Abstract
Background:
Informed consent forms are required in all clinical trials which are approved by an independent Ethics Committee before practical use in the trials. However, how much the average subject actually understands of the information contained in these informed consent forms is uncertain.
Aim:
In a cross sectional study, the translated informed consent forms used in psychiatric clinical trials were assessed with respect to their ease of readability.
Materials and Methods:
We analyzed 30 informed consent forms translated from English to Hindi used in multinational and multicentre psychiatric clinical trials sponsored by different sponsors. We examined consent forms for readability scores and factors that might relate to readability.
Results:
The mean readability score for the informed consent forms, determined by the Flesch-Kincaid Grade Level Index (FKGL) was grade levels of 13.66. The ease of readability assessed by the Flesch Reading Ease Score (FRES) was 46.08 suggesting significant complexity of the texts. These values carry even more significance when the average years of schooling for India as a whole are 6.2 years.
Conclusion:
Our results show that the most informed consent forms were too complex to understand by an average adult subject. We suggest reducing this complexity and increasing the ease of readability so those average subjects receive the intended information as exactly as it could be. This can be achieved by few simple measures like improving the deficiencies in translation processes, encouraging the investigators to participate while preparing these forms, and enhanced understanding of the site specific requirements, namely culture, language (dialect), general literacy rate, etc.
Keywords: Clinical research, informed consent document, readability
INTRODUCTION
There is a consensus regarding the importance of informed consent in clinical research. The process of obtaining informed consent from subjects is a critical point of entry for research participants.[1] Patients learn about clinical trials from numerous ways and also there are several factors affecting their decision-making capacity. Educational materials for patients and informed-consent documents present highly complex information that must be understood by patients.[2,3] Kuczewski and Marshall[4] recommend adopting the approach that consent is an interactive and dynamic process and many factors can influence the study participant's willingness to sign the document. These factors include socioeconomic background, cultural traditions, literacy and language ability, and interactions with physicians, and other healthcare professionals.
The manner and context in which information is conveyed is as important as the information itself. The ability to understand is dependent upon patients′ intelligence, rationality, maturity, and language, and it is necessary to adopt the presentation of information in subject's capacity to understand. (Belmont report).[5] The informed consent process presents some major challenges for study participants and research staff. Several researchers have addressed these problems with need to improve upon them. Among them, Brady[6] identifies the following issues:
Subject's hesitation to ask detailed questions
Variable presentation of the content
Difficulty verifying the subject's comprehension.
A vital component of the informed consent process in participant's comprehension of the given information, without which it may not be completely “informed” consent. It becomes more so important when a literacy rate for adults over age of 15 years was 66% in India in 2007 (UNESCO Institute for Statistics). For males, it was 76.9% but only 54.5% females were literate. Average years of schooling have commonly been used as a proxy for educational attainment. The average years of schooling for India as a whole are 6.2 years (National Sample Survey Organisation). Sixteen of the 27 states have an average that is lower than the national average including the states of Uttar Pradesh and Bihar. Almost all of the subjects enrolled in the clinical trials, approved by the mentioned independent ethics committee, come from these two states. Standard consent forms are written at a level too difficult for many patients to read and comprehend, especially those with low literacy skills.[7–9] The literacy rate and years of schooling figures indicate that a substantial proportion of patients may not be able to read and understand the consent forms that are currently used in clinical research[10–12] Despite recommendations that forms be simplified to a 6th to 8th-grade level, most forms continue to be written at or above a 12th-grade level.
This incongruence between the national literacy level and the average ICF reading level requires that the documents should be composed in “understandable” language (USDHH).[13] It is also despite the recommendation issued in 1998 by the Informed Consent Working Group (formed by the National Cancer Institute [NCI], the Office of Human Research Protections [OHRP], and the U.S. Food and Drug Administration [FDA]) to keep language at or below an 8th-grade reading level, and evaluate and guarantee this level by software programs or other methodologies.[14] The independent ethics committees approve these informed consent documents since the legal aspects are emphasized over communicative aspect. Few people hypothesize that ethics committee members may actually judge the language to be acceptable simply because the majority of such members are usually professionals who understand the language, and the minority who are not find the language more acceptable over time with repeated exposure.[15]
Empirical research has demonstrated that patients are able to understand and use only a portion of the information provided by consent forms;[16–18] this is especially true for patients with the more debilitating mental illnesses such as schizophrenia. Most of psychiatric illnesses including schizophrenia involve impairment of cognition of variable degrees and that acts as an impediment in explicit understanding of information.[19] With slowed information processing, these subjects face more difficulty understanding the complex language despite of their education grades.[20,21]
Translation must take into account constraints that include context, the rules of grammar of the two languages, their writing conventions, and their idioms. A common misconception is that there exists a simple word-for-word correspondence between any two languages, and that translation is a straightforward mechanical process; such a word-for-word translation, however, cannot take into account context, grammar, conventions, and idioms
Numbers of methods have been developed by the researchers to calculate the complexity of documents and ease of readability. But, most of them were objective and quantitative tests and do not require testing readers.[22]
The objective of this study was to evaluate the comprehensibility of consent forms used at our clinical research site by average adult male and female patients. We try to find out the average level of education required to understand them completely and the factors deterring their understanding.
