Abstract
Objectives
To evaluate the histopathology of the temporal bones of a patient with documented superficial siderosis of the central nervous system who underwent right cochlear implantation six years before death.
Background
Superficial siderosis of the central nervous system is due to chronic or repeated subarachnoid hemorrhage and results in sensorineural deafness in 95% of affected individuals in addition to other neurologic findings. The deposition of hemosiderin in the meninges and around cranial nerves is thought to be causative. There have been no previous reports of temporal bone pathology in this disorder.
This 57 year old man developed progressive, bilateral hearing loss starting in his 30's with loss of pure tone thresholds and word recognition. He underwent a right cochlear implant at age 51 with full insertion of the device.
Methods
The temporal bones and brainstem were fixed in formalin and prepared for histologic study by standard techniques. Special stains, including Gomori stain for iron were performed on sections of the temporal bones and cochlear nucleus.
Results
There was severe bilateral degeneration of the organ of Corti, spiral ligament, stria vascularis, and spiral ganglion cells. Gomori stain revealed iron deposits within the spiral ligament, stria vascularis and in the subepithelial mesenchymal tissue of the maculae of the vestibular system. Evaluation of the cochlear nucleus revealed iron deposits within glial cells and larger cells, probably macrophages, near the CSF surface. On the right side, the track created by the cochlear implant entered the scala tympani and continued to mm17, as measured from the round window.
Discussion and Conclusions
This is the first known case of superficial siderosis with documented temporal bone histopathology. Hearing loss was likely caused by severe degeneration of spiral ganglion cells in both ears, despite the presence of remaining hair cells in the middle and apical turns. This was consistent with cochlear neuronal degeneration and retrograde degeneration of spiral ganglion cells within the inner ear, or alternatively, consistent with primary degeneration of hair cells and neural structures within the cochlea. Despite the presence of neural degeneration, the patient achieved a word recognition score of 28% six months following implantation.
Keywords: Superficial siderosis, histopathology, temporal bone, hearing loss, cochlear implantation
Introduction
Superficial siderosis of the central nervous system is due to chronic or repeated subarachnoid hemorrhage and results in sensorineural deafness in 95% of patients, cerebellar ataxia in 88%, pyramidal signs in 76%, dementia in 24%, bladder disturbances in 24%, anosmia in 17%, anisocoria in 10%, and sensory signs in 13% (1). The deposition of hemosiderin in the meninges, brainstem, cerebellum, spinal cord, and around cranial nerves is thought to be causative (2). Diagnosis is confirmed by the presence of xanthochromic spinal fluid and the loss of signal intensity on T2 weighted MRI images due to magnetic susceptibility effects of iron (3,4). The pathogenesis of tissue destruction in superficial siderosis of the CNS may include effects of hemosiderin itself, but also iron catalyzed lipid peroxidation (5). The localization of tissue damage perhaps is also mediated by ferritin reactive microglia which permits the rapid conversion of iron to hemosiderin (5). The high vulnerability of the eighth nerve is thought due to its long glial segment and the fact that the eighth nerve passes through the pontine cistern which has a greater flow of CSF than for other cranial nerves (1). A progressive, high frequency sensorineural hearing loss has been described in a number of case reports (6-8).
There are case reports of both successful (9/10) as well as unsuccessful (11) cochlear implantation in this disorder.
To date, the temporal bone histopathology in superficial siderosis of the CNS has not been described. However, there have been descriptions of pathology of the CNS in this disorder. Thus, Revesz et al, (12) describes intense deposition of hemosiderin with reactive gliosis in the leptomeninges, subpial and subependymal areas of the hemispheres, brainstem, cerebellum, spinal cord and selectively the first, second, fifth, seventh, eighth, and tenth cranial nerves, and also spinal nerves. Tomlinson et al, (13) described pigmentation of cranial nerves, hyalin bodies, macrophage reaction, astrocytic proliferation, and severe gliosis and degeneration of myelin. We report the first description of the temporal bone histopathology in a well documented case of superficial siderosis in a 57 year-old man who underwent unilateral cochlear implantation.
Case report
This 57 year old man first noted a hearing loss in both ears in his late 30's. There was a history of loud noise exposure during a 14 month period in military service at 20 years of age. The diagnosis of superficial siderosis was made on the basis of MR scans of the CNS and the presence of xanthochromic spinal fluid on lumbar puncture. Although there was a history of lumbar disc surgery with fusion at age 42, complete angiography of the brain and spinal cord failed to reveal a source of bleeding. His other neurologic symptoms included progressive weakness of the lower extremities resulting in spastic paraparesis, a neurogenic bladder, and progressive sensory loss in the lower body below the umbilicus. At age 51, he had a transient loss of vision in his left eye attributed to possible migraine.
