Abstract
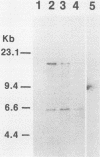
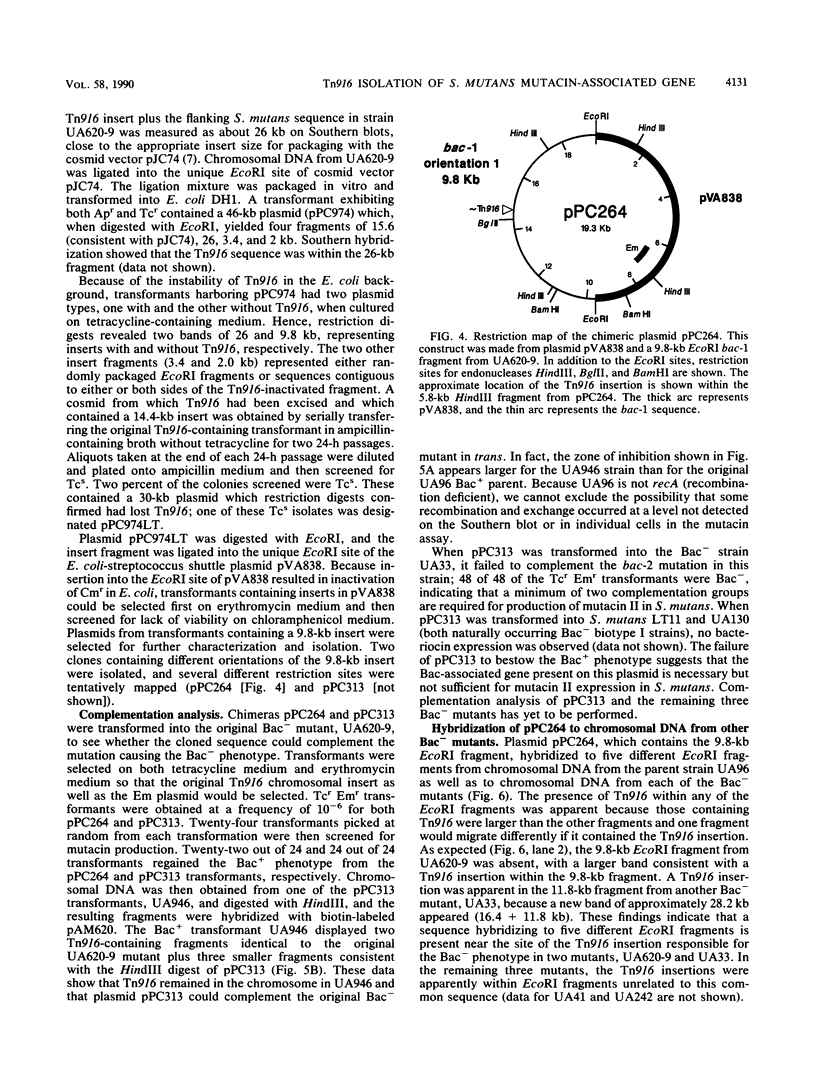
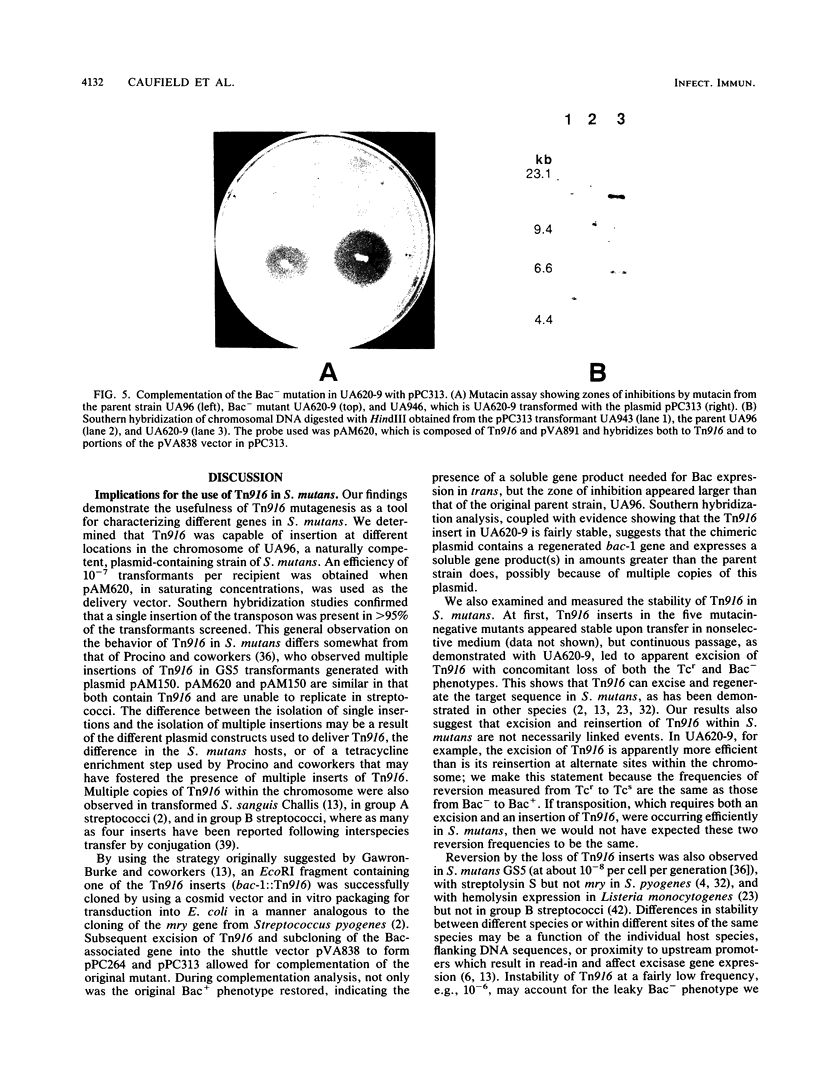
Among the attributes thought to contribute to the virulence of Streptococcus mutans is its ability to elaborate bacteriocinlike substances, which may provide a selective force enhancing its colonization potential. One such inhibitory substance, mutacin II, is produced by certain plasmid-containing strains of S. mutans. We introduced insertional mutations into a mutacin II-producing strain of S. mutans (UA96) by transformation with a plasmid carrying Tn916, resulting in transformants bearing single inserts of the transposon at different sites within the chromosome. The insertions identify five different EcoRI fragments required for production of mutacin II (Bac phenotype; bac-1 to bac-5 genotypes). The EcoRI fragments, containing bac-1::Tn916 was ligated into a cosmid vector, pJC74, and transduced into Escherichia coli DH1, where Tn916 is known to be unstable. The loss of Tn916 resulted in a 30-kb plasmid, pPC974, containing approximately 15 kb of S. mutans DNA. A Bac-associated DNA fragment was then subcloned into the streptococcus-E. coli shuttle vector pVA838 and transformed into S. mutants, where it was capable of complementing the bac mutation in the Bac- parent. These findings suggest that we have isolated at least one gene associated with mutacin production.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield P. W., Childers N. K., Allen D. N., Hansen J. B. Distinct bacteriocin groups correlate with different groups of Streptococcus mutans plasmids. Infect Immun. 1985 Apr;48(1):51–56. doi: 10.1128/iai.48.1.51-56.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield P. W., Walker T. M. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989 Feb;27(2):274–278. doi: 10.1128/jcm.27.2.274-278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Flannagan S. E., Ike Y., Jones J. M., Gawron-Burke C. Sequence analysis of termini of conjugative transposon Tn916. J Bacteriol. 1988 Jul;170(7):3046–3052. doi: 10.1128/jb.170.7.3046-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. Escherichia coli plasmids packageable in vitro in lambda bacteriophage particles. Methods Enzymol. 1979;68:309–326. doi: 10.1016/0076-6879(79)68022-9. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253–277. doi: 10.1007/978-3-642-70586-1_14. [DOI] [PubMed] [Google Scholar]
- Delisle A. L. Properties of mutacin b, an antibacterial substance produced by Streptococcus mutans strain BHT. Microbios. 1986;46(186):21–28. [PubMed] [Google Scholar]
- Fukushima H., Fukushima S., Umemoto T., Fukuhara H., Sagawa H. Purification and chemical analysis of a bacteriocin from the oral bacterium streptococcus mutans rm-10. Arch Oral Biol. 1982;27(9):721–727. doi: 10.1016/0003-9969(82)90020-6. [DOI] [PubMed] [Google Scholar]
- Fukushima H., Kelstrup J., Fukushima S., Umemoto T., Kaibori A., Sagawa H. Characterization and mode of action of a purified bacteriocin from the oral bacterium Streptococcus mutans RM-10. Arch Oral Biol. 1985;30(3):229–234. doi: 10.1016/0003-9969(85)90038-x. [DOI] [PubMed] [Google Scholar]
