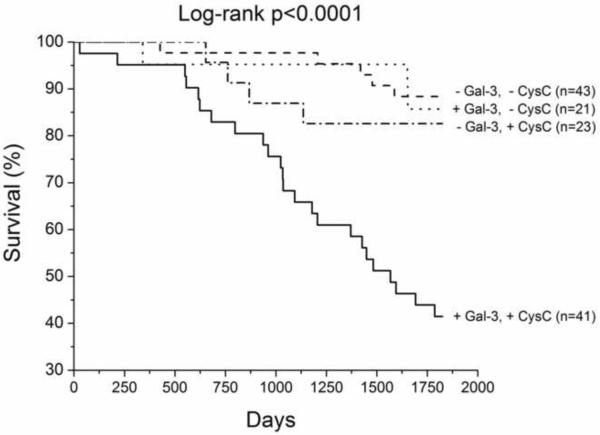
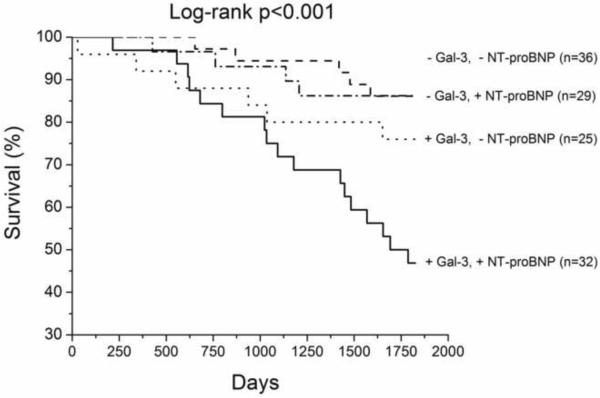
We have up to 5-year long-term follow-up of our chronic HF cohort, which demonstrated that higher galectin-3 levels were associated with higher all-cause mortality, particularly in those with elevated cystatin C levels (Table 3, Figure 1). In multivariate analysis, galectin-3 remained an independent predictor of mortality after adjusting for age, eGFR, LVEF, and ratio of the mitral inflow early wave to the early diastolic myocardial relaxation velocity at septal mitral annulus (Table 3). Galectin-3 also predicted mortality independent of age and cardiac structure, or age and cystatin C levels in this cohort (Table 3). However, galectin-3 did not predict the composite endpoint of all-cause mortality, cardiac transplantation, or HF hospitalization (p=0.34).
Table 3.
Univariate and Multivariate Cox Proportional Hazards Analyses of 5 Year All-Cause Mortality for Patients with Chronic Systolic Heart Failure (37 events; n=133)
| Natural logarithm galectin-3 (pg/mL)* | Hazard Ratio (95% Confidence interval) | p-value |
|---|---|---|
| Univariate Model | 1.87 (1.36 – 2.55) | <0.001 |
| Multivariate Model 1: Adjusted for age, eGFR, and NT-proBNP. | 1.75 (1.18 – 2.58) | 0.005 |
| Multivariate Model 2: Adjusted for age, eGFR, left ventricular ejection fraction, and ratio of the mitral inflow E wave to the early diastolic myocardial relaxation velocity at septal mitral annulus | 1.94 (1.30 – 2.91) | 0.001 |
| Multivariate Model 3: Adjusted for age, left ventricular mass index, left ventricular end-systolic volume index, and left ventricular end-diastolic volume index | 1.56 (1.09 – 2.23) | 0.016 |
| Multivariate Model 4: Adjusted for age and cystatin C | 1.60 (1.09 – 2.34) | 0.015 |
Hazard ratios per 1 standard deviation increments (1 standard deviation for natural logarithm galectin-3 = 0.26 ng/mL; 1 standard deviation for age = 13 years; 1 standard deviation for estimated glomerular filtration rate [eGFR] = 30 ml/min/1.73m2; 1 standard deviation for natural logarithm aminoterminal pro-B-type natriuretic peptide [NT-proBNP] = 1.36 pg/mL; 1 standard deviation for left ventricular ejection fraction = 6.4%; 1 standard deviation for ratio of the mitral inflow E wave to the early diastolic myocardial relaxation velocity at septal mitral annulus = 12; 1 standard deviation for left ventricular end-systolic volume index = 30 mL/m2; 1 standard deviation for left ventricular end-diastolic volume index = 34 mL/m2; 1 standard deviation for cystatin C = 0.94 mg/L).
Figure 1.
Kaplan-Meier analysis of 5-year all-cause mortality in chronic systolic HF patients (n=133) with patients stratified according to optimal receiver operating characteristic curve galectin-3 cut-off value for prediction of all-cause mortality (14.4ng/mL, Figure 1A), with median cystatin C levels (1.23ng/mL, Figure 1B) and amino-terminal pro-B-type natriuretic peptide (1,240pg/mL, Figure 1C).
Abbreviations: +, above or equal to cut-off or median; −, below cut-off or median; Gal-3, Galectin-3; CysC, Cystatin C; NT-proBNP, amino-terminal pro-B-type natriuretic peptide.