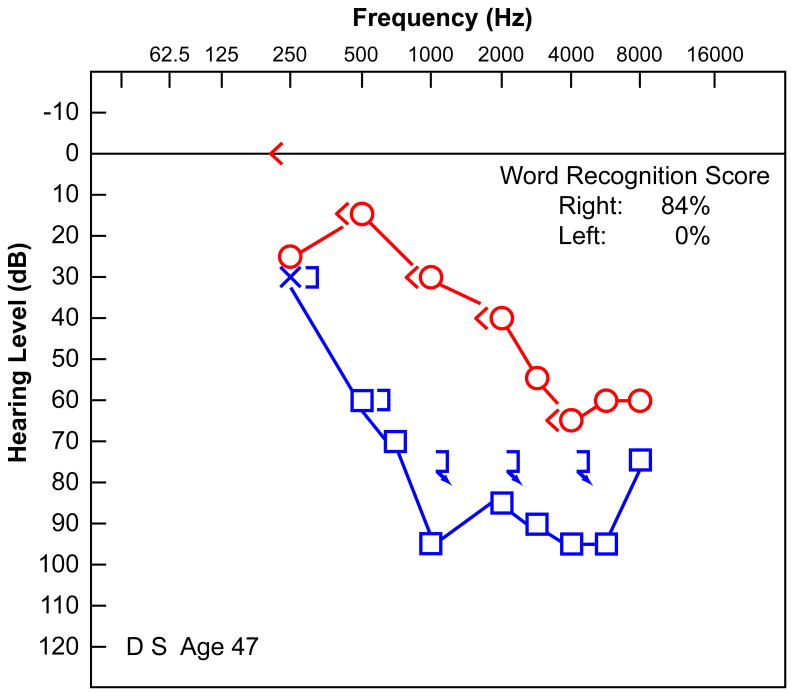
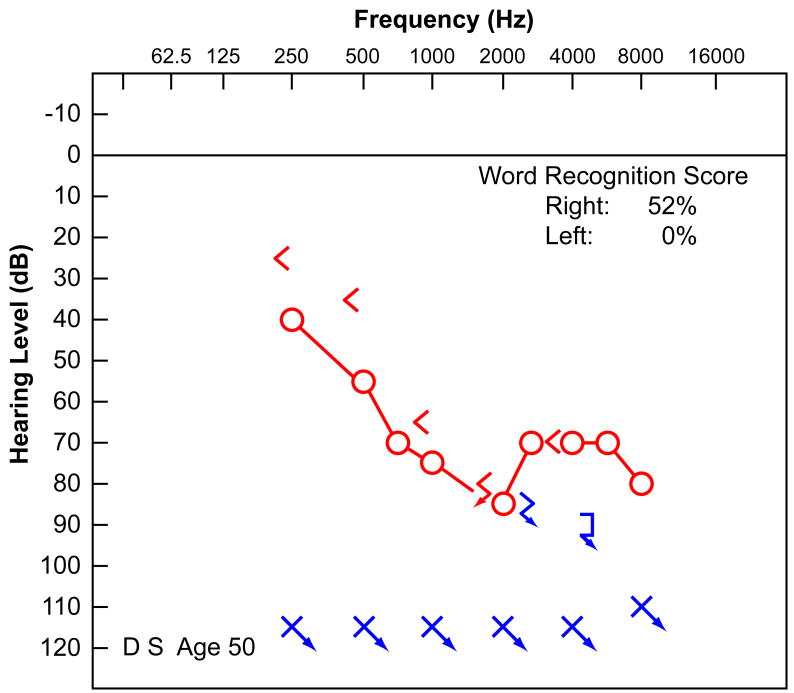
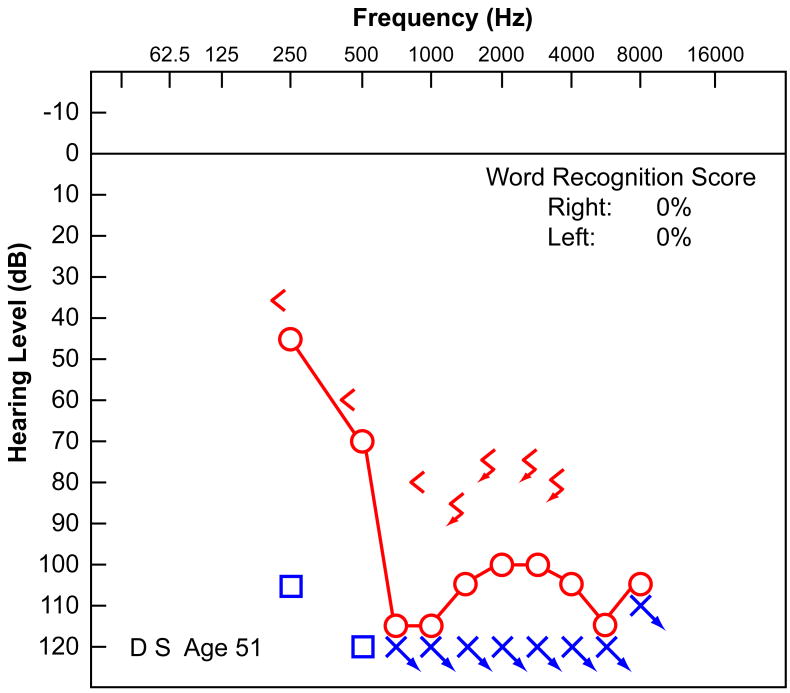
His audiometry was well documented. At age 47 (Fig. 1a), he had a bilateral, downsloping, sensorineural hearing loss worse in the left ear, with reduced speech discrimination (0% left ear, 84% right ear). At the age of 50 (Fig. 1b), the hearing in both ears had worsened. There was no residual hearing on the left and a more severe downsloping sensorineural hearing loss on the right with a speech discrimination of 52%. At the age of 51 (Fig. 1c), his residual hearing on the right side had deteriorated further, and word recognition scores had dropped to 0% in both ears. Audiologic evaluation at age 51 demonstrated absent distortion product otoacoustic emissions bilaterally. In addition to his hearing loss, he had a history of non-vertiginous disequilibrium. Vestibular testing at age 51 revealed that using bithermal calorics there was 100% left unilateral weakness and a 12% right beating directional preponderance consistent with either a left peripheral vestibular deficit or, more likely, a bilateral reduced vestibular response. There was no response to ice water calorics on the left and only a mild response to ice water on the right.
FIG. 1.
Audiometry demonstrated a progressive sensorineural hearing loss in this 57-year-old man with well-documented superficial siderosis. A, Audiogram at age 47 years. B, Audiogram at age 50 years. C, Audiogram at age 51 years before cochlear implantation.
Failure of fixation suppression for warm water irrigations on the right was suggestive of central involvement.
He underwent a right cochlear implantation at age 51 via a transmastoid approach with placement of a Nucleus 24 straight electrode array within the cochlea. Eight stiffening rings remained external to the cochleostomy. Fascia was used to seal the cochleostomy.
Six months following cochlear implantation, the patient's consonant/nucleus/consonant (CNC) word score was 28%, and connected speech test (CST) sentence score was 61%. He was unable to participate in continued audiologic follow up because of his ongoing medical problems.
Materials and methods
The temporal bones and brainstem were removed three hours postmortem. The right temporal bone specimen was received with the cochlear implant already removed, presumably during removal of the specimen. The temporal bone specimens were complete, including part of the external auditory canal and mastoid, the entire cochlea, and vestibular system.
The temporal bones were fixed in formalin and prepared for histologic study by standard techniques including embedment in celloidin. The specimens were serially sectioned at a thickness of 20 micrometers in the axial (horizontal) plane and reconstructed by two dimensional methods.
Results
Left ear
Cochlear capsule
There was a focus of otosclerosis which involved the anterior and posterior stapediovestibular joint and the footplate itself resulting in fixation. The otosclerosis extended inferiorly to the stapes to include the otic capsule and endosteum of the basal turn. The round window was patent.
Cochlea and spiral ganglion
The configuration of the cochlea was normal with 2.5 turns. The length of the cochlear duct was 33.1 mm, and the length of Rosenthal's canal was 14.0 mm.