- Garnier T., Cole S. T. Complete nucleotide sequence and genetic organization of the bacteriocinogenic plasmid, pIP404, from Clostridium perfringens. Plasmid. 1988 Mar;19(2):134–150. doi: 10.1016/0147-619x(88)90052-2. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Imanishi H., Ooshima T. Isolation and mode of action of a cell-free bacteriocin (mutacin) from serotype g Streptococcus mutans MT3791. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 May;261(3):287–298. doi: 10.1016/s0176-6724(86)80046-3. [DOI] [PubMed] [Google Scholar]
- Hamada S., Ooshima T. Production and properties of bacteriocins (mutacins) from Streptococcus mutans. Arch Oral Biol. 1975 Oct;20(10):641–648. doi: 10.1016/0003-9969(75)90131-4. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Dzuback A. L., Andrews S. W. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J Dent Res. 1987 Jun;66(6):1092–1094. doi: 10.1177/00220345870660060101. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Johnson K. P., Yaphe B. I. Isolation of a Streptococcus mutans strain producing a novel bacteriocin. Infect Immun. 1984 Apr;44(1):141–144. doi: 10.1128/iai.44.1.141-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Yaphe B. I., Johnson K. P. Colonization of the human oral cavity by a strain of Streptococcus mutans. J Dent Res. 1985 Nov;64(11):1272–1274. doi: 10.1177/00220345850640110301. [DOI] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B., Segarra R. A., Gilmore M. S. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J Bacteriol. 1990 Jan;172(1):155–163. doi: 10.1128/jb.172.1.155-163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Iwanami T., Hirasawa M., Watanabe C., McGhee J. R., Shiota T. Purification and certain properties of a bacteriocin from Streptococcus mutans. Infect Immun. 1982 Mar;35(3):861–868. doi: 10.1128/iai.35.3.861-868.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Koulourides T., Kurita T., Housch T., Hirasawa M. Anti-dental caries effect in rats and man of a bacteriocin purified from the oral bacterium Streptococcus mutans C3603. Arch Oral Biol. 1985;30(5):381–384. doi: 10.1016/0003-9969(85)90063-9. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Hara N., Iwabuchi T. Molecular cloning and expression in Escherichia coli of the Bacillus licheniformis bacitracin synthetase 2 gene. J Bacteriol. 1989 Mar;171(3):1705–1711. doi: 10.1128/jb.171.3.1705-1711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T., Hirasawa M. Biological and biochemical characterization of novel lipid-like antibacterial substances (mutalipocins) produced by Streptoccus mutans strain 32K. J Gen Microbiol. 1988 Jan;134(1):213–220. doi: 10.1099/00221287-134-1-213. [DOI] [PubMed] [Google Scholar]
