Abstract
Study Design
Immunohistochemical analysis of type IX collagen in disc tissue from spinal fusion patients.
Objective
To determine if collagen IX can be detected in adult disc tissue removed at spinal fusion surgery from patients either with or without degeneration-associated tryptophan SNPs and whether the distribution is associated either with severity of degeneration or incidence of a collagen IX SNP genotype.
Summary of Background Data
Genetic factors are strongly associated with risk of development and/or progression of disc degeneration. Two single nucleotide polymorphisms that introduce tryptophan polymorphisms in COL9A2 and COL9A3 are independently linked to an increased risk of lumbar disc disease. Although tryptophan variants are associated with accelerated degeneration, it is not known if collagen IX can be detected in adult disc tissue.
Methods
We selected age-matched disc samples from five clinical groups: Fracture with Trp(−) (6 cases), Herniation (6 cases), Degeneration (5 cases), Spondylolisthesis with Trp(−) (8 cases) and Spondylolisthesis/Herniation/Fracture with Trp(+) (6 cases of Trp3 allele and 1 case of Trp2 allele). Using H&E staining and immunohistochemical staining (collagens IX and IIA), 78 sections from 32 patients were analyzed. Selected disc tissues were assayed biochemically for collagen IX.
Results
Focal deposition of collagen IX was observed in regions of adult human disc tissue from spines showing degenerative changes in patients whether or not they were positive for a tryptophan SNP. However, in non-degenerative control disc tissue from fracture cases, little or no collagen IX was detected. The latter finding was confirmed by direct biochemical analyses for collagen IX in pooled samples of normal adult human annulus fibrosus or nucleus pulposus.
Conclusion
During growth and maturation of the disc, collagen IX is presumably removed completely during matrix remodeling so that the protein is absent from normal adult annulus and nucleus but can reappear at sites of degeneration presumably as part of a repair response to mechanical injury.
Introduction
It is now well recognized that genetic factors are important determinants of the risk of degenerative disc disease in the lumbar spine. Heritability is responsible for over 70% of lumbar spine degeneration after adjusting for environmental factors (1). The genetics are complex, however, and only weak risk associations for polymorphic variants of multiple genes have been found. For example, two single nucleotide polymorphisms (SNPs) that produce tryptophan polymorphisms in different collagen type IX genes, Gln326Trp in COL9A2 (Trp2 allele) and Arg103Trp in COL9A3 (Trp3 allele), have been linked to increased risk of lumbar disc disease assessed clinically and by MRI in several studies of different populations (2–6).
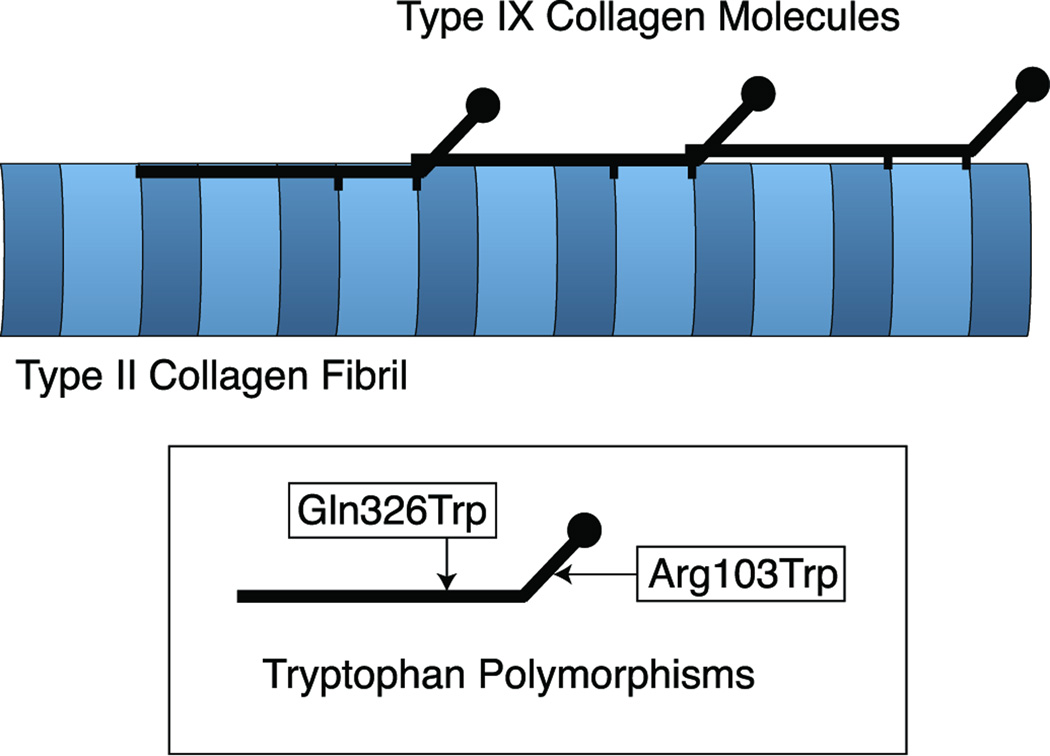
Collagen IX consists of a trimer of α1(IX), α2(IX) and α3(IX) chains, the products of three different genes (7). It functions as a fibril-associated molecule covalently cross-linked to newly formed type II collagen fibrils in cartilages where it acts as a collagen network/matrix bridging molecule that is required for tissue integrity and longevity (8–12, Fig. 1). It is possible that the tryptophan-containing allelic variants of collagen IX are detrimental to the collagen architecture of the disc established during tissue formation (13) or, for instance, after neo-expression in the adult tissue during healing to repair local injury, perhaps by sustaining an inflammatory response. Inflammatory mediators, TNF-α, IL-1 and IL-6 do appear to play a significant role in the pathogenesis of degenerative disc disease (14–16).