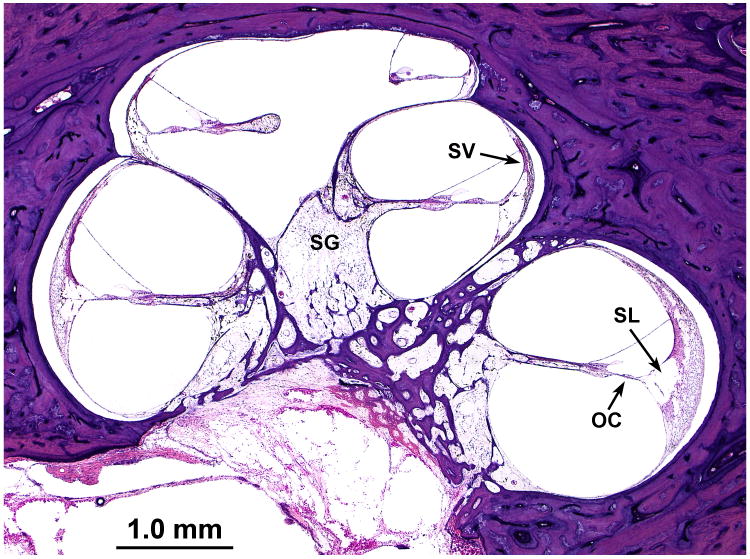
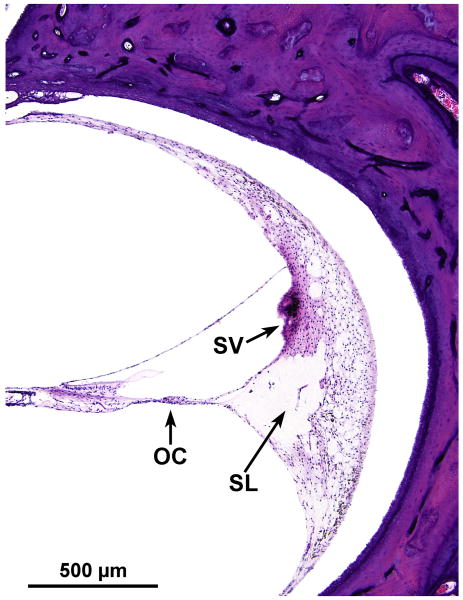
There was severe degeneration of the organ of Corti, particularly in the basal turn, where the basilar membrane was covered by a layer of squamous epithelial cells with no hair cells. There were islands of preserved inner and outer hair cells in the middle and apical turns. There was mild apical hydrops (Fig. 2). There was marked degeneration of the spiral ligament in all turns, particularly involving the region located near the attachment of the basilar membrane to the spiral ligament (Figs. 2,3). Perhaps because of this degeneration there was artifactual separation of the spiral ligament from the otic capsule (Figs. 2,3). There was severe degeneration of the stria vascularis in all turns. In some areas, there were granular inclusions within the stria vascularis (Fig. 3).
FIG. 2.
Midmodiolar section of left cochlea demonstrated severe atrophy of the spiral ganglion (SG), the organ of Corti (OC), stria vascularis (SV), and the spiral ligament (SL), especially in the area near its attachment to the basilar membrane.
FIG. 3.
Higher power photomicrograph of the basal turn of the organ of Corti. This demonstrated severe degeneration of the organ of Corti (OC) and stria vascularis (SV) and severe atrophy of the spiral ligament (SL), especially in the area of its attachment to the basilar membrane, perhaps resulting in artifactual separation of the spiral ligament from the lateral cochlear bony wall. Granular inclusions were common within the stria.
The most conspicuous correlate of the sensorineural loss reported during life was severe atrophy of spiral ganglion cells and sensory cells. The corrected spiral ganglion cell counts were: segment I–20; segment II-34; segment III-102; segment IV-102 cells, and a total of 258 spiral ganglion cells within the entire Rosenthal's canal. There was marked atrophy of the auditory nerve in the internal canal with hyalinization of the cribrose area (Fig. 2).
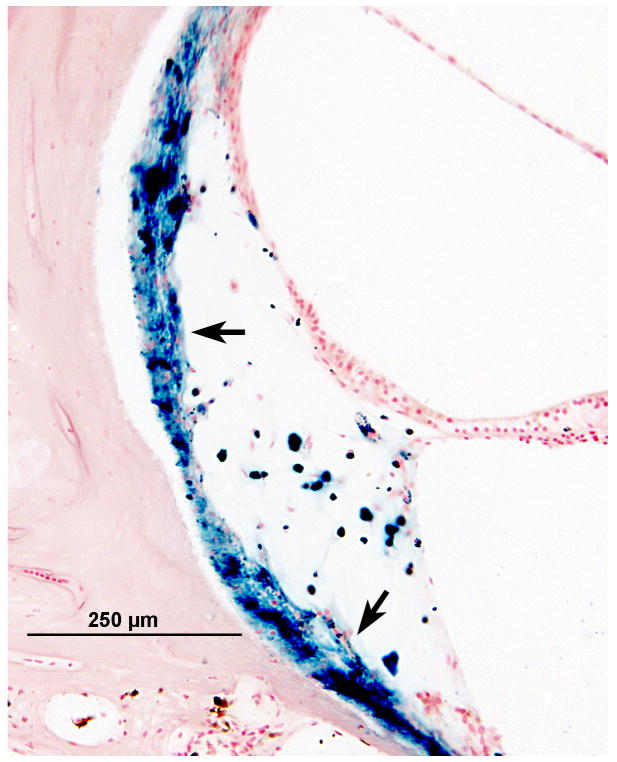
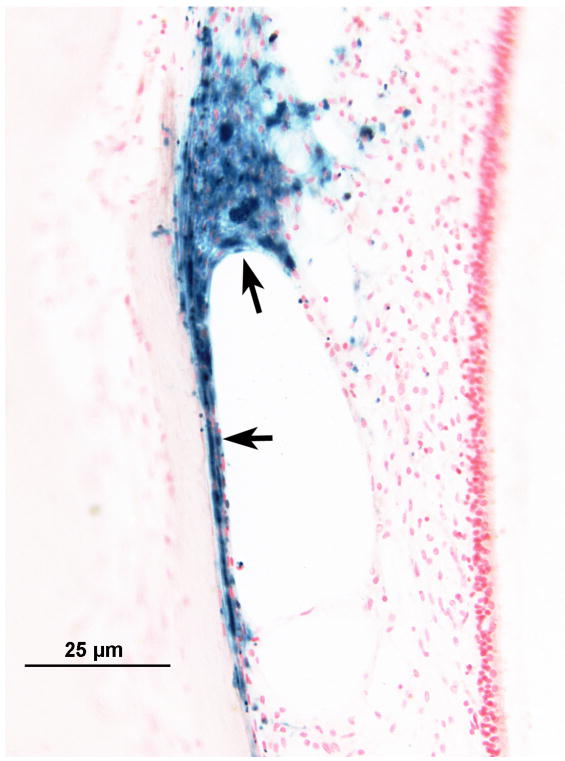
Iron stain of the cochlea and vestibular labyrinth
Gomori's iron stain of the cochlea and vestibular labyrinth revealed iron deposits within the spiral ligament (Fig. 4a), in the stria vascularis, and also in the subepithelial mesenchymal tissue of the maculae (Fig. 4b). There was no significant staining seen around the auditory or vestibular nerves, although both of these were markedly atrophied.