Discussion
The major findings of this study include: 1) the lack of a strong relation between galectin-3 and echocardiographic measurements of cardiac structure and function in chronic stable systolic HF, and between galectin-3 and invasive hemodynamic measurements in advanced decompensated HF; and 2) the relatively stronger relation between galectin-3 and indices of renal dysfunction including cystatin C levels. Taken together, these data emphasize that galectin-3 is a pleotropic molecule mediating immune response, inflammation, and fibrogenesis rather than specific to cardiac (or renal) performance alone.
Galectin-3 is a macrophage-derived mediator, which can activate fibroblasts and induce their proliferation and collagen deposition1,5,15. In pre-clinical studies, increased expression of galectin-3 is associated with subsequent development of cardiac dysfunction1. Several human studies have indicated that elevated levels of galectin-3 provide independent prognostic value in HF. In the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study, higher galectin-3 levels measured by the same research assay were associated with poorer survival at 1-year follow-up in 209 subjects with a diagnosis of acute decompensated heart failure seen at the Emergency Department, as well as at 4-year follow-up in a subset of patients6,16. In the Deventer-Alkmaar heart failure study (DEAL-HF), 232 elderly subjects with advanced symptoms from chronic HF were followed for 6.5 years, and elevated galectin-3 was also found to be a significant predictor of mortality risk even following adjustment for age, eGFR, and NT-proBNP (hazard ratio 1.24, 95% confidence interval 1.03–1.50, p=0.026)8. The results within our chronic systolic HF cohort corroborate those from DEAL-HF, with higher plasma galectin-3 levels predicting increased risk of all-cause mortality. In contrast, we found a lack of relation between plasma galectin-3 levels and some of the strongest cardiac-specific echocardiographic or hemodynamic predictors of poor survival in systolic HF. The lack of any clear association with cardiac-specific indices in our study is surprising considering the fact that cardiac fibrosis is an important component of myocardial stiffness, and our findings are discrepant with prior reports. In the echocardiographic subset of the PRIDE study, where 76 subjects with acute decompensated HF and 39 non-HF patients underwent echocardiographic evaluation between 23–73 hours after hospital contact, galectin-3 levels were modestly associated with early diastolic myocardial relaxation velocity at mitral annulus (r=−0.246, p=0.03) and ratio of the mitral inflow early wave to early diastolic myocardial relaxation velocity at mitral annulus (r=0.345, p=0.01), as well as tricuspid and mitral regurgitation that was not seen in our cohort. It is important to recognize that the patient population in the PRIDE study was very different from our studies, with a mean LVEF 45.5±15%, a mean LV end-diastolic volume of 109±44ml, and a mean ratio of the mitral inflow early wave to early diastolic myocardial relaxation velocity at mitral annulus of 12.3±6 in the acute HF cohort (n=76)16. Only 15% of subjects in PRIDE admitted for acute decompensated HF had history of cardiomyopathy. In comparison, our study populations consisted of more advanced systolic HF (mean LVEF 26 and 28%, mean LV end-diastolic volume of 218 and 231ml, and mean ratio of the mitral inflow early wave to early diastolic myocardial relaxation velocity at mitral annulus of 15.5 and 20.3 in our chronic and acute systolic HF cohorts, respectively for acute and chronic HF), with echocardiographic measurements performed at the time of blood draw. Different assays used between different studies may also affect the findings, even though all the studies to date showed consistency in demonstrating relations between galectin-3 with renal function and all-cause mortality. Nevertheless, the mere differences in baseline echocardiographic characteristics may potentially explain the marked differences in the observations regarding the relationships between galectin-3 and cardiac structure and function.
While this discrepancy requires confirmation in future human studies, we hypothesize a few potential explanations. First, not all diastolic dysfunction in chronic systolic HF can be explained by myocardial fibrosis. In fact, extensive remodeling with cardiac enlargement inevitably causes myocyte stretch and altered relaxation as a result of myocardial deformities. Other non-fibrotic contributors of diastolic dysfunction include ischemia, altered energetics, and even disruption of normal calcium homeostasis. In addition, there may be a disassociation between tissue and circulating galectin-3 levels such that systemic levels of galectin-3 may not reflect the myocardial expression and consequences of galectin-3. An alternative explanation for our observation of a discrepancy between the ability of galectin-3 to predict risk without clear association with cardiac structure and function may lie in the relatively stronger relation between galectin-3 levels and renal function. This relation observed in our study is consistent with prior reports. In both PRIDE and DEAL-HF study, there was a strong inverse relation between galectin-3 levels and eGFR8,16. Interestingly, a recent report from the same investigators of DEAL-HF identified stronger prognostic value within subgroup of patients with HF and preserved ejection fraction, who may be more dependent on renal insufficiency as contributor of congestive symptoms17. Indeed, galectin-3 expression and secretion has been linked to the development of renal fibrosis in animal models5. In an experimental glomerulonephritis model, galectin-3 has been shown to modulate renal mesangial cell proliferation and led to alterations in extracellular matrix18. The availability of cystatin C measurements in our cohort, which demonstrated a strong correlation with galectin-3, further illustrated the tight association between circulating galectin-3 levels and renal insufficiency. It is interesting to note that cystatin C itself is a cysteine protease inhibitor endogenously and ubiquitously produced in all nucleated cells, which has been associated with alterations in extracellular matrix production9,19,20 and the presence of left ventricular hypertrophy in non-heart failure subjects21,22. It is therefore conceivable that the pathophysiologic role of galectin-3 leading to progressive renal compromise may be more prominent than its impact on cardiac pathology, and such an association with renal impairment may in part determine galectin-3's prognostic role in heart failure. Further investigations regarding the clinical utility of galectin-3 are warranted.