Figure 1.
Diagram showing type IX collagen molecules covalently link to the surface of type II collagen fibrils. Also shown are the two identified sites of tryptophan polymorphisms in type IX collagen.
In the present study the question we address is whether collagen type IX can be detected in adult disc tissue removed at spinal fusion surgery from non-degenerative controls and from patients genotyped for either of the two tryptophan polymorphisms and whether the tissue distribution of collagen type IX can shed light on the mechanism through which a tryptophan-encoded SNP can accelerate degeneration.
Materials and Methods
Study Design
Human disc tissue was available for analysis from a completed clinical study of collagen type IX genetic variants in patients requiring spinal fusion surgery (4). Patients were enrolled in the study to determine whether the clinical severity of lumbar spine pathology was associated with heterozygosity for either the COL9A2 or COL9A3 SNP variant that previous genetic studies have linked to severity of lumbar disc disease. The results of that study had shown a strong association of heterozygosity for the COL9A3 Trp-encoded SNP (α3(IX) W103) with spondylolisthesis, the most severe of the five grades of lumbar spine disease that were classified (4).
A cross-section of the surgical disc tissue samples were examined by immunohistochemistry and Western blot analysis to determine if: 1) collagen type IX could be detected in adult annulus fibrosus and nucleus pulposus. Spinal fracture cases resulting from traumatic injury were included in the clinical study, so disc tissue from these served as non-degenerative controls. 2) collagen type IX was abnormally accumulated or focally distributed in association with clinical disease severity or collagen type IX SNP genotype.
Tissue Samples
Disc tissue was obtained from 133 patients undergoing spinal fusion surgery (4). The Institutional Review Board at the University of Washington approved this study. The diagnostic classification was made by four surgeons independently within a hierarchical range of five disease severities (17, 18): (1) Fracture (no degeneration); (2) Disc degeneration; (3) Disc herniation; (4) Spinal stenosis without spondylolisthesis; (5) Spinal stenosis with spondylolisthesis. Of 133 patients, 11 patients were identified with either positive SNP Trp2 (4 cases) or SNP Trp3 (7 cases) polymorphism.
We selected disc samples for analysis in each group matched by age (see Tables 1 and 2): Fracture (6 cases), Herniation (6 cases), Degeneration (5 cases), Spondylolisthesis with Trp(−) (8 cases) and Spondylolisthesis/Herniation/Fracture with Trp(+) (6 cases of Trp3 allele and 1 case of Trp2 allele). Due to the difficulty in judging the exact origin and orientation of surgical samples, we grossly selected tissue samples that best represented nucleus pulposus and annulus fibrosus based on gross appearance and texture. To compare type IX collagen distribution in a normal young mammalian intervertebral disc as a reference control, an intact ox tail disc (18 mo steer, local meat supplier) was analyzed.
Table 1.
Patient characteristics and findings for the fracture group (Trp−) and lumbar degenerative spondylolisthesis/disc herniation/fracture group (Trp+)
| IIA |
Type IX |
α1(IX)NC4 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Age | Sex/Race | Dx | Trp | Location | Procedure | NP | EP | IA | NP | EP | IA | NP | EP | IA |
| D7933 | 16 | F(c) | Fx | (−) | T11-L1 | Fusion | − | n/a | − | ± | n/a | − | − | n/a | ± |
| D1388 | 19 | F(c) | Fx | (−) | L2-4 | Fusion | − | − | − | − | − | − | − | ± | − |
| D1447 | 26 | M(b) | Fx | (−) | L3-4 | Fusion | − | − | +* | − | − | +* | − | + | +* |
| D8969 | 47 | F(c) | Fx | (−) | L1-2 | Fusion | − | n/a | − | − | n/a | − | − | n/a | − |
| D1110 | 56 | M(a) | Fx | (−) | L 1-2 | Fusion | − | − | ±* | ± | − | +* | − | − | +* |
| D6044 | 64 | F(c) | Fx | (−) | L1-2 | Fusion | − | n/a | − | − | n/a | − | − | n/a | − |
| D9341 | 24 | F(c) | SP | W3 | L4-S1 | Fusion | ++ | − | ++ | ± | ± | ± | − | − | − |
| D7063 | 33 | M(b) | H | W3 | L4-S1 | Fusion | ++ | ± | ++ | + | ± | + | + | − | ++* |
| D9612 | 44 | F(c) | SP | W3 | L5-S1 | Fusion | ++ | ± | ++ | − | ± | − | − | − | − |
| D8544 | 50 | F(c) | SP | W3 | L5-S1 | Fusion | + | − | ++ | − | ± | + | − | − | + |
| D3806 | 52 | F(b) | SP | W2 | L4-5 | Fusion | + | ± | + | ± | ± | ++ | − | + | ++ |
| D5965 | 66 | M(c) | Fx | W3 | T4-6 | Fusion | − | − | − | − | + | − | − | − | − |
| D3002 | 77 | M(c) | SP | W3 | L1-S1 | Fusion | ++ | ± | − | − | − | ± | − | − | ± |
F: Female; M: Male; a: Asian; b: African American; c: Caucasian; Fx: Fracture; H: Disc Herniation; SP: Spondylolisthesis; W2: Tryptophan polymorphism at residue 326 of the α2(IX) chain; W3: Tryptophan polymorphism at residue 103 of the α3(IX) chain; IIA: type IIA procollagen; α1(IX) NC4: long form of α1(IX) chain; NP: nucleus pulposus; EP: end plate; IA: inner annulus; (−): negative; (±): weak positive staining; (+): Clear positive staining; (++): strong positive staining; n/a: tissue is not available;
Positive staining on annulus just adjacent to EP.