FIG. 4.
Left ear. Gomori iron stain of the organ of Corti and vestibular labyrinth. A, The blue deposit, indicating the presence of iron, was seen within and beneath the degenerate spiral ligament (arrows). B, The iron deposits also were seen beneath degenerating mesenchymal cells of the saccule (arrows).
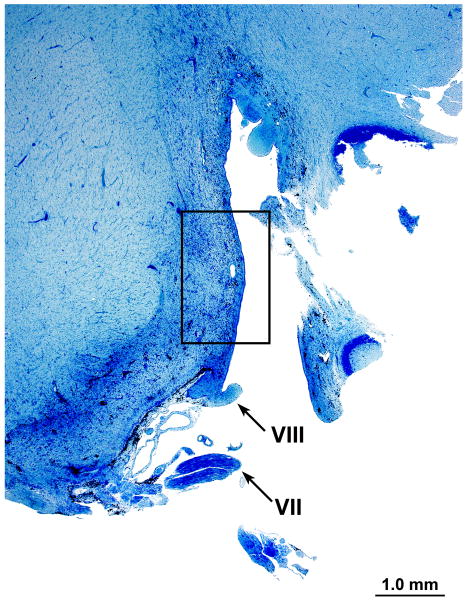
Cochlear nucleus
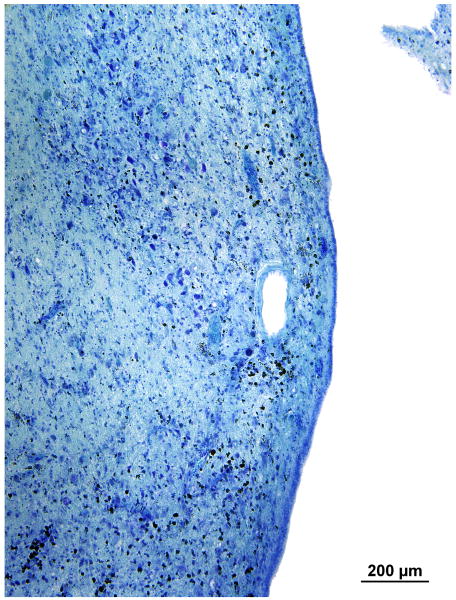
Azure stain (for nucleic acid)
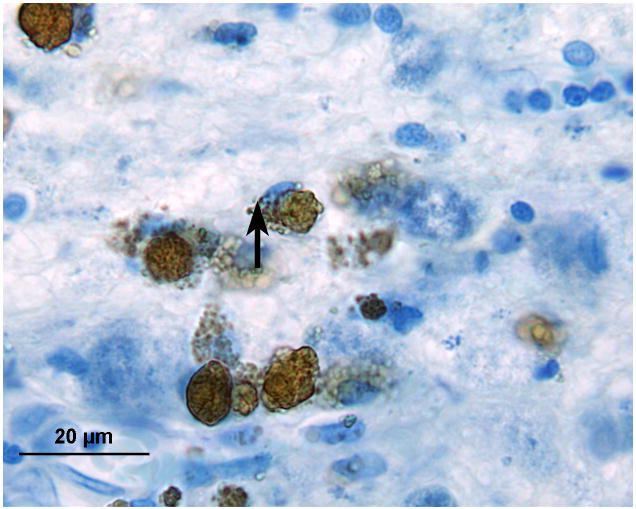
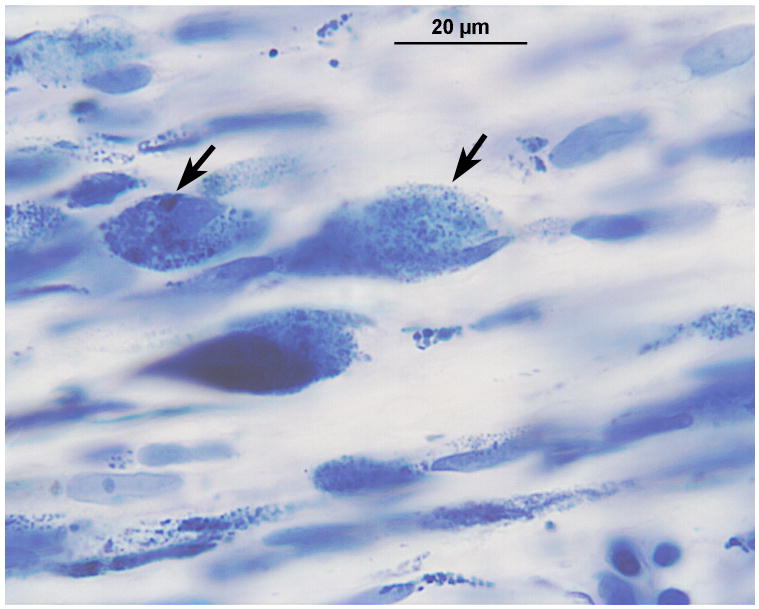
In the area of the cochlear nucleus (Fig. 5a,b) there were iron deposits within glial cells, and larger cells, probably macrophages especially near the CSF surface (Figs. 5c,d). There were also large flocculent bodies, probably identical to the ovoid bodies described by Koeppen et al, (1971) (Fig. 5d).
FIG. 5.
