Abstract
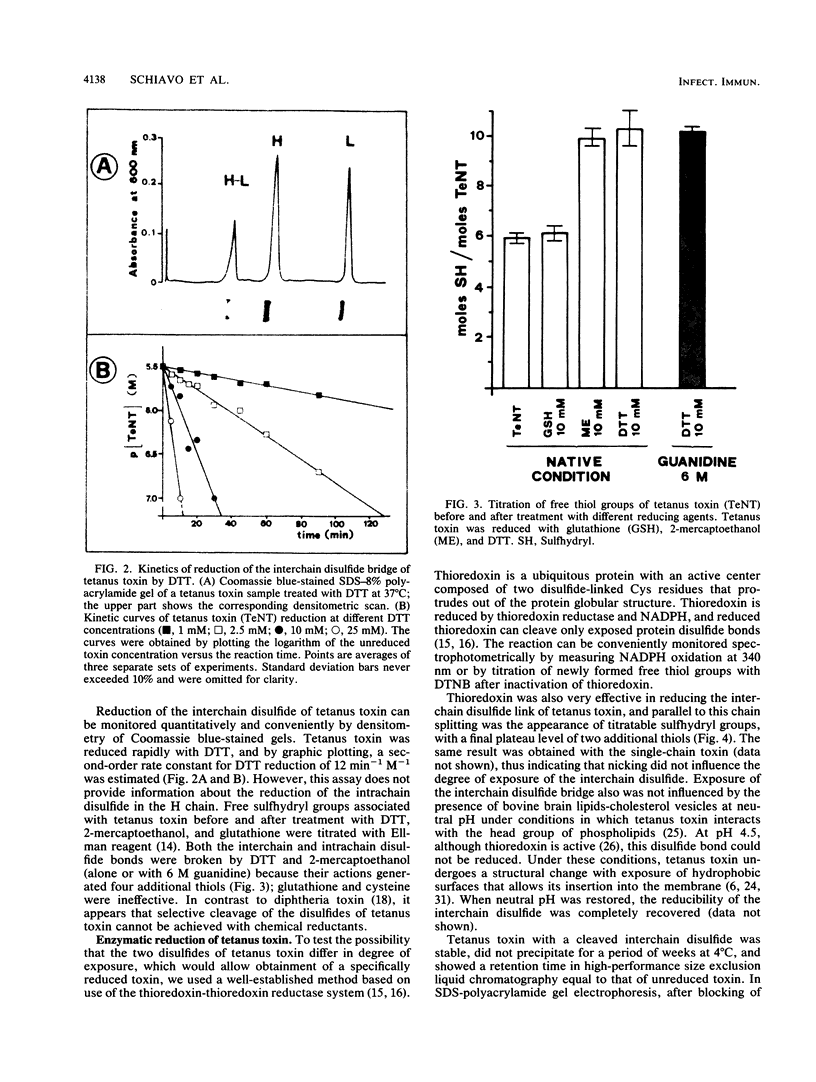
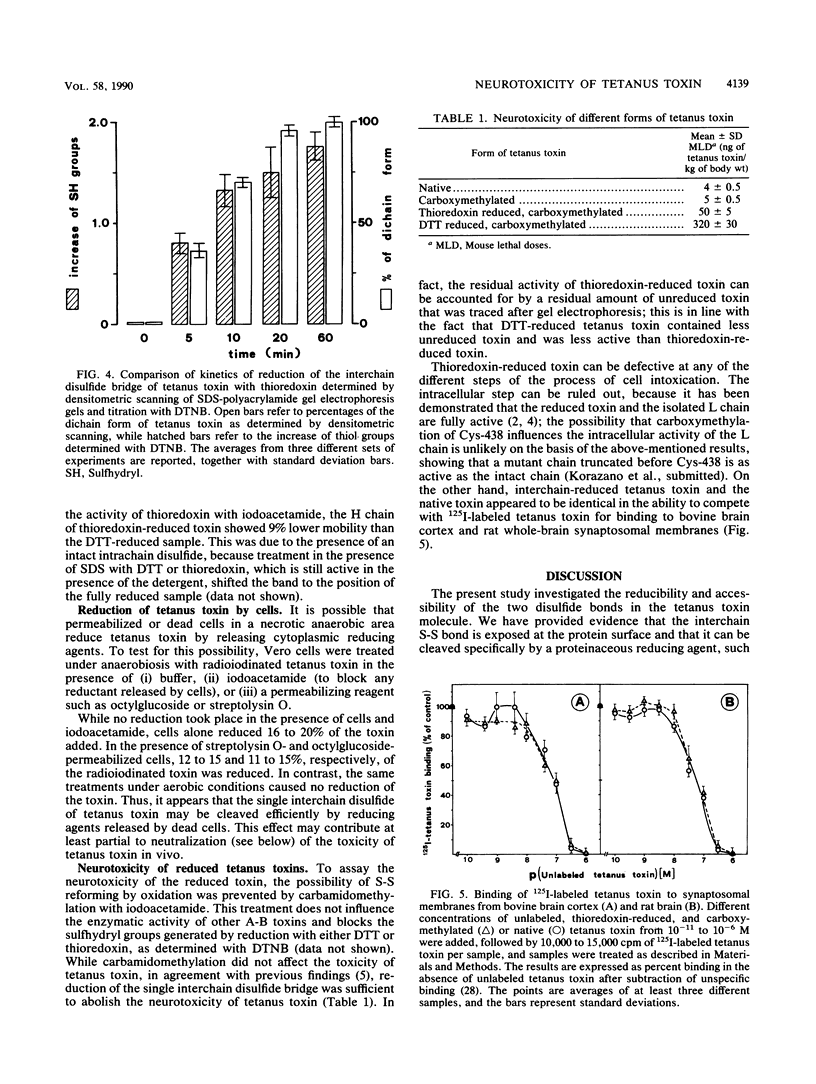
Tetanus toxin is composed of a heavy chain (100 kDa) and a light chain (50 kDa) held together by a single interchain disulfide bridge. An additional intrachain disulfide is present in the carboxy-terminal part of the heavy chain. Reduction of the two disulfide bonds in tetanus toxin with both chemical and proteinaceous reducing agents was studied. Dithiothreitol and 2-mercaptoethanol cleaved both the inter- and intrachain disulfide bridges of the toxin, while glutathione and cysteine were ineffective. Specific reduction of the single interchain disulfide link was achieved with the thioredoxin-thioredoxin reductase system, thus indicating that this bond is exposed at the protein surface. Also, dead or permeabilized cells were able to reduce the toxin. Such reduced toxin bound to neuronal membranes as well as the native toxin but was not neurotoxic. These findings open the possibility that reduction by cytoplasmic agents released by dead cells contributes to detoxification of tetanus toxin. Moreover, together with the notion that the light chain is the active form of the toxin in the cytoplasm, these results suggest that the interchain disulfide bond of tetanus toxin plays a role in nerve cell penetration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bader M. F., Bhakdi S., Gratzl M. Introduction of macromolecules into bovine adrenal medullary chromaffin cells and rat pheochromocytoma cells (PC12) by permeabilization with streptolysin O: inhibitory effect of tetanus toxin on catecholamine secretion. J Neurochem. 1989 Jun;52(6):1751–1758. doi: 10.1111/j.1471-4159.1989.tb07253.x. [DOI] [PubMed] [Google Scholar]
- Ahnert-Hilger G., Weller U., Dauzenroth M. E., Habermann E., Gratzl M. The tetanus toxin light chain inhibits exocytosis. FEBS Lett. 1989 Jan 2;242(2):245–248. doi: 10.1016/0014-5793(89)80478-8. [DOI] [PubMed] [Google Scholar]
