Abstract
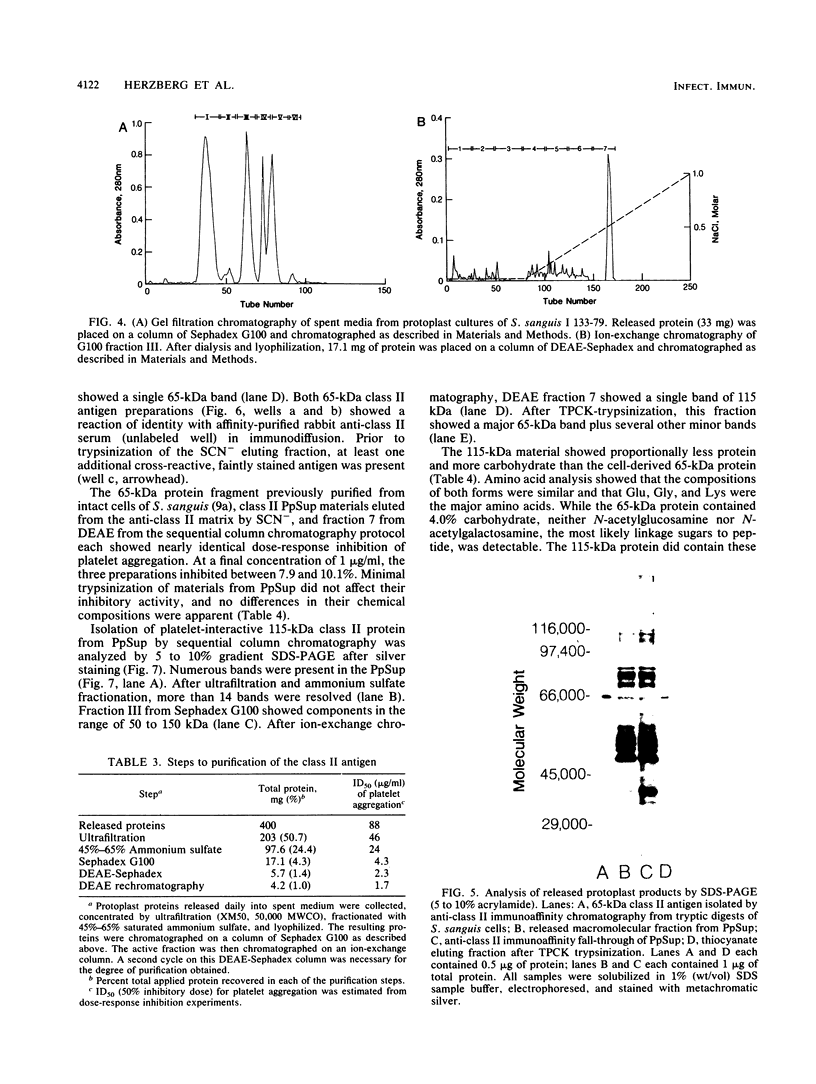
To isolate a more native, platelet-interactive macromolecule (class II antigen) of Streptococcus sanguis, cultured protoplasts were used as a source. Protoplasts were optimally prepared from fresh washed cells by digestion with 80 U of mutanolysin per ml for 75 min at 37 degrees C while osmotically stabilized in 26% (wt/vol) raffinose. Osmotically stabilized forms were surrounded by a 9-nm bilaminar membrane, as shown by transmission electron microscopy. Protoplasts were cultured in chemically defined synthetic medium and osmotically stabilized by ammonium chloride. Spent culture media were harvested daily for 7 days. Each day, soluble proteins were isolated from media, preincubated with platelet-rich plasma, and tested for inhibition of platelet aggregation induced by S. sanguis cells. Products released from S. sanguis protoplasts and reactive with an anti-class II antigen immunoaffinity matrix were able to inhibit S. sanguis-induced platelet aggregation. As resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, anti-class II-reactive protoplast products included silver-stained bands of 67, 79, 115, 216, and 248 kDa. The 115-kDa protein fraction was isolated by gel filtration and ion-exchange chromatography. This form of the class II antigen contained N-formylmethionine at its amino terminus. Rhamnose constituted 18.2% of the total residual dry weight and nearly half of its carbohydrate content. Diester phosphorus constituted 1% of this fraction. After trypsinization of the protoplast products from either preparation, a 65-kDa protein fragment was recovered. This protoplast protein fragment and the S. sanguis cell-derived 65-kDa class II antigen, previously implicated in the induction of platelet aggregation, were shown to be functionally and immunologically identical.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeygunawardana C., Bush C. A., Cisar J. O. Complete structure of the polysaccharide from Streptococcus sanguis J22. Biochemistry. 1990 Jan 9;29(1):234–248. doi: 10.1021/bi00453a032. [DOI] [PubMed] [Google Scholar]
- Alpenfels W. F. A rapid and sensitive method for the determination of monosaccharides as their dansyl hydrazones by high-performance liquid chromatography. Anal Biochem. 1981 Jun;114(1):153–157. doi: 10.1016/0003-2697(81)90466-8. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Calandra G. B., Cole R. M. Lysis and protoplast formation of group B streptococci by mutanolysin. Infect Immun. 1980 Jun;28(3):1033–1037. doi: 10.1128/iai.28.3.1033-1037.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassels F. J., London J. Isolation of a coaggregation-inhibiting cell wall polysaccharide from Streptococcus sanguis H1. J Bacteriol. 1989 Jul;171(7):4019–4025. doi: 10.1128/jb.171.7.4019-4025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G., Herzberg M. C. Platelet interaction with bacteria. VI. contrasting the role of fibrinogen and fibronectin. Am J Hematol. 1980;9(1):43–53. doi: 10.1002/ajh.2830090106. [DOI] [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C. A collagen-like immunodeterminant on the surface of Streptococcus sanguis induces platelet aggregation. J Immunol. 1987 May 15;138(10):3360–3366. [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C. Purification and partial characterization of a 65-kDa platelet aggregation-associated protein antigen from the surface of Streptococcus sanguis. J Biol Chem. 1990 Aug 25;265(24):14080–14087. [PubMed] [Google Scholar]