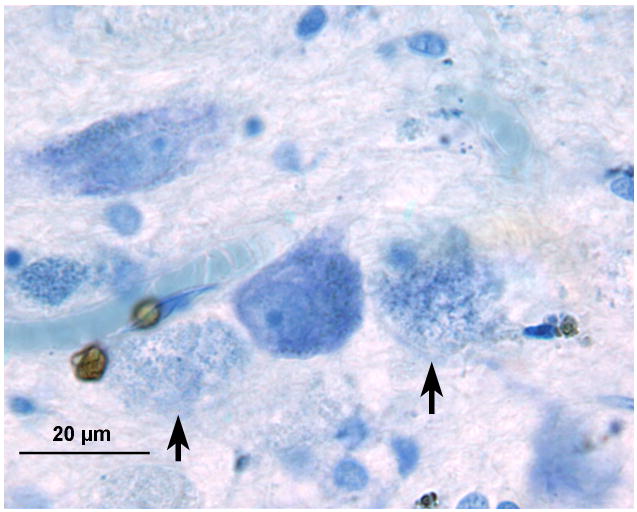
Left cochlear nucleus. Azure stain for nucleic acid. A, Area of cochlear nucleus near entry of the VIIth and VIIIth cranial nerves. B, Higher power of boxed area seen in Figure 5A. Iron deposits were shown in brown/black within macrophages and glial cells, especially in the subpial cortex. C, Most cells that were filled with iron deposits (brown inclusions) were small (5–7 μm) and were probably macrophages, with the likely exception of an occasional glial cell (arrow). D, Large neurons showed no evidence of iron accumulation. The large flocculent figures indicated by the arrows are strikingly similar in size to the large neurons but may correspond to the “ovoid bodies” or “anuclear foamy bodies” described by Koeppen et al. (2,14). No figures of this size stained for iron by the Gomori stain.
Such ovoid bodies or axonal swellings did not contain myelin as judged by Luxol fast blue staining, nor did they contain neurofilaments as demonstrated by anti-neurofilament staining.
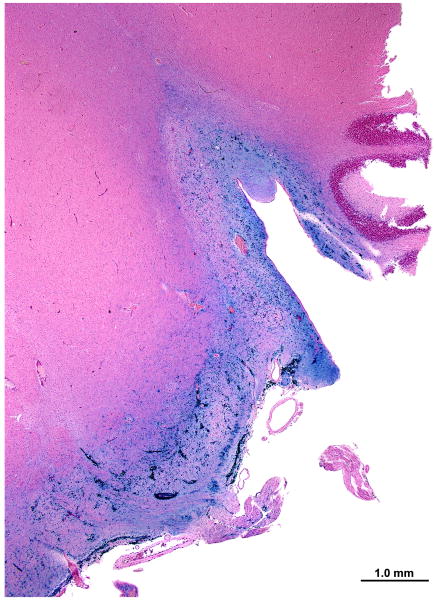
Gomori's iron stain
The large flocculent bodies were negative for iron deposit. There was, however, glial, and perhaps macrophage-associated iron staining, particularly in the subpial zone (Fig. 6a,b). Iron deposition appeared to be particularly prominent near capillaries within the pons (Fig. 6b).
FIG. 6.
Left cochlear nucleus. Gomori iron stain. A, The iron deposits (blue) were concentrated in the subpial cortex of the cochlear nucleus. B, The iron staining was particularly prominent around capillaries within the medulla (arrows).
Vestibular system
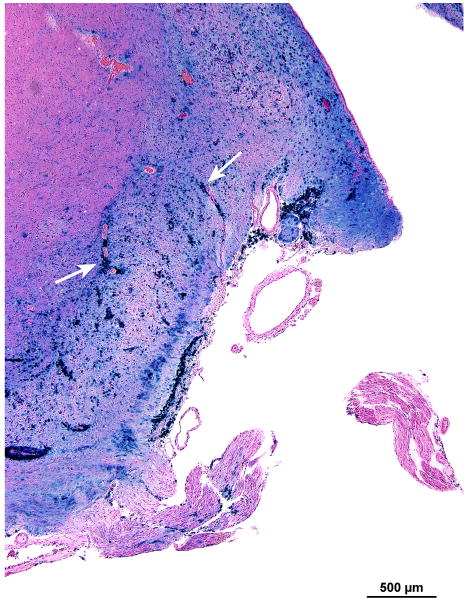
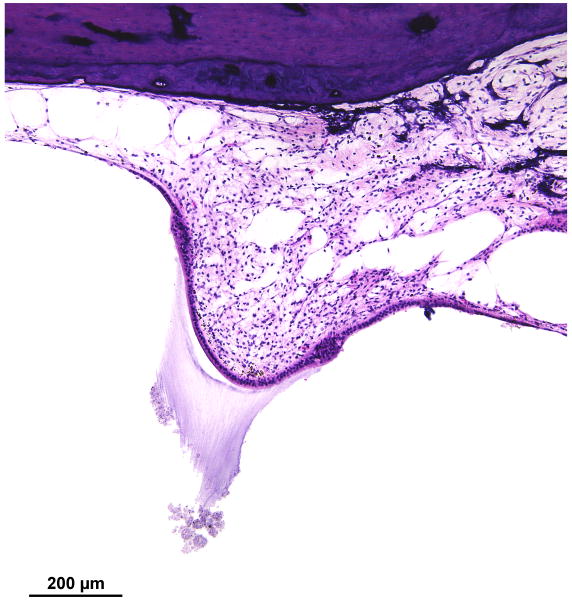
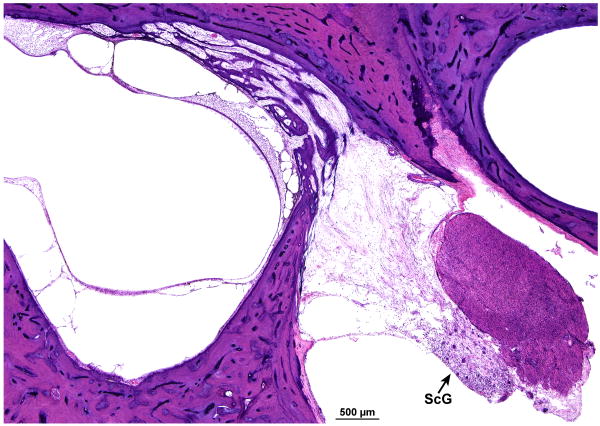
There was marked degeneration of the neuroepithelium of all five end organs of the vestibular system. The sensory epithelium was recognizable, but was nearly completely devoid of hair cells (Figs. 7,8). In addition, there was marked atrophy of both the superior and inferior vestibular nerves. Although Scarpa's ganglion cells were visible (Fig. 8a) they appeared pyknotic. Swellings of peripheral vestibular axons, similar to those found within the cochlear nucleus (Fig. 5d) were found (Fig. 8b). Whereas Figure 8b shows swollen nerve fibers, Figure 5d shows presumed swollen axons in the cochlear nucleus, but not positively identified as peripheral axons. There was osteoid and new bone formation in the ampullated end of the superior semicircular canal.