Table 2.
Summary of patient’s characteristics and immunohistochemistry of disc herniation, lumbar degeneration and spondylolisthesis groups without tryptophan polymorphism (Trp−)
| IIA |
Type IX |
α1(IX) NC4 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Age | Sex/Race | Dx | Trp | Location | Procedure | NP | EP | IA | NP | EP | IA | NP | EP | IA |
| D5646 | 23 | F (x) | H | (−) | L4-5 | Discectomy | + | + | ++ | + | + | + | ± | + | ± |
| D3550 | 37 | M(c) | H | (−) | L5-S1 | Discectomy | + | n/a | ++ | ++ | n/a | ++ | ± | n/a | + |
| D8892 | 40 | M(c) | H | (−) | L4-5 | Fusion | ++ | + | ++ | ++ | + | +* | ± | + | +* |
| D3035 | 45 | M(c) | H | (−) | L5-S1 | Discectomy | ++ | ++ | ± | ± | + | − | − | − | − |
| D2440 | 57 | F (c) | H | (−) | L 4-5 | Discectomy | + | ± | + | + | ++ | − | ± | ± | − |
| D5641 | 66 | F (c) | H | (−) | L3-5 | Discectomy | ++ | + | + | − | ± | ± | − | − | − |
| D2496 | 48 | M(c) | D | (−) | L2-4 | Fusion | + | + | + | ± | ++ | ± | − | ±/+ | ± |
| D4885 | 55 | M(c) | D | (−) | L3-4 | Discectomy | ++ | n/a | + | + | n/a | ± | − | n/a | − |
| D1370 | 59 | F (c) | D | (−) | L4-5 | Fusion | ++ | + | + | ± | − | − | − | − | − |
| D9039 | 62 | M(c) | D | (−) | L3-S1 | Fusion | + | ± | − | ± | + | + | − | − | ± |
| D6786 | 76 | M(c) | D | (−) | L4-5 | Fusion | + | + | ± | + | ++ | ± | − | − | − |
| D8581 | 28 | M(x) | SP | (−) | L4-5 | Fusion | ± | ± | − | − | ± | − | − | ± | − |
| D6403 | 24 | M(a) | SP | (−) | L4-S1 | Fusion | + | + | ++ | − | + | ± | − | + | + |
| D1937 | 36 | M(c) | SP | (−) | L4-5 | Fusion | ++ | + | + | + | + | ± | − | ± | − |
| D5713 | 47 | F (c) | SP | (−) | L5-S1 | Fusion | ± | ± | − | − | + | ± | − | ± | − |
| D3830 | 55 | F (c) | SP | (−) | L4-S2 | Fusion | ± | − | − | − | + | − | − | − | − |
| D3210 | 64 | M(c) | SP | (−) | L4-5 | Fusion | ++ | − | − | + | + | − | − | − | − |
| D1365 | 71 | F (c) | SP | (−) | L4-5 | Fusion | + | − | − | − | − | − | − | − | − |
| D9713 | 78 | F (c) | SP | (−) | L4-S1 | Fusion | ++ | + | + | + | + | − | − | ± | ± |
F: Female, M: Male, a: Asian; b: African American; c: Caucasian; x: Hispanic; Fx: Fracture, H: Disc Herniation, D: Disc Degeneration, SP: Spondylolisthesis; Trp: Tryptophan polymorphism; IIA: type IIA procollagen, α1(IX) NC4: long form of α1(IX) chain; NP: nucleus pulposus, EP: end plate, IA: inner annulus; (−): negative, (±): weak positive staining, (+): Clear positive staining, (++): strong positive staining, n/a: tissue is not available,
Positive staining in annulus just adjacent to EP.