- FREIMER E. H., KRAUSE R. M., McCARTY M. Studies of L forms and protoplasts of group A streptococci. I. Isolation, growth, and bacteriologic characteristics. J Exp Med. 1959 Dec 1;110:853–874. doi: 10.1084/jem.110.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachon-Kalweit S., Elder B. L., Fives-Taylor P. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect Immun. 1985 Jun;48(3):617–624. doi: 10.1128/iai.48.3.617-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzija O. A simple method for the quantitative determination of muramic acid. Anal Biochem. 1974 Aug;60(2):512–517. doi: 10.1016/0003-2697(74)90261-9. [DOI] [PubMed] [Google Scholar]
- Handley P. S., Jacob A. E. Some structural and physiological properties of fimbriae of Streptococcus faecalis. J Gen Microbiol. 1981 Dec;127(2):289–293. doi: 10.1099/00221287-127-2-289. [DOI] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Cell-free released components of Streptococcus sanguis inhibit human platelet aggregation. Infect Immun. 1983 Oct;42(1):394–401. doi: 10.1128/iai.42.1.394-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Levine M. J., Ellison S. A., Tabak L. A. Purification and characterization of monkey salivary mucin. J Biol Chem. 1979 Mar 10;254(5):1487–1494. [PubMed] [Google Scholar]
- Iwasaki H., Shimada A., Yokoyama K., Ito E. Structure and glycosylation of lipoteichoic acids in Bacillus strains. J Bacteriol. 1989 Jan;171(1):424–429. doi: 10.1128/jb.171.1.424-429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linder L., Andersson C., Sund M. L., Shockman G. D. Protoplast formation and localization of enzymes in Streptococcus mitis. Infect Immun. 1983 Jun;40(3):1146–1154. doi: 10.1128/iai.40.3.1146-1154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A., DORFMAN A. Synthesis of capsular polysaccharide (hyaluronic acid) by protoplastmembrane preparations of group A Streptococcus. J Biol Chem. 1962 Feb;237:273–279. [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E., Cisar J. O., McNeil M. R., Bush C. A., Tjoa S. S., Fennessey P. V. A polysaccharide from Streptococcus sanguis 34 that inhibits coaggregation of S. sanguis 34 with Actinomyces viscosus T14V. J Bacteriol. 1988 May;170(5):2229–2235. doi: 10.1128/jb.170.5.2229-2235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Parks L. C., Shockman G. D., Higgins M. L. Growth of Streptococcus mutans protoplasts is not inhibited by penicillin. J Bacteriol. 1980 Sep;143(3):1491–1497. doi: 10.1128/jb.143.3.1491-1497.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberay A. H., Herzberg M. C., Rudney J. D., Nieuwenhuis H. K., Sixma J. J., Seligsohn U. Responses of platelets to strains of streptococcus sanguis: findings in healthy subjects, Bernard-Soulier, Glanzmann's, and collagen-unresponsive patients. Thromb Haemost. 1987 Apr 7;57(2):222–225. [PubMed] [Google Scholar]
- Takemoto H., Hase S., Ikenaka T. Microquantitative analysis of neutral and amino sugars as fluorescent pyridylamino derivatives by high-performance liquid chromatography. Anal Biochem. 1985 Mar;145(2):245–250. doi: 10.1016/0003-2697(85)90357-4. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Lactose metabolism in Streptococcus lactis: phosphorylation of galactose and glucose moieties in vivo. J Bacteriol. 1979 Dec;140(3):774–785. doi: 10.1128/jb.140.3.774-785.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Yeung M. K., Mattingly S. J. Biosynthesis of cell wall peptidoglycan and polysaccharide antigens by protoplasts of type III group B Streptococcus. J Bacteriol. 1983 Apr;154(1):211–220. doi: 10.1128/jb.154.1.211-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Fischetti V. A. Immunochemical analysis of intact M protein secreted from cell wall-less streptococci. Infect Immun. 1981 Apr;32(1):86–91. doi: 10.1128/iai.32.1.86-91.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]