FIG. 7.
Left ear. There was severe degeneration of the neuroepithelium of the lateral semicircular canal with almost no hair cells present. There was likewise degeneration of the superior division of the vestibular nerve. Compare with corresponding section from the right lateral ampulla seen in Figure 12.
FIG. 8.
Vestibular system, left ear. A, There was severe degeneration of the superior vestibular nerve innervating the macula utriculi. No hair cells were present within the macula. The few remaining cells of Scarpa's ganglion (ScG) were pyknotic. B, The arrows indicate swellings of the peripheral axons of the vestibular nerve that were similar to those found within the cochlear nucleus (Azure stain).
Right ear
Cochlear capsule
As on the left, there was a focus of otosclerosis at the oval window and inferior to it on the promontory. The footplate was not fixed on the right side.
Cochlea and spiral ganglion
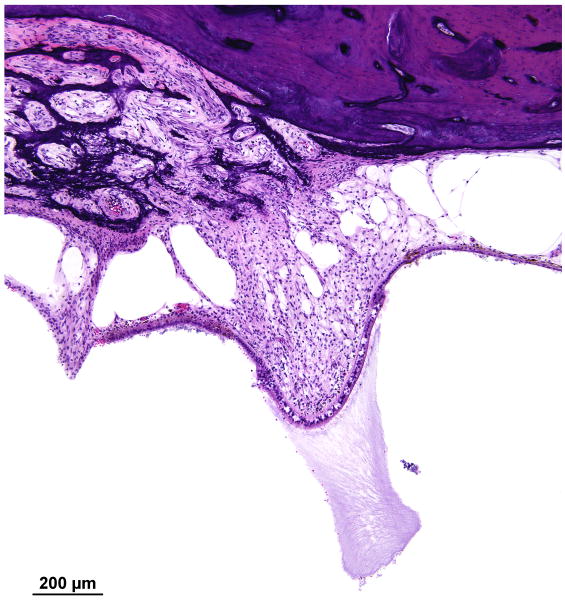
The cochlea had a normal configuration consisting of 2.5 turns. The cochlear duct measured 33.4mm in length, and Rosenthal's canal measured 13.9mm in length. There was mild to moderate endolymphatic hydrops involving the entire cochlea (Fig. 9a,b). There was collapse of the ductus reuniens, perhaps secondary to trauma induced by cochlear implantation (Fig. 10).
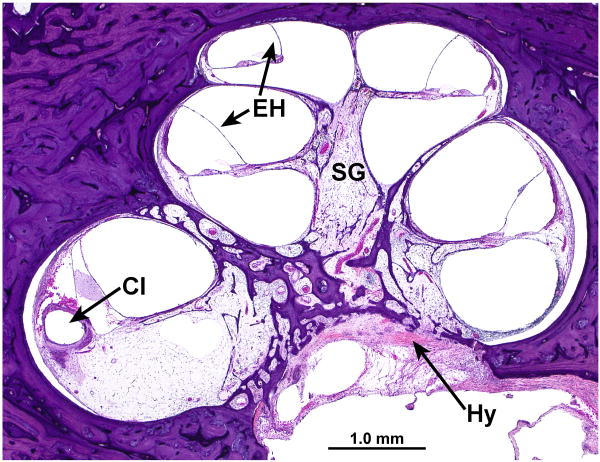
FIG. 9.
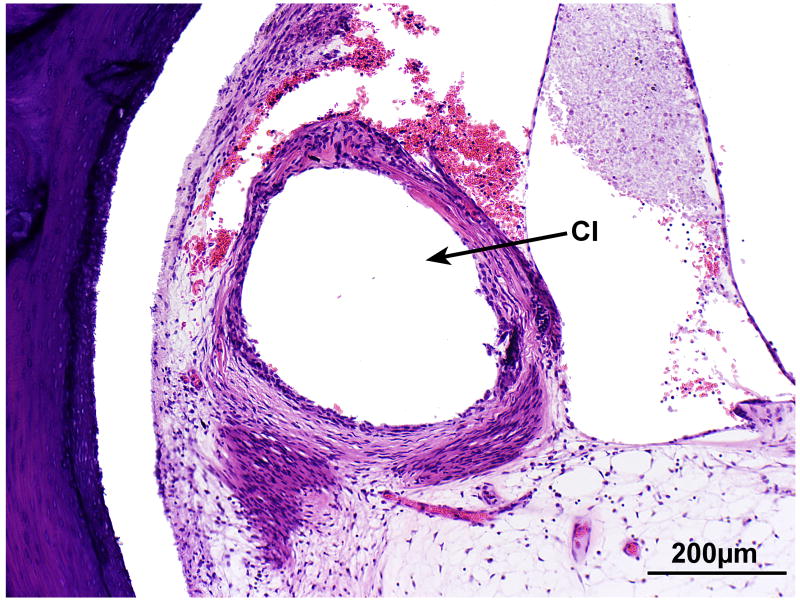
Cochlea, right ear. A, Midmodiolar section. There was severe degeneration of the spiral ganglion (SG) within the Rosenthal's canal. There was mild endolymphatic hydrops (EH) in all turns. There was severe degeneration of the auditory nerve with hyalinization (Hy) at the cribrose area. A track made by the cochlear implant (CI) was visible within the spiral ligament of the basal turn. The scala tympani was largely filled with fibrous tissue and some new bone. There was severe degeneration of the organ of Corti in all turns. B, High power of fibrous track around the cochlear implant (CI).
