Abstract
Radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC) is an established alternative treatment to surgery and intra-arterial chemotherapy, usually performed under contrast-enhanced CT scan guidance. We describe our experience with the use of contrast-enhanced ultrasound and contrast dynamics analysis for planning and monitoring RFA in a patient with HCC.
Keywords: Contrast-enhanced ultrasound, hepatocellular carcinoma, radiofrequency ablation
Introduction
Radiofrequency ablation (RFA) is a proven method of treatment of hepatocellular carcinoma (HCC) in patients who are not candidates for liver transplantation or those in whom surgical resection cannot be performed.[1] The major limitation of RFA has been the small volume of ablation of tumor and its dependence on imaging for planning, monitoring, and assessment of the final outcome.[2]
Although there are studies in literature describing the use of contrast-enhanced ultrasound (CE-US) for RFA monitoring, these have used only visual interpretation of the pre- and post-procedural images.[3,4] In India, contrast-enhanced computer tomography (CECT) scan has been traditionally used as the imaging tool for the monitoring and follow-up of RFA.[5] CE-US has recently been made available in our country, and we would like to report its use along with a software package to analyze contrast dynamics, for assessing treatment response, during RFA of liver HCC.
Case Report
A 55-year-old man who was hepatitis C virus HCV positive with cirrhosis (Child Pugh B) was found to have a 4×3.5-cm hypoechoic nodule in the left lobe of liver in segment 2, on routine USG surveillance [Figure 1]. His alphafetoprotein AFP level was 18 IU/mL. A prior USG guided fine-needle aspiration cytology had shown HCC. The patient was referred to us for RFA.
Figure 1.
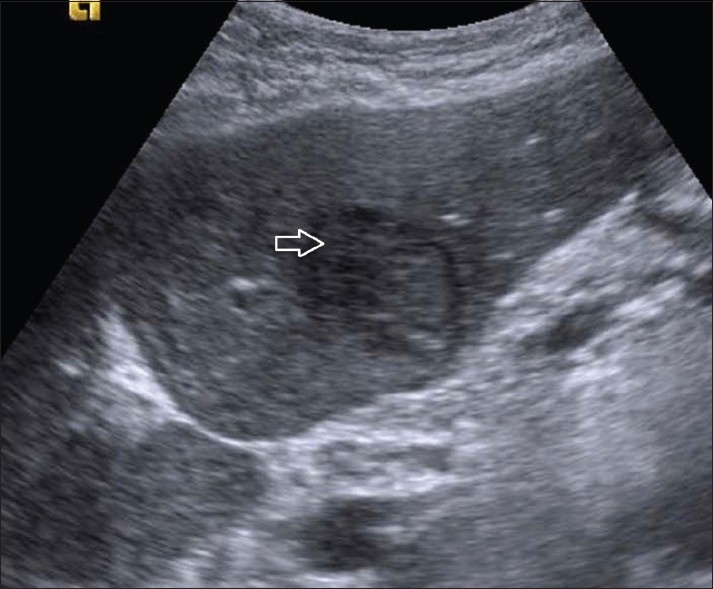
Grey scale USG image shows a hypoechoic nodule (arrow) in the left lobe of the liver
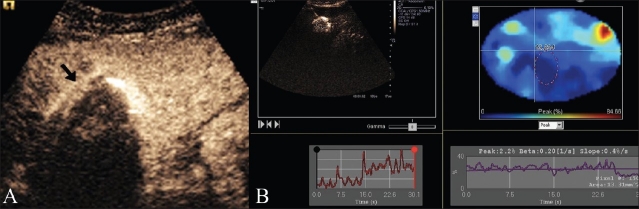
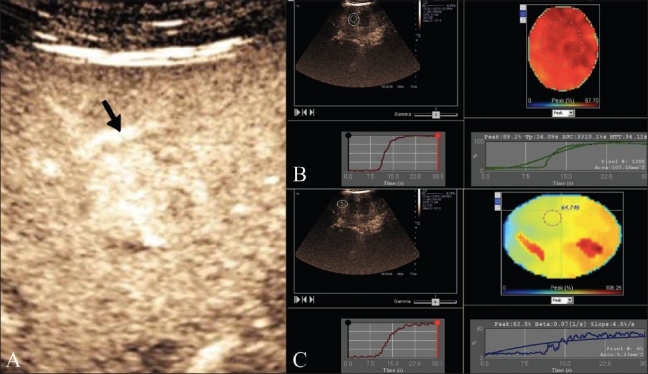
RFA was planned using conscious sedation with a cool tip RF system (Valleylab Tyco, Colorado, USA) with a 200-KW generator and a 4-cm tip cluster electrode connected to a peristaltic pump with rotating rollers to push cold saline water to the tip of the electrode to achieve a uniform ablation zone. An intravenous line was maintained for sedation and for the injection of 2 mL of microbubbles (Sonovue, Bracco, Italy) followed by 10 mL of saline. Imaging was done using an Acuson S2000 scanner (Siemens, Mountain View, CA, USA) with contrast pulse sequence technology, using a low mechanical index MI of 0.6. All images were stored in a cine loop video of 130 frames and analyzed offline using dynamic contrast analysis software (Siemens AG). These images showed intense arterial enhancement of the HCC nodule in the arterial phase at 18 s [Figure 2A]. The contrast dynamics evaluation showed marked arterial enhancement with a peak of 97.7% compared to peak enhancement of the normal liver parenchyma of 64.7% [Figure 2B, C]. Five minutes after the completion of ablation and before removing the RFA electrode, a baseline USG image was obtained, followed by CE-US with a second injection of 2 mL of Sonovue followed by 10 mL saline. Real-time images were acquired and stored as cine loop videos, in the arterial and portal phases, in the same way as the pre-procedure images and were then analyzed for contrast dynamics. The baseline pre-contrast post-RFA image showed the nodule to be hypoechoic with a peripheral echogenic rim of hemorrhage and necrosis, while the post CE-US image [Figure 3A] showed no enhancement of the nodule in the arterial phase, with a markedly reduced contrast signal peak of only 0.4% on the contrast dynamics software [Figure 3B]. Following this, the electrodes were safely removed. Post-procedure recovery was uneventful, and the patient was discharged after 2 hours of observation. The first follow up of the patient was done at 3 months using CE-US and showed no arterial enhancement at the RFA scar site.