MATERIALS AND METHODS
Materials and participants
Thirty informed consent documents, all translated from English to Hindi and customized for our site were assessed for readability. All of them were approved by an independent ethics committee for their use with potential subjects for respective studies. All the participants were graduates and were employee of the hospital working as research coordinator, clinical psychologists, and psychiatric social workers. They were randomly given the consent documents without revealing the identity of source. Each of the participants were given sufficient time to read the whole document carefully and count the total number of sentences, total number of words, and total number of syllables for all words of the document. All the 15 participants had Hindi as their mother tongue and had sufficient understanding of language and all the locally prevalent dialects. The minimum level of education grade was 15 and average was 16.7.
Evaluation of readability
For each of the 30 English to Hindi translated informed consent documents, readability scores were assigned by using: the Flesch Reading Ease Score (FRES) and the Flesch-Kincaid Grade Level Index (FKGL). Both FRES and the FKGL determine readability by examining the average number of words per sentence and the average number of syllables per word. The FRES score is a number from 0 to 100, with higher scores indicating easier readability. The FKGL each give the U.S. grade level required to read text, with a higher grade level indicating more difficult readability.
We used these tests since we do not have such an easy to apply indexes to measure ease of readability of Hindi language texts. There are no supporting data or validation measure advocating their use in Hindi texts. FKGL give the US grade level and we assumed the congruency of grade levels of India and US.
FKGL scores range from minimum of one but have no maximum grade level that can be calculated. However, it was originally designedto measure readability of elementary and secondary school texts.Thus one may expect scores falling beyond the 12th-grade levelto have less practical validity. Both the FRES and FKGL havebeen used extensively to calculate readability of informed consentforms,[7,23–25] patient education materials,[26] andmedical literature.[27]
Analysis
We performed a descriptive analyses of range, mean, minimum and maximum values of readability scores determined by FRES and FKGL.
RESULTS
The mean+/– SD FRES for 30 tested documents was 46.08+/– 15.76 which indicated a “difficult” reading level with wide variations in different documents [Figure 1]. The range of FRES scores was from 6.34 to 71.99.
Figure 1.
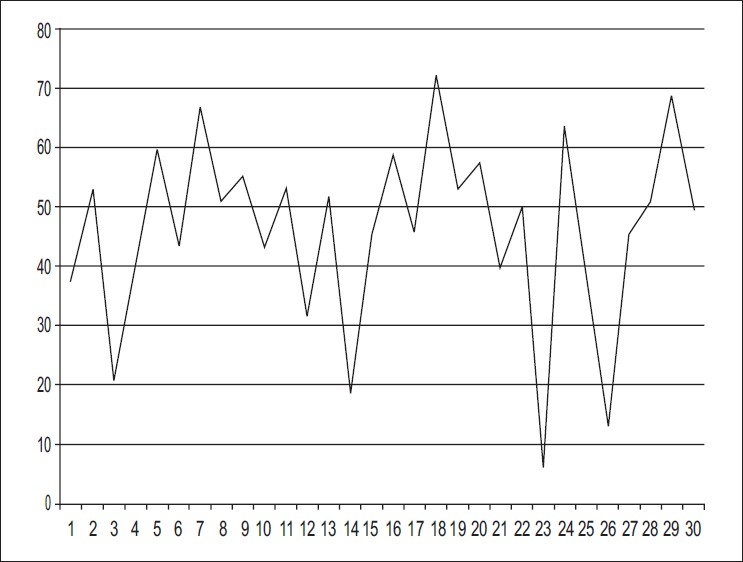
FRES
The corresponding FKGL scores mean +/– was 13.67+/– 2.06 which indicated that the person should be graduating to completely understand the documents [Figure 2]. The range was from 9.30 to 17.96 grade levels.