Biochemical analysis
Selected disc samples were assayed for type IX collagen. Samples were minced and extracted in 4 M guanidine HCl, 0.05 M Tris-HCl, pH 7.4, containing protease inhibitors (2 mM phenylmethylsulfonyl fluoride, 10 mM o-phenanthroline, 2 mM EDTA, 5 mM benzamidine), at 4°C for 48 h to remove non-cross-linked matrix proteins and proteoglycans. Residues were washed thoroughly with water, digested with CNBr in 70% formic acid under N2 at room temperature for 24 h and freeze-dried. The CNBr digests were dissolved in 1% (v/v) trifluoroacetic acid (TFA) and chromatographed on a C8 reverse-phase HPLC column (Brownlee Aquapore RP-300) using a linear gradient (10–60%) of acetonitrile:n-propanol (3:1, v/v) in aqueous 0.1% (v/v) TFA at 1 ml/min over 60 min, monitoring 220-nm absorbance as described previously (19). Reverse-phase HPLC fractions of CNBr-peptides were run on SDS-12.5% (w/w) polyacrylamide gels and transblotted to a nitrocellulose membrane (Bio-Rad Laboratories) using a Milliblot-SDE electroblotting apparatus (Millipore). Western blot analysis was performed using a polyclonal antiserum that recognizes multiple epitopes in all three chains of human and bovine collagen type IX (UW 186; 20) and a peroxidase-conjugated secondary antibody. Immuno-reactive CNBr-peptide bands were detected using chemiluminescence Western blot assay.
In addition, we used pepsin digestion in an attempt to isolate collagen IX from adult human annulus and nucleus (after 4 M guanidine HCl extraction) following a procedure developed for articular cartilage, in which the 1.8 M NaCl precipitate of a pepsin digest contains most of the type IX collagen (8).
Antibodies
Three antibodies were used in this study. To identify the biosynthetic activity of disc cells, rabbit anti-type IIA antiserum, which was kindly provided by Linda Sandell (21), was used. This antibody has been demonstrated to identify type II collagen procollagen NH2-peptide intracellularly and extracellularly (21, 22). A polyclonal rabbit anti-type IX serum (UW186) was used to recognize collagenous domains of all three chains of type IX collagen and a rabbit anti-α1(IX) NC4 serum (UW176) raised against a recombinant protein according to human α1(IX) NC4 sequences was used to demonstrate the presence of the long form of α1(IX) chain that contains the NC4 globular domain.
Immunohistochemistry
Disc samples were embedded in OCT compound (Miles Laboratories, Inc., Elkhart, IL) and frozen at −70°C. 10µm frozen sections were cut, mounted on polylysine-coated slides (Fisher Scientific Co.) and stored at −70°C before analysis.
Frozen sections were air dried and fixed in 10% PBS-buffered formalin, pH 7.4, for 30 minutes at room temperature. After washing in water to remove residual formalin, endogenous peroxidase was blocked by 3% H2O2 in water for 30 minutes at room temperature. Tissue sections then were digested with 1% hyaluronidase in PBS, pH 7.4, for 45 minutes at 37°C. After several PBS washing steps, sections were blocked with 2.5% goat serum (Universal Elite®, ABC Kit, Vector, Inc.) at 37°C for one hour. A series of sections were then incubated with different concentrations of each primary antibody, anti-type IIA at 1:1000, UW186 at 1:500 and UW176 at 1:500 overnight at 4°C. All antibodies were diluted in PBS with 1% BSA. Thereafter, to detect primary antibody, the avidin-biotin system (Universal Elite®, ABC Kit, Vector, Inc.; and Peroxidase Substrate Kit Vector® VIP, Vector, Inc.) was applied according to manufacturer’s instructions. The photo images were viewed and recorded by a digital imaging system.
A ranked scoring system based on the degree of positive staining was applied: 1) (−) absent staining; 2) (±) weak positive staining; 3) (+) clear positive staining; 4) (++) strong positive staining. For a negative control, we treated parallel sections with normal rabbit serum (Sigma) at the same dilution but without the primary antibody.
Results
Histology and immunohistochemistry
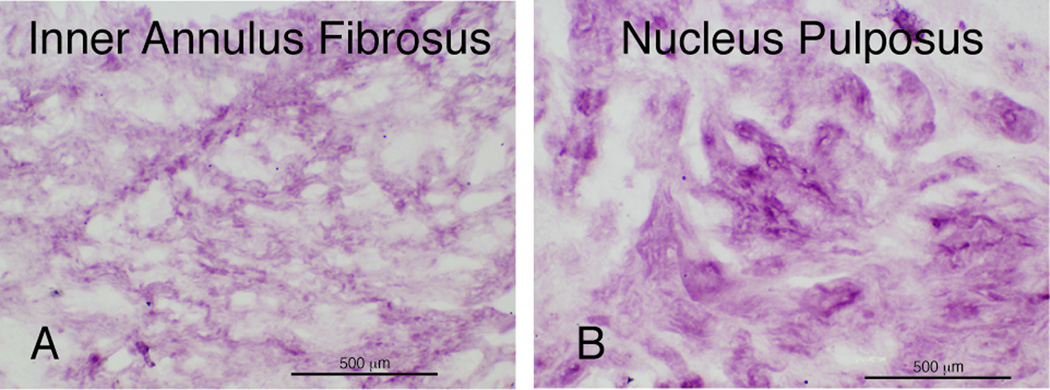
The control bovine disc tissue showed a histological gradation in matrix appearance from NP to annulus typical for a young, recently developed disc (23). The immunolocalization of type IX collagen in ox tail disc tissue revealed a distribution pattern expected for the radial gradient of increasing type II collagen concentration from outer annulus to nucleus pulposus (Fig. 2, A and B). Type IX collagen was strongly detected in pericellular and inter-territorial matrix in NP and inner annulus, but not in outer annulus (not shown) where type I collagen is more prominent (24).