Figure 2 (A-C).
Pre-RFA evaluation. CE-US (A) shows the enhancing liver nodule (arrow) in the arterial phase. The contrast dynamics software shows analysis of the peak enhancement of the HCC nodule -97.7% (B) at 15 seconds compared tonormal liver enhancement pattern with peak enhancement of the normal liver of 64.7% (C)
Figure 3 (A,B).
Post-RFA evaluation. CE-US (A) shows a non-enhancing nodule (arrow) in the arterial phase. Please note that the post-ablation contrast dynamics (B) of the HCC nodule shows no arterial (0.4%) enhancement
Discussion
The final goal of all ablative treatments is to obtain complete necrosis of the malignant nodule by disruption of the blood supply to the tumor tissue. Ideally, this should be accurately demonstrated by post-procedure imaging. So far, CECT has been used for the above purpose, and the absence of arterial enhancement has been the sine qua non of a successful procedure.[6,7] However, there are limitations with the use of CT, namely the use of radiation, administration of iodinated contrast repeatedly, and the lack of real-time monitoring. Any demonstration of residual enhancement/tumor tissue is an indication to perform the RFA again, preferably in the same session. This reduces the number of subsequent ablations and also improves patient survival.[8] So far, CE-US is the only imaging modality that not only monitors the procedure in real time but also accurately shows the treatment response at the end of the procedure.[6] To be able to improve the differentiation between complete and incomplete treatment response, the use of second-generation contrast agents like perfluoro agents has been suggested, and the images can now be analyzed using dedicated contrast dynamics software programs, as shown in our patient. In our initial experience, we felt that this was of tremendous help to the operator and helped him decide immediately whether a further RFA session was required or not even before the electrodes were removed. Earlier studies using first- and second-generation USG contrast have also reported a higher specificity of CE-US, namely 94%, to assess immediate post-procedure tumor response, while CECT shows a much lower specificity, namely 20% to 24%.[5,8] The reasons for the latter have been reported to be the presence of peripheral hyperemia, the presence of gas, and the uncooperative nature of patients while on the CT scan gantry table.
CE-US has so far been used for RFA only in limited institutes world wide mainly due to the lack of availability of ultrasound contrast media and dedicated CE-US hardware and software. However, these limitations are offset by the reduced cost of CE-US vis a vis CECT (using 1:2).
The case highlights the usefulness of CE-US as an imaging modality to monitor RFA in patients with HCC. With the use of contrast dynamics analysis, one can accurately detect the presence of any residual tumor immediately at the end of the procedure. Our initial experience suggests high operator comfort and patient ease during ablation with improved visualization of the end point of the procedure with the use CE-US.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 2.Ng KK, Poon RT. Radiofrequency ablation for malignant liver tumor. Surg Oncol. 2005;14:41–52. doi: 10.1016/j.suronc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Wen YL, Kudo M, Zheng RQ, Minami Y, Chung H, Suetomi Y, et al. Radiofrequency ablation of hepatocellular carcinoma: Therapeutic response using contrast-enhanced coded phase-inversion harmonic sonography. AJR Am J Roentgenol. 2003;181:57–63. doi: 10.2214/ajr.181.1.1810057. [DOI] [PubMed] [Google Scholar]
- 4.Cioni D, Lencioni R, Rossi S, Garbagnati F, Donati F, Crocetti L, et al. Radiofrequency thermal ablation of hepatocellular carcinoma: Using contrast-enhanced harmonic power Doppler sonography to assess treatment outcome. AJR Am J Roentgenol. 2001;177:783–8. doi: 10.2214/ajr.177.4.1770783. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra DG, Shah RC, Parikh V, Jagannath P. Radiofrequency ablation of liver tumors: Experience with open and percutaneous approach. Indian J Gastroenterol. 2006;25:66–70. [PubMed] [Google Scholar]
- 6.Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51(Suppl):S19–23. doi: 10.1016/j.ejrad.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Kim SK, Lim HK, Kim YH, Lee WJ, Lee SJ, Kim SH, et al. Hepatocellular carcinoma treated with radio-frequency ablation: Spectrum of imaging findings. Radiographics. 2003;23:107–21. doi: 10.1148/rg.231025055. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: Radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–8. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]