Figure 2.
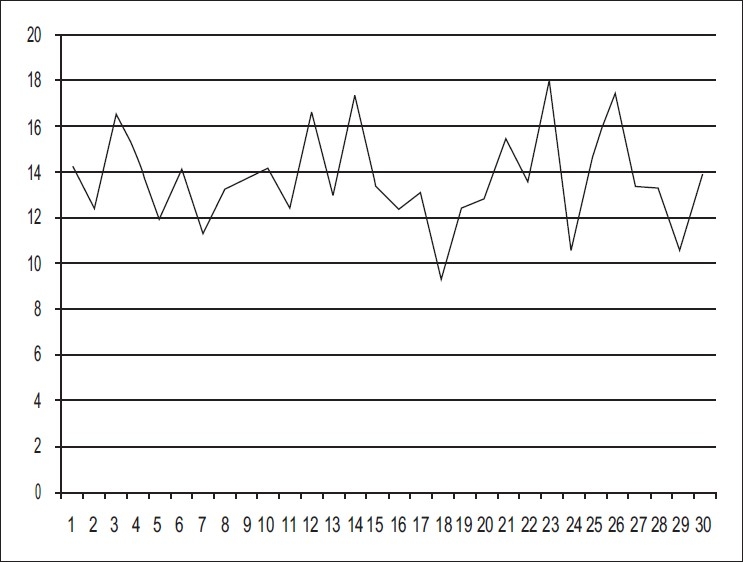
FKGL
DISCUSSION
Informed consent is an interactive, multifaceted process, of which one important element is the informed consent form.[28,29] In clinical research, the participants must be informed using a documents they can understand completely. Word by word translating of the documents may not leave any piece of information behind and make it easier to read but it did not show the adequate comprehensibility.
The results show that the perception of the readability and comprehensibility of a standard information sheet can be improved by professional linguistic revision of the sheet. This result is not surprising since a number of studies have shown that the average information sheet is difficult to read and understand.[30,31] It is interesting that there is a correlation between the respondents′ own assessment of the comprehensibility of the form and their actual comprehension. This indicates that it may be useful simply to ask whether or not a prospective research subject has found the information leaflet easy or difficult. The answer to this question could be used as a rough indication of the person's level of understanding.[32]
This result empirically supports the advice given by Wager et al. that it is useful to test the understanding of prospective research subjects after giving information but before eliciting consent.
The results of this study confirm with those of previous studies that showed comprehension of informed consent documents poses problems for many participants. Cassileth et al.[33] concluded that the difficulty of the material and its legalistic wording imposed barriers to the patients’ comprehension of information intended to facilitate informed decisions.
To improve comprehension, consent forms should be brief and direct. They should avoid legal jargon and should be written at appropriate reading levels using plain English.[11,34] Following these recommendations and those in the participant education literature, we developed a more suitable form written on a 7th-grade level.
Research is lacking as to the methods by which participant comprehension of informed consent documents can be increased to a clinically acceptable level or even what a clinically acceptable level might be. Williams et al. suggested that simplifying the text to an approximately 6th-grade level might allow marginally literate participants to comprehend the documents.
Given the large number of adult population in the India with inadequate literacy skills, the comprehensibility of informed consent documents and its ability to aid subjects in decision making impose serious ethical questions. These adults may not be able to read and understand the forms they are signing, and they may not let clinicians or researchers know their problem since most participants who have reading difficulties are embarrassed to admit it.[35] Clinicians should also be aware that patients with inadequate literacy skills may be anxious about being expected to read and sign documents and to communicate with physicians.[12,35,36] Patients with low literacy may also have problems with basic physician/participant communication. Recent studies[37,38] have shown that participants with extremely limited literacy skills may have limited health knowledge and may not understand basic concepts of research such as the phases of clinical trials. Such individuals often do not understand what the physician has said and may not be willing to ask physicians for clarification of information.[35]
Ethicists and patient educators[8,24,33,39–43] are recommending that patients be included in the development of patient education materials and forms to ensure that the materials include information important to them and are more understandable, appealing, and culturally sensitive.
Research is needed to determine the methods to increase comprehension, especially for participants with inadequate or marginal reading skills. Input from participants and recognition of hidden illiteracy will be critical in the development of better informed consent.