- Kuypers J. M., Heggen L. M., Rubens C. E. Molecular analysis of a region of the group B streptococcus chromosome involved in type III capsule expression. Infect Immun. 1989 Oct;57(10):3058–3065. doi: 10.1128/iai.57.10.3058-3065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Reider J. L., Virgili S. S., Kopecko D. J. Survey of the extrachromosomal gene pool of Streptococcus mutans. Infect Immun. 1977 Jul;17(1):215–226. doi: 10.1128/iai.17.1.215-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Murchison H. H., Barrett J. F., Cardineau G. A., Curtiss R., 3rd Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect Immun. 1986 Nov;54(2):273–282. doi: 10.1128/iai.54.2.273-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nida K., Cleary P. P. Insertional inactivation of streptolysin S expression in Streptococcus pyogenes. J Bacteriol. 1983 Sep;155(3):1156–1161. doi: 10.1128/jb.155.3.1156-1161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Date S., Abiko Y., Takiguchi H., Taniguchi S. Cloning and characterization of Streptococcus mutans LM7 plasmid pAM7. Infect Immun. 1987 Oct;55(10):2538–2540. doi: 10.1128/iai.55.10.2538-2540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrot M., Caufield P. W., Lavoie M. C. Preliminary characterization of four bacteriocins from Streptococcus mutans. Can J Microbiol. 1990 Feb;36(2):123–130. doi: 10.1139/m90-022. [DOI] [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procino J. K., Marri L., Shockman G. D., Daneo-Moore L. Tn916 insertional inactivation of multiple genes on the chromosome of Streptococcus mutans GS-5. Infect Immun. 1988 Nov;56(11):2866–2870. doi: 10.1128/iai.56.11.2866-2870.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., van der Hoeven J. S., Mikx F. H. Effect of bacteriocin production by Streptococcus mutans on the plaque of gnotobiotic rats. Infect Immun. 1979 Mar;23(3):571–576. doi: 10.1128/iai.23.3.571-576.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Heggen L. M., Kuypers J. M. IS861, a group B streptococcal insertion sequence related to IS150 and IS3 of Escherichia coli. J Bacteriol. 1989 Oct;171(10):5531–5535. doi: 10.1128/jb.171.10.5531-5535.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Kirchman P. A., Caparon M. G. An intermediate in transposition of the conjugative transposon Tn916. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4809–4813. doi: 10.1073/pnas.85.13.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser J. N., Rubens C. E. Transposon mutagenesis of group B streptococcus beta-hemolysin biosynthesis. Infect Immun. 1987 Sep;55(9):2314–2316. doi: 10.1128/iai.55.9.2314-2316.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Jones J. M., Senghas E., Gawron-Burke C., Clewell D. B. Generation of Tn5 insertions in streptococcal conjugative transposon Tn916. Appl Environ Microbiol. 1987 May;53(5):1069–1072. doi: 10.1128/aem.53.5.1069-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven J. S., Rogers A. H. Stability of the resident microflora and the bacteriocinogeny of Streptococcus mutans as factors affecting its establishment in specific pathogen-free rats. Infect Immun. 1979 Feb;23(2):206–213. doi: 10.1128/iai.23.2.206-212.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]