Acknowledgments
Dr. Tang has previously received research grant support from Abbott Laboratories, which has a licensing agreement with BG Medicine in the development of galectin-3 assay.
Funding sources: This work was supported by the American Society of Echocardiography Sonographer's Grant, the National Institutes of Health Clinical and Translational Science Award (CTSA UL1-RR024989), and the National Space Biomedical Research Institute (NASA NCC 9-58).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures All other authors declared no relationships to disclose.
References
- 1.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 2.Thandavarayan RA, Watanabe K, Ma M, Veeraveedu PT, Gurusamy N, Palaniyandi SS, Zhang S, Muslin AJ, Kodama M, Aizawa Y. 14-3-3 protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochem Pharmacol. 2008;75:1797–1806. doi: 10.1016/j.bcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, Carretero OA. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol. 2008;294:H1226–1232. doi: 10.1152/ajpheart.00305.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YH, D'Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, Andre S, Gabius HJ, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296:H404–412. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kimmenade RR, Januzzi JL, Jr., Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Lainscak M, Coletta AP, Sherwi N, Cleland JG. Clinical trials update from the Heart Failure Society of America Meeting 2009: FAST, IMPROVE-HF, COACH galectin-3 substudy, HF-ACTION nuclear substudy, DAD-HF, and MARVEL-1. Eur J Heart Fail. 2010;12:193–196. doi: 10.1093/eurjhf/hfp185. [DOI] [PubMed] [Google Scholar]
- 8.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010 doi: 10.1007/s00392-010-0125-y. Published online on May 21, 2010 at doi:10.1007/s00392-010-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YH, Lin LY, Wu YW, Chien KL, Lee CM, Hsu RB, Chao CL, Wang SS, Hsein YC, Liao LC, Ho YL, Chen MF. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009;409:96–99. doi: 10.1016/j.cca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Troughton RW, Prior DL, Pereira JJ, Martin M, Fogarty A, Morehead A, Yandle TG, Richards AM, Starling RC, Young JB, Thomas JD, Klein AL. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. 2004;43:416–422. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M, Fogarty A, Morehead AJ, Starling RC, Young JB, Thomas JD, Lauer MS, Klein AL. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96:257–262. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Tang WH, Van Lente F, Shrestha K, Troughton RW, Francis GS, Tong W, Martin MG, Borowski AG, Jasper S, Starling RC, Klein AL. Impact of myocardial function on cystatin C measurements in chronic systolic heart failure. J Card Fail. 2008;14:394–399. doi: 10.1016/j.cardfail.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010 doi: 10.1093/eurjhf/hfq091. Published online on June 5, 2010 at doi:10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki S, Bao Q, Hughes RC. Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J Pathol. 1999;187:481–489. doi: 10.1002/(SICI)1096-9896(199903)187:4<481::AID-PATH263>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Cheng XW, Obata K, Kuzuya M, Izawa H, Nakamura K, Asai E, Nagasaka T, Saka M, Kimata T, Noda A, Nagata K, Jin H, Shi GP, Iguchi A, Murohara T, Yokota M. Elastolytic cathepsin induction/activation system exists in myocardium and is upregulated in hypertensive heart failure. Hypertension. 2006;48:979–987. doi: 10.1161/01.HYP.0000242331.99369.2f. [DOI] [PubMed] [Google Scholar]
- 20.Sam F, Siwik DA. Digesting the remodeled heart: role of lysosomal cysteine proteases in heart failure. Hypertension. 2006;48:830–831. doi: 10.1161/01.HYP.0000242332.19693.e4. [DOI] [PubMed] [Google Scholar]
- 21.Ix JH, Shlipak MG, Chertow GM, Ali S, Schiller NB, Whooley MA. Cystatin C, left ventricular hypertrophy, and diastolic dysfunction: data from the Heart and Soul Study. J Card Fail. 2006;12:601–607. doi: 10.1016/j.cardfail.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel PC, Ayers CR, Murphy SA, Peshock R, Khera A, Balko JA, Gupta S, Mammen PP, Drazner MH, Markham DW. Association of cystatin C with left ventricular structure and function: the Dallas Heart Study. Circ Heart Fail. 2009;2:98–104. doi: 10.1161/CIRCHEARTFAILURE.108.807271. [DOI] [PubMed] [Google Scholar]