Kuczewski and Marshall[4] suggest that misunderstandings are more likely to occur when investigators and participants speak different languages or even different dialect, especially when “there are no equivalent expressions for particular biomedical concepts or when the notion of informed consent is unfamiliar.” They offer strategies to help minimize language barriers:
Use an effective process of translation and back-translation when an informed consent document must be translated from one language to another. This process must include adequate pretesting of the consent document to determine that it is comprehensible to individuals who will be recruited for a research project.
Enlist the help of individuals who can act as “cultural experts” on ways in which to communicate difficult scientific concepts for study populations who may be unfamiliar with the biomedical problem being investigated.
Keep the consent document as short as possible, using simple language and a format that is clear and understandable for potential research participants.
We propose the following essential components to be integrated while writing the informed consent document or translating from one language to another.
The informed consent documents should be viewed as moral rather than legal element of clinical research.
The adequacy of comprehensibility should be tested before presenting it to ethics committee or review board with the help of patients.
Cultural differences at different sites using same language must be taken into account.
Extensive pre-testing before actually using it in practice.
Involvement of investigators from the site while customizing it for the site.
Use of supportive documents/instruments to explain technical terms/procedures.
Dialects
While we often tend to think of languages as singularities understandable by everyone who calls him or herself a “native speaker,” this is not always the case; for instance Hindi, may be broken up into dozens of smaller dialects, each with its own quirks that make it unique and distinct from the spoken by other groups and communities. Dialect refers to a variety of a language that is characteristic of a particular group of the language's speakers. The term is applied most often to regional speech patterns, but a dialect may also be defined by other factors, such as social class. In India, since a big city like ours caters health services to a large geographical area and hence people with various dialects. These sites should be allowed to participate in the process of writing the informed consent document to make it suitable for people from all dialects.
Translation
The translation services need to follow certain proficiency level for translation of scientific texts. The translation should involve bilingual proofreaders with basic scientific knowledge and in-house proofreaders for quality control. To translate the scientific text from English to Hindi, keeping it understandable by patients with same education grade level, it must be translated and re-written. Effective scientific translators must understand not only the fundamental science they are translating but also the principles of two written languages: the source language and the target language. The technical knowledge of the subject is extremely vital for keeping the text comprehensible.
The translation process must involve successive phases according to the level of complexity of the text to be localized, always using a mother tongue translator, with possible collaboration by specialist text revision editors, proof readers, and scientific and technical consultants for the target group. The objective of the process should be to develop the language and tone that suits the destined population. It is the difference between a simple text conversion and a complete content adaptation that can determine the effectiveness of the message.
Limitations
Though the study had significant lacunae in its analysis and applicability, still it suggests a need to take up this issue more seriously for research and provide practical solutions. Few of the limitations, we identified during the study processes were as follows.
Readability statistics available may provide a quick solution to evaluating the readability score but there are some caveats. An ideal scale would take into account more than just simple calculations of words and syllables.
One problem with readability formulae is that they ignore the actual vocabulary used in writing. The problem identified here is that the test assumes that there is a strong negative correlation between word length and a word's readability. Readability scores depend on the writing style rather than the content of written material.
Applicability of the readability tests used is not tested or validated for text written in Hindi.
Manual counting of words and syllables by different persons inducted significant inter-individual variations.