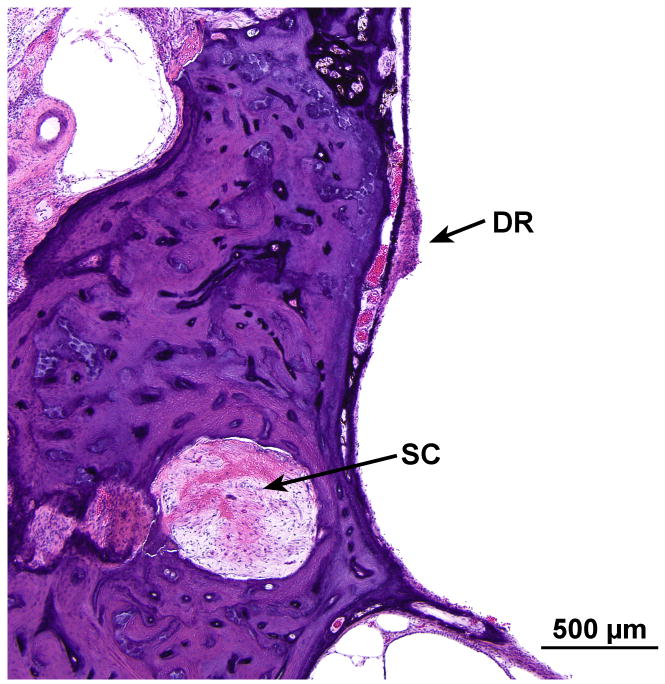
FIG. 10.
There was collapse of the ductus reuniens (DR), perhaps secondary to trauma induced by cochlear implantation. There also was degeneration of the posterior ampullary nerve with hyalinization of the singular canal (SC).
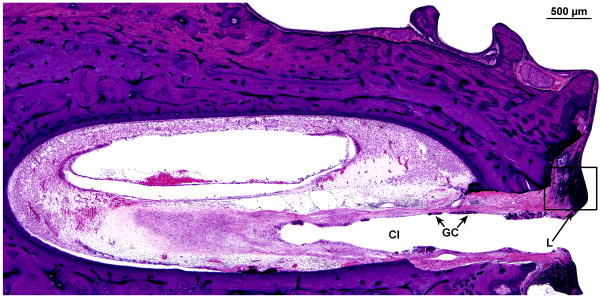
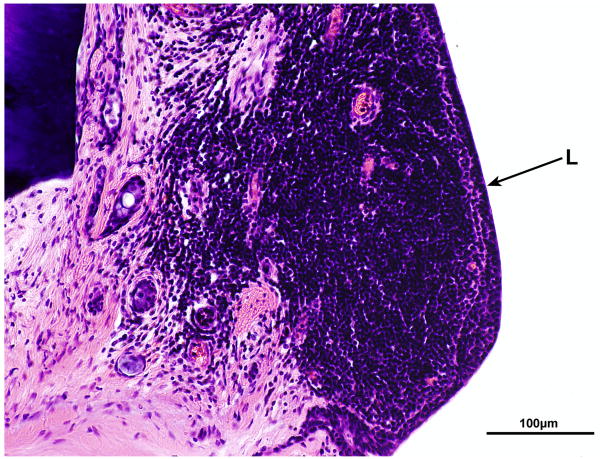
There was a track formed by the cochlear implant which entered the scala tympani at approximately 4.75mm from the round window (Fig. 11a). The track continued to millimeter 17 at the junction of the basal and middle turns. There was a marked inflammatory cell infiltrate at the circumference of the cochlear implant track throughout its length (Fig.11a,b). There was an intense fibrous tissue reaction around the electrode which remained largely within the scala tympani, except in the ascending basal turn where it had dissected the spiral ligament (Fig.9b).
FIG. 11.
Right cochlea. A, Section of the basal turn near the cochleostomy site. There was an intense fibrous reaction and foreign body giant cells (GC) and lymphocytes (L) around the cochlear implant (CI) as it entered the scala tympani. B, High power of boxed area shown in Figure 11A. An intense infiltrate of lymphocytes (L) was adjacent to the implant track.
Hair cells were absent in the basal turn and present in the middle and apical turns. As on the opposite left side, there was atrophy of the stria vascularis (Fig. 9a) and spiral ligament, particularly near its attachment to the basilar membrane.
The corrected spiral ganglion cell counts were: Segment I-367; segment II-680; segment III-136; segment IV-286 cells, and a total of 1,469 spiral ganglion cells in Rosenthal's canal. The auditory nerve was markedly atrophied within the internal auditory canal, and there was hyalinization near the cribrose area of the auditory nerve (Fig. 9a).
Vestibular system
There was no hydrops of the vestibular system.
The vestibular neuroepithelium and innervating neurons were degenerated in all five of the end organs, but much less than seen in the opposite left ear (Fig. 12,13). In contrast to the eighth nerve, the geniculate ganglion was normal in appearance.
FIG. 12.
Right ear. Although some hair cells were missing in the ampulla of the lateral semicircular canal and there was degeneration of the superior division of the vestibular nerve, the degeneration of both the end organ and the nerve was much less severe than seen on the opposite left side (Fig. 7).
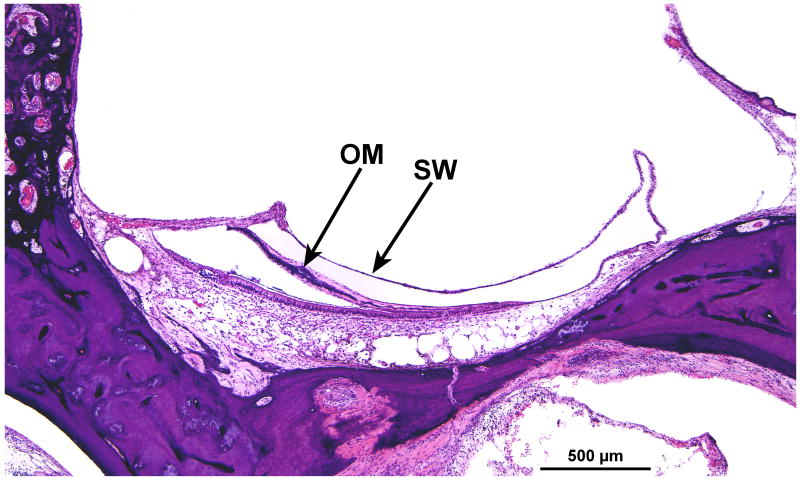
FIG. 13.
Right ear. There was moderate degeneration of the macula saccula with collapse of the saccular wall (SW) and encapsulation of the otolithic membrane (OM).
Discussion
Although the pathophysiology of neural degeneration in superficial siderosis is incompletely understood, it is considered secondary to hemosiderin deposit within the CNS and perhaps iron catalyzed lipid peroxidation (2,5,14). Previous reports documented success of cochlear implantation (9,10). However, Wood et al, (11) reported that in two cases of superficial siderosis, the initial results following cochlear implantation were promising, but deteriorated rapidly over a short period of time.