Figure 2.
Immunolocalization of type IX collagen on ox tail disc. Panel A: inner annulus. Panel B: Nucleus pulposus.
All the human disc tissue analyzed was from discs in the spinal segment L3 to S1 except one fracture sample from T11-L1 and one Trp3-positive sample from T4T5 or T5T6. A total of 78 sections from 32 patients were analyzed by H&E staining and immunohistochemistry.
Three histological structures, nucleus pulposus (NP), end plate (EP) and inner annulus (IA), were identified microscopically. As we expected, tissue alterations consistent with degenerative and reparative lesions, such as clusters of chondrocytes (cloning), clefts and tears in NP and IA, were evident in most specimens from herniated, degenerative and spondylolisthesis cases. From the spinal fracture cases, only older individuals (50–60 years) showed signs of chondrocyte cloning. In some cases, some focal mucoid degeneration of the NP was evident. Regions of advanced degeneration and reparative change were seen in disc tissue from most degenerative and spondylolisthesis cases. Large chondrocyte clusters (clones) were most commonly seen in disc tissue from older individuals.
Tables 1 and 2 summarize the immunohistochemistry results for type IIA procollagen, type IX collagen and the NC4 domain of the long form of the α1(IX) chain in control (fracture) and degenerative human disc samples. Fig. 3 shows examples of the immuohistochemical staining on tissues from herniated discs either with (D7063) or without (D8892, D3550) a Trp3 allele and a spondylolisthesis disc with a Trp3 allele (D3806).
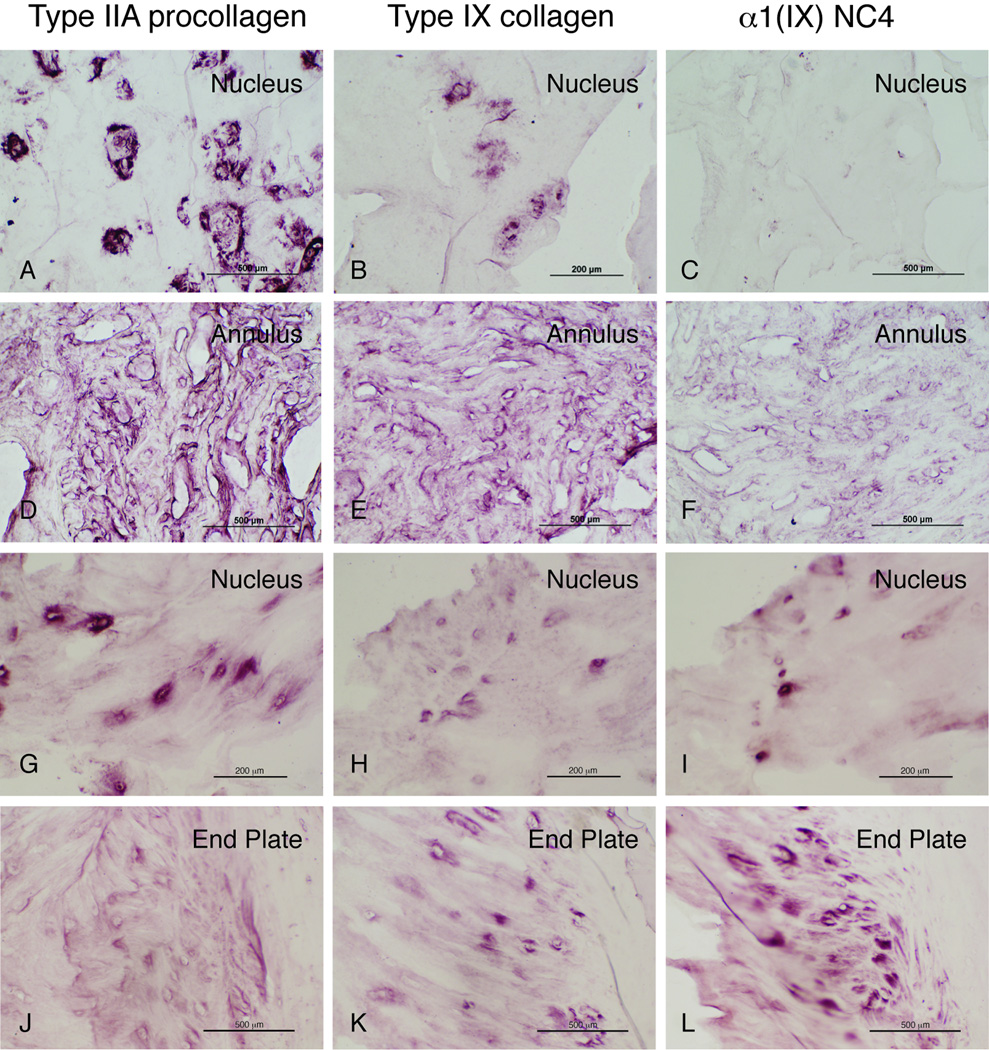
Figure 3.
Immunohistochemistry of type IIA procollagen, type IX collagen and the long form of α1(IX) chain that contain the NC4 globular domain.