The grade level assessed by the readability tests was US grade level and its equivalence class level of India was not determined.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Berg JW, Appelbaum PS, Lidz CW, Parker L. Informed consent: Legal theory and clinical practice. 2nd ed. New York: Oxford University Press; 2001. [Google Scholar]
- 2.Fitzmaurice DA, Adams JL. A systematic review of patient information leaflets for hypertension. J Hum Hypertens. 2000;14:259–62. doi: 10.1038/sj.jhh.1001003. [DOI] [PubMed] [Google Scholar]
- 3.Andrus MR, Roth MT. Health literacy: a review. Pharmacotherapy. 2002;22:282–302. doi: 10.1592/phco.22.5.282.33191. [DOI] [PubMed] [Google Scholar]
- 4.Kuczewski MG, Marshall P. The decision dynamics of clinical research: the context and process of informed consent. Med Care. 2002;40:45–54. doi: 10.1097/01.MLR.0000023955.04138.AF. [DOI] [PubMed] [Google Scholar]
- 5.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (National Commission). Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, D.C.: U.S. Government Printing Office; 1979. [PubMed] [Google Scholar]
- 6.Brady JS. Multimedia delivery can enhance the consent process. Applied Clinical Trials. 2003 Jan;:36–42. [Google Scholar]
- 7.Hammerschmidt DE, Keane MA. Institutional Review Board (IRB) review lacks impact on the readability of consent forms for research. Am J Med Sci. 1992;304:348–51. doi: 10.1097/00000441-199212000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Young DR, Hooker DT, Freeberg FE. Informed consent documents: increasing comprehension by reducing reading level. IRB. 1990;12:1–5. [PubMed] [Google Scholar]
- 9.Morrow GR. How readable are subject consent forms? JAMA. 1980;244:56–8. [PubMed] [Google Scholar]
- 10.Williams MV, Baker DW, Parker RM, Nurss JR. Relationship of functional health literacy to patients’ knowledge of their chronic disease: a study of patients with hypertension and diabetes. Arch Intern Med. 1998;158:166–72. doi: 10.1001/archinte.158.2.166. [DOI] [PubMed] [Google Scholar]
- 11.Meade C, Howser DM. Consent forms: how to determine and improve their readability. Oncol Nurs Forum. 1992;19:1523–8. [PubMed] [Google Scholar]
- 12.Doak CC, Doak LG, Root JH. Teaching participants with low-literacy skills. 2nd ed. Philadelphia, PA: JB Lippincott; 1996. [Google Scholar]
- 13.U.S. Department of Health and Human Services; 2005. United States Department of Health and Human Services, Code of Federal Regulations Title 45, Public Welfare, Part 46, Protection of Human Subjects, Subpart A, Section 46.116. General requirement for informed consent. [Google Scholar]
- 14.National Cancer Institute. Simplification of informed consent documents. [last cited on 2004]. Available from: http://www.cancer.gov/clinicaltrials/understanding/simplification-ofinformedconsent-docs.
- 15.Tarnowski KJ, Allen DM, Mayhall C, Kelly PA. Readability of pediatric biomedical research informed consent forms. Pediatrics. 1990;85:58–62. [PubMed] [Google Scholar]
- 16.Grossman L, Summers F. A study of the capacity of schizophrenic patients to give informed consent. Hosp Community Psychiatry. 1980;31:205–206. doi: 10.1176/ps.31.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Munetz MR, Roth LH. Informing patients about tardive dyskinesia. Arch Gen Psychiatry. 1985;42:866–71. doi: 10.1001/archpsyc.1985.01790320034005. [DOI] [PubMed] [Google Scholar]
- 18.Irwin M, Lovitz A, Marder SR, Mintz J, Winslade WJ, Van Putten T, et al. Psychotic patients’ understanding of informed consent. Am J Psychiatry. 1985;142:1351–4. doi: 10.1176/ajp.142.11.1351. [DOI] [PubMed] [Google Scholar]
- 19.Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III. Abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19:149–74. doi: 10.1007/BF01499323. [DOI] [PubMed] [Google Scholar]
- 20.Vollmann J, Bauer A, Danker-Hoipfe H, Helmchen H. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33:1463–71. doi: 10.1017/s0033291703008389. [DOI] [PubMed] [Google Scholar]
- 21.Appelbaum PS, Grisso T, Frank E, O’Donnell S, Kupfer DJ. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156:1380–4. doi: 10.1176/ajp.156.9.1380. [DOI] [PubMed] [Google Scholar]
- 22.Rush RT. Assessing readability: formulas and alternatives. Read Teach. 1985;39:274–83. [Google Scholar]