A prominent finding in this first known case with documented temporal bone histopathology in superficial siderosis was severe degeneration of the spiral ganglion cells in both ears, despite the presence of remaining hair cells in the middle and apical turns, consistent with a primary cochlear neuronal degeneration and retrograde degeneration of spiral ganglion cells within the inner ear, secondary to a primary insult to the auditory axons in the pontine cistern (15-17). Alternatively, these results are also consistent with the hypothesis that the cause of deafness was loss of sensory and neural structures within the cochlea rather than eighth nerve axonal pathology. Although the last known documentation of the patient's word recognition score occurred six years before death, nevertheless, despite a significant degeneration of spiral ganglion cells, the patient had a CNC word score of 28%. The lack of a negative correlation between spiral ganglion cell count and word recognition scores during life is consistent with previous observations in specimens with other etiologies of deafness (18).
Conclusions
This is the first known case of superficial siderosis with documented temporal bone histopathology and the hearing loss was most likely correlated with a severe degeneration of spiral ganglion cells of both ears despite the presence of remaining hair cells of the middle and apical turns. This is consistent with cochlear neuronal degeneration and retrograde degeneration of spiral ganglion cells within the inner ear or consistent with primary degeneration of hair cells and neural structures within the cochlea. Despite neuronal degeneration as a primary histologic correlate of deafness, the patient achieved a CNC word score of 28%, at least initially.
Acknowledgments
This work was supported by funding from the NIH (NIDCD) Grant #R01-DC-000152 Electron Microscopy of the Human Inner Ear.
References
- 1.Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain. 1995;118:1051–1066. doi: 10.1093/brain/118.4.1051. [DOI] [PubMed] [Google Scholar]
- 2.Koeppen AH, Michael SC, Danhong LZC, Cusack MJ, Gibson WM, et al. The pathology of superficial siderosis of the central nervous system. Acta Neuropathol. 2008;116:371–382. doi: 10.1007/s00401-008-0421-z. [DOI] [PubMed] [Google Scholar]
- 3.Uchino A, Aibe H, Itoh H, Aiko Y, Tanaka M. Superficial siderosis of the central nervous system; its MRI manifestations. Clin Imaging. 1997;21:241–245. doi: 10.1016/s0899-7071(96)00050-2. [DOI] [PubMed] [Google Scholar]
- 4.Grisoli M, Maccagnano E, DeSimone T, Savoiardo M. Superficial siderosis of the CNS: selective central myelin vulnerability and peripheral myelin sparing demonstrated by MRI. European J Neurol. 2007;14:e2–e3. doi: 10.1111/j.1468-1331.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- 5.Koeppen AH, Hurwitz CG, Dearborn RE, Dickson AC, Borke RC, Chu RC. Experimental superficial siderosis of the central nervous system: biochemical correlates. J Neurological Sciences. 1992;112:38–45. doi: 10.1016/0022-510x(92)90129-9. [DOI] [PubMed] [Google Scholar]
- 6.Weekamp HH, Huygen PLM, Merx JL, Kremer HPH, Cremers, Cor WRJ. Longitudinal analysis of hearing loss in a case of hemosiderosis of the central nervous system. Otol Neurotol. 2003;24:738–742. doi: 10.1097/00129492-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Vibert D, Hausler R, Lovblad KO, Schroth G. Hearing loss and vertigo in superficial siderosis of the central nervous system. Am J Otolaryngol. 2004;25(2):142–149. doi: 10.1016/j.amjoto.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Ayache D, Blaivie C, Kohen A, Tosella L, Williams MT. Auditory manifestations of superficial hemosiderosis of the central nervous system. Eur Arch Otorhinolaryngol. 2007;264:701–704. doi: 10.1007/s00405-006-0238-0. [DOI] [PubMed] [Google Scholar]
- 9.Irving RM, Graham JM. Cochlear implantation in superficial siderosis. J Laryngol Otol. 1996 December;(110):1151–1153. doi: 10.1017/s0022215100135996. [DOI] [PubMed] [Google Scholar]
- 10.Kim CS, Song JJ, Park MH, Kim YH, Koo JW. Cochlear implantation in superficial siderosis. Acta Oto-Laryngol. 2006;126:892–896. doi: 10.1080/00016480500529330. [DOI] [PubMed] [Google Scholar]
- 11.Wood VH, Bird PA, Giles EC, Baber WJ. Unsuccessful cochlear implantation in two patients with superficial siderosis of the central nervous system. Otol Neurotol. 2008 Aug;29(5):622–625. doi: 10.1097/MAO.0b013e3181758e7e. [DOI] [PubMed] [Google Scholar]
- 12.Revesz T, Earl CJ, Barnard RO. Superficial siderosis of the central nervous system presenting with longstanding deafness. J Royal Soc Medicine. 1988 Aug;(81):479–481. doi: 10.1177/014107688808100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson BE, Walton JN. Superficial haemosiderosis of the central nervous system. J Neurol Neurosurg Psychiat. 1964;27:332–339. doi: 10.1136/jnnp.27.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koeppen AH, Dickson AC, Chu RC, Thach RE. The pathogenesis of superficial siderosis of the central nervous system. Ann Neurol. 1993;34(5):646–653. doi: 10.1002/ana.410340505. [DOI] [PubMed] [Google Scholar]
- 15.Spoendlin H. Degeneration behaviour of the cochlear nerve. Eur Archives Oto-Rhino-Laryngol. 1971;200(4):275–291. doi: 10.1007/BF00373310. [DOI] [PubMed] [Google Scholar]
- 16.Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- 17.Sekiya T, Yagihashi A, Shimamura N, Asano K, Suzuki S, Matsubara A, Namba A, Shinkawa H. Apoptosis of auditory neurons following central process injury. Exp Neurol. 2003 Dec;184(2):648–658. doi: 10.1016/S0014-4886(03)00288-7. [DOI] [PubMed] [Google Scholar]
- 18.Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005 Apr;(4):672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- 19.Koeppen AH, Barron KD. Superficial siderosis of the central nervous system. A histological, histochemical and chemical study. J Neuropathol Exp Neurol. 1971 Jul;30(3):448–469. doi: 10.1097/00005072-197107000-00010. [DOI] [PubMed] [Google Scholar]