Panel A, B and C: disc herniation (D8892)
Panel D, E and F: disc herniation (D3550)
Panel G, H and I: disc herniation with Trp3 polymorphism (D7063)
Panel J, K and L: spondylolisthesis disc with Trp2 polymorphism (D3806).
The fracture group, with no clear clinical history of back pain, was considered to be age-matched normal controls. By immunohistochemistry these disc samples showed no type IIA procollagen in nucleus pulposus (NP) or end plate (EP). In two cases, D1447 and D1110, some IIA was localized in the annulus adjacent to EP, where type IX collagen and type IX NC4 domain were also detected. Immunohistostaining of type IX collagen and type IX NC4 were not detected in NP and EP of fracture cases.
In contrast to the fracture group, there was very strong immunohistostaining of type IIA procollagen in herniated, degenerative and spondylolisthesis discs (Table 2). Type IIA procollagen was extensively localized intracellularly and in the pericellular matrix of NP and inner annulus (Fig. 3, A, D, G and J) in almost all cases and in EP in some cases. Again, some type IIA procollagen was evident in annulus tissue adjacent to EP in the fracture group samples.
There tended to be a co-localized immunostaining distribution of type IX collagen and type IIA collagen to the same tissue region, though type IX was less intensive. Type IX collagen was clearly detected in NP, EP and annulus. This was most evident in herniated discs from young to middle aged individuals (D5646, D3550, D8892) in the pericellular matrix (Fig. 3, Panel A–F). In contrast, there was less immunostaining of type IX collagen in degenerative discs, and least in spondylolisthesis discs with or without a Trp-encoding SNP polymorphism. Type IX collagen was detected in EP of most cases (Tables 1 and 2). Comparing this to the spondylolisthesis diagnostic group there was a possible trend for more collagen type IX staining in the subset without a Trp-encoding SNP than in those with a Trp-encoding SNP (lower set in Table 2 vs. lower set of Dx SP in Table 1). Interestingly, the long form of α1(IX) chain that contains the NC4 globular domain was mainly detected intracellularly and in the pericellular matrix of EP and annulus, most consistently seen in annulus adjacent to EP (Fig. 3, panel L). There was no clear immunolocalization of α1(IX) NC4 globular domain in NP in most samples except one case (D7063) of spondylolisthesis with a Trp SNP (Fig. 3, panel I). Herniated discs tended to show more staining for the α1(IX) NC4 domain (Table 2).
Isolation and Western blot analysis of type IX collagen from disc tissue
No type IX collagen could be detected in the 1.8M NaCl precipitate of a pepsin digest of a large sample of either annulus fibrosus or nucleus pulposus from adult human disc tissue by Coomassie Blue staining. From the results in comparison with yields from adult articular cartilage (8) we could estimate that no more than 0.01% of the total collagen in adult disc tissue could be type IX collagen. This contrasts with immature disc tissue, which does yield significant amounts by this procedure.
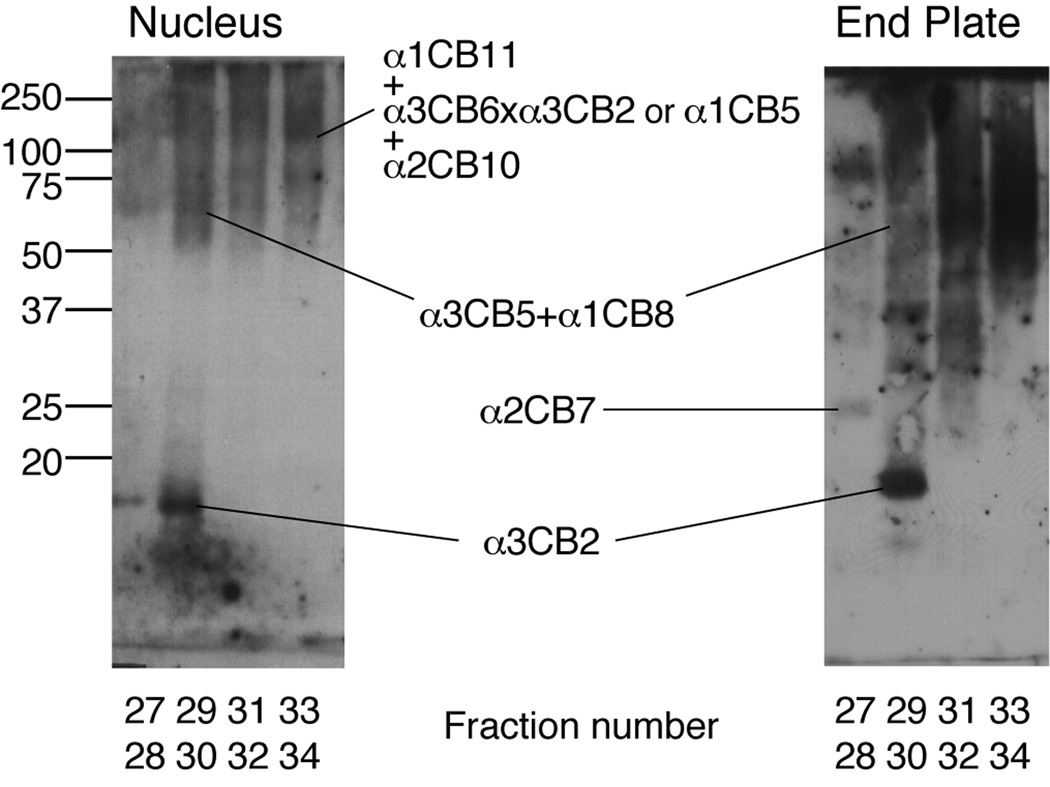
Figure 4 shows the results of Western blot analysis for type IX collagen in an SDS-PAGE separation of a CNBr-digest of spondylolisthesis disc, from a Trp(+) patient (D9341). After reverse-phase HPLC to concentrate type IX collagen peptides by resolving the bulk of the type II collagen CB peptides (19), several linear and cross-linked type IX collagen CNBr-peptides could be identified from nucleus pulposus and end plate using the anti-collagen IX serum (Fig. 4). A particularly strong epitope is recognized in a fragment (CB2) of α3(IX) by this polyclonal antiserum as a definitive marker of type IX collagen (19). The findings confirm the positive identification of type IX collagen in the disc tissue samples examined immunohistochemically.