- 23.Goldstein AO, Frasier P, Curtis P, Reid A, Kreher NE. Consent form readability in university-sponsored research. J Fam Pract. 1996;42:606–11. [PubMed] [Google Scholar]
- 24.Hopper KD, TenHave TR, Hartzel J. Informed consent forms for clinical and research imaging procedures: how much do participants understand? AJR Am J Roentgenol. 1995;164:493–6. doi: 10.2214/ajr.164.2.7839996. [DOI] [PubMed] [Google Scholar]
- 25.Davis TC, Arnold C, Berkel HJ, Nandy I, Jackson RH, Glass J. Knowledge and attitude on screening mammography among low-literate, low-income women. Cancer. 1996;78:1912–20. doi: 10.1002/(sici)1097-0142(19961101)78:9<1912::aid-cncr11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Kusec S, Brborovic O, Schillinger D. Diabetes websites accredited by the Health on the Net Foundation Code of Conduct: readable or not? Stud Health Technol Inform. 2003;95:655–60. [PubMed] [Google Scholar]
- 27.Roberts JC, Fletcher RH, Fletcher SW. Effects of peer review and editing on the readability of articles published in Annals of Internal Medicine. JAMA. 1994;272:119–21. [PubMed] [Google Scholar]
- 28.Taub HA, Baker MT. The effect of repeated testing upon comprehension of informed consent materials by elderly volunteers. Exp Aging Res. 1983;9:135–8. doi: 10.1080/03610738308258441. [DOI] [PubMed] [Google Scholar]
- 29.“Informed consent in research involving human participants.”. 32 (RFA OD97001) Vol. 25. Bethesda, MD: National Institutes of Health; 1996. Sep 27, NIH Guide. [Google Scholar]
- 30.Arthur VA. Written patient information: a review of the literature. J Adv Nurs. 1995;21:1081–6. doi: 10.1046/j.1365-2648.1995.21061081.x. [DOI] [PubMed] [Google Scholar]
- 31.Mumford ME. A descriptive study of the readability of patient information leaflets designed by nurses. J Adv Nurs. 1997;26:985–91. doi: 10.1046/j.1365-2648.1997.00455.x. [DOI] [PubMed] [Google Scholar]
- 32.Wager E, Tooley PJH, Emanuel MB, Wood SF. Get patients’ consent to enter clinical trials. BMJ. 1995;311:734–7. doi: 10.1136/bmj.311.7007.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassileth BR, Zupkis RV, Sutton-Smith K, March V. Informed consent-why are its goals imperfectly realized? N Eng J Med. 980(302):896–900. doi: 10.1056/NEJM198004173021605. 980. [DOI] [PubMed] [Google Scholar]
- 34.LoVerda ME, Prochazka AV, Byyny RL. Research consent forms: continued unreadability and increasing length. J Gen Int Med. 989(4):410–2. doi: 10.1007/BF02599693. [DOI] [PubMed] [Google Scholar]
- 35.Parikh NS, Parker RM, Nurss JR, Baker DW, Williams MV. Shame and health literacy: the unspoken connection. Patient Educ Couns. 1996;27:33–9. doi: 10.1016/0738-3991(95)00787-3. [DOI] [PubMed] [Google Scholar]
- 36.Weiss BD, Coyne C. Communicating with patients who cannot read. N Engl J Med. 1997;337:272–4. doi: 10.1056/NEJM199707243370411. [DOI] [PubMed] [Google Scholar]
- 37.Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: a comparative study of standard versus simplified forms. J Natl Cancer Inst. 1998;90:668–74. doi: 10.1093/jnci/90.9.668. [DOI] [PubMed] [Google Scholar]
- 38.Williams MV, Parker RM, Baker DW, Parikh NS, Pitkin K, Coates WC, et al. Inadequate functional health literacy among participants at two public hospitals. JAMA. 1995;274:1677–82. [PubMed] [Google Scholar]
- 39.Llewellyn-Thomas HA, Thirel EC, Sem FW, Woermke DE. Presenting clinical trial information: a comparison of methods. Patient Educ Couns. 1995;25:92–107. doi: 10.1016/0738-3991(94)00705-q. [DOI] [PubMed] [Google Scholar]
- 40.Cassileth BR, Lusk EY, Miller DS, Witz S. Attitudes toward clinical trials among patients and the public. JAMA. 1982;248:968–70. [PubMed] [Google Scholar]
- 41.Rudd RE, Comings JP. Learner developed materials: an empowering product. Health Educ Q. 1994;21:313–27. doi: 10.1177/109019819402100304. [DOI] [PubMed] [Google Scholar]
- 42.Reid JC, Klachko DM, Kardash CA, Robinson RD, Scholes R, Howard D. Why people don’t learn from diabetes literature: influence of text and reader characteristics. Patient Educ Couns. 1995;25:31–8. doi: 10.1016/0738-3991(94)00688-i. [DOI] [PubMed] [Google Scholar]
- 43.Turner S, Maher E, Young T, Young J, Vaughan Hudson G. What are the information priorities for cancer patients involved in treatment decisions? An experienced surrogate study in Hodgkin's disease. Br J Cancer. 1996;73:222–7. doi: 10.1038/bjc.1996.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