Figure 4.
Western blot analysis of type IX collagen CB-peptides from spondylolisthesis disc with Trp3 polymorphism (D9341). The collagen CB-peptides were partially resolved by reverse-phase HPLC, column fractions were run on SDS- 12.5% PAGE and then transblotted to nitrocellulose membrane, and the collagen IX fragments were revealed using an anti-type IX collagen rabbit antiserum (UW 186).
Discussion
The findings indicate deposition of type IX collagen in regions of adult human disc tissue where degenerative changes are occurring. In control tissue from fracture cases, little or no collagen IX was detected in disc tissue itself, only in end- plate cartilage. This conclusion was supported by the direct biochemical analyses for type IX collagen, which could barely detect the protein in normal adult human annulus fibrosus and nucleus pulposus. Our findings are consistence with an earlier report that type IX collagen can be detected in adult rat and bovine disc tissue but not in adult human discs (25). This presumably reflects the large chronological difference in age of mature human discs versus rat and bovine. It is notable that young nucleus pulposus and young discs generally, which do contain type IX collagen (26, 27), differ from hyaline cartilage in the molecular form of type IX collagen deposited during disc development. The molecule contains a short chain form of α1(IX), the product of an alternative promoter, that lacks the amino terminal NC4 globular domain present in cartilage type IX collagen (12). Notably, the NC4 domain was detected mainly associated with cells and pericellular matrix of EP and in annulus adjacent to EP (Fig. 3, panel L) with no clear immunolocalization of α1(IX) NC4 in NP.
Strong staining for type IIA procollagen and type IX collagen was seen in disc samples from patients with disc herniation, degeneration and spondylolisthesis but not from the control fracture group. The most likely explanation for this is the expression of type IIA and type IX collagen in regions of disc tissue undergoing degenerative changes in response to injury, for example, caused by herniation and on exposure to macrophages and the innate immune system. It has been shown that in osteoarthritic (OA) joints there is a stimulated expression in articular cartilage of several collagen genes, including the IIA form of COL2A1 (28, 29).
During growth and maturation of the collagen fibril network, therefore, collagen IX is presumably removed completely by matrix remodeling so that it is virtually absent from normal adult annulus and nucleus but can make a reappearance at sites of degeneration and mechanical injury. This contrasts with the situation in adult human articular cartilage where about 1% of the adult collagen is still type IX (9). The amount of collagen IX deposited in degenerate discs does not appear to be related to the patient’s Trp allele genotype status. The main determinant appears to be whether degenerative changes have occurred or not, with herniation apparently resulting in the strongest collagen IX presence (Table 2).
The findings may be relevant in understanding the mechanism through which SNPs encoding tryptophan in collagen type IX are associated with risk of progressive, early onset lumbar disc degeneration (2–6). Though an impaired anatomical structure during development is one possibility (13), the Trp-containing allelic product is known to be incorporated into cross-linked collagen in fetal cartilage (20) and, as shown here, can be deposited and apparently cross-linked into reparative tissue in adult discs (Fig. 4). In considering how the neo-expression of collagen IX containing a chain from a Trp-positive allele in an injured disc could promote and sustain further damage, one possibility is through chronic activation of macrophages, but only in individuals with a genetically susceptible innate immune system.
Key Points.
In non-degenerative control disc tissue from fracture cases, little or no collagen IX was detected either by immunohistostaining or direct biochemical analysis.
Deposition of type IX collagen was found in inner regions of adult human disc tissue showing degenerative changes where type IIA procollagen was also expressed.
The results support a concept that re-expression of type IX collagen occurs at sites of disc degeneration as part of a repair response to mechanical injury.
We speculate that the products of COL9 Trp alleles associated with lumbar disc disease may compound degeneration in genetically susceptible individuals.
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grants AR37318 and AR36794 (DRE); and the Burgess Chair Endowment of the University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalichman L, Hunter DJ. The genetics of intervertebral disc degeneration. Familial predisposition and heritability estimation. Joint Bone Spine. 2008;75:383–387. doi: 10.1016/j.jbspin.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Annunen S, Paassilta P, Lohiniva J, et al. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285:409–412. doi: 10.1126/science.285.5426.409. [DOI] [PubMed] [Google Scholar]
- 3.Paassilta P, Lohiniva J, Goring HH, et al. Identification of a novel common genetic risk factor for lumbar disc disease. JAMA. 2001;285:1843–1849. doi: 10.1001/jama.285.14.1843. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y, Mirza SK, Wu JJ, et al. The association of lumbar spondylolistesis with collagen IX tryptophan alleles. J Bone Joint Surg Br. 2004;86-B:1021–1026. doi: 10.1302/0301-620x.86b7.14994. [DOI] [PubMed] [Google Scholar]
- 5.Jim JJ, Noponen-Hietala N, Cheung KM, et al. The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine. 2005;30:2735–2742. doi: 10.1097/01.brs.0000190828.85331.ef. [DOI] [PubMed] [Google Scholar]
- 6.Higashino K, Matsui Y, Yagi S, et al. The alpha2 type IX collagen tryptophan polymorphism is associated with the severity of disc degeneration in younger patients with herniated nucleus pulposus of the lumbar spine. Int Orthop. 2007;31:107–111. doi: 10.1007/s00264-006-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Rest M, Mayne R. Type IX collagen. In: Mayne R, Burgeson RE, editors. Structure and Function of Collagen Types. Orlando, FL: Academic Press; 1987. pp. 195–221. [Google Scholar]
- 8.Wu JJ, Eye DR. Cartilage type IX collagen is cross-linked by hydroxypyridinium residues. Biochem Biophys Res Comm. 1984;123:1033–1039. doi: 10.1016/s0006-291x(84)80237-5. [DOI] [PubMed] [Google Scholar]
- 9.Wu JJ, Woods PE, Eyre DR. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J Biol Chem. 1992;267:23007–23014. [PubMed] [Google Scholar]
- 10.Diab M, Wu JJ, Eyre DR. Collagen type IX of human cartilage: A structure profile of intermolecular cross-linking sites. Biochem J. 1996;314:327–332. doi: 10.1042/bj3140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre DR, Pietka T, Weis MA, et al. Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J Biol Chem. 2004;1:2568–2574. doi: 10.1074/jbc.M311653200. [DOI] [PubMed] [Google Scholar]
- 12.Wu JJ, Eyre DR. Intervertebral disc collagen: Usage of the short form of α1(IX) in bovine nucleus pulposus. J Biol Chem. 2003;278:24521–24545. doi: 10.1074/jbc.M302431200. [DOI] [PubMed] [Google Scholar]
- 13.Aladin DM, Cheung KM, Chan D, et al. Expression of the trp2 allele of COL9A2 is associated with alterations in the mechanical properties of human intervertebral discs. Spine. 2007;32(25):2820–2825. doi: 10.1097/BRS.0b013e31815b75c5. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 15.Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 16.Kang JD, Georgescu HI, McIntrye-Larke L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6 and prostaglandin E2. Spine. 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology: Recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26:93–113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Wiltse LL, Winter RB. Terminology and measurement of spondylolisthesis. J Bone Joint Surg (Am) 1983;65-A:768–772. [PubMed] [Google Scholar]
- 19.Ichimura S, Wu JJ, Eyre DR. Two-dimensional peptide mapping of cross-linked type IX collagen in human cartilage. Arch Biochem Biophys. 2000;378:33–39. doi: 10.1006/abbi.2000.1805. [DOI] [PubMed] [Google Scholar]
- 20.Matsui Y, Wu J, Weis MA, et al. Matrix deposition of tryptophan-containing allelic variants of type IX collagen in developing human cartilage. Matrix Biol. 2003;22:122–128. doi: 10.1016/s0945-053x(02)00102-6. [DOI] [PubMed] [Google Scholar]
- 21.Oganesian A, Zhu Y, Sandell LJ. Type IIA procollagen amino propeptide is localized in human embryonic tissues. J Histochem Cytochem. 1997;45:1469–1480. doi: 10.1177/002215549704501104. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Oganesian A, Keene DR, et al. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TFG-beta1 and BMP-2. J Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88:10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 24.Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts S, Menage J, Duance V, et al. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine. 1991;16:1030–1038. [PubMed] [Google Scholar]
- 26.Eyre DR. The intervertebral disc and spinal disease: Biochemical concepts. In: Gatchel R, Mayer T, Mooney V, editors. Contemporary Conservative Care for Spinal Disorders: Concepts, Diagnosis and Treatment. Philadelphia: Lea & Febiger; 1991. pp. 74–83. [Google Scholar]
- 27.Eyre DR, Matsui Y, Wu JJ. Collagen polymorphisms of the intervertebral discs. Biochem Soc Trans. 2002;30:844–848. doi: 10.1042/bst0300844. [DOI] [PubMed] [Google Scholar]
- 28.Sharif M, Kirwin J, Charni N, et al. A 5-yr longitudinal study of type IIA collagen synthesis and total type II collagen degradation in patients with knee osteoarthritis—association with disease progression. Rheumatology. 2007;46:938–943. doi: 10.1093/rheumatology/kel409. [DOI] [PubMed] [Google Scholar]
- 29.Sive JI, Baird P, Jeziorsk M, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]