- Barbieri L., Battelli M. G., Stirpe F. Reduction of ricin and other plant toxins by thiol:protein disulfide oxidoreductases. Arch Biochem Biophys. 1982 Jun;216(1):380–383. doi: 10.1016/0003-9861(82)90224-7. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., Habig W. H., Holz R. W. Isolated light chain of tetanus toxin inhibits exocytosis: studies in digitonin-permeabilized cells. J Neurochem. 1989 Sep;53(3):966–968. doi: 10.1111/j.1471-4159.1989.tb11800.x. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Blass J., Turpin A., Raynaud M. Chemical characterization of tetanus toxin and toxoid. Amino acid composition, number of SH and S-S groups and N-terminal amino acid. Eur J Biochem. 1970 Nov;17(1):100–105. doi: 10.1111/j.1432-1033.1970.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroff D. A., Fleck U. Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol. 1966 Nov;92(5):1580–1581. doi: 10.1128/jb.92.5.1580-1581.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Cotman C. W., Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972 Dec;55(3):696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisel U., Jarausch W., Goretzki K., Henschen A., Engels J., Weller U., Hudel M., Habermann E., Niemann H. Tetanus toxin: primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 1986 Oct;5(10):2495–2502. doi: 10.1002/j.1460-2075.1986.tb04527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N. F., Lyness V. A. The complete nucleotide sequence of tetanus toxin. Nucleic Acids Res. 1986 Oct 10;14(19):7809–7812. doi: 10.1093/nar/14.19.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Habermann E., Dreyer F. Clostridial neurotoxins: handling and action at the cellular and molecular level. Curr Top Microbiol Immunol. 1986;129:93–179. doi: 10.1007/978-3-642-71399-6_2. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Enzymatic reduction-oxidation of protein disulfides by thioredoxin. Methods Enzymol. 1984;107:295–300. doi: 10.1016/0076-6879(84)07019-1. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Kadenbach B., Jarausch J., Hartmann R., Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal Biochem. 1983 Mar;129(2):517–521. doi: 10.1016/0003-2697(83)90586-9. [DOI] [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- Krieglstein K., Henschen A., Weller U., Habermann E. Arrangement of disulfide bridges and positions of sulfhydryl groups in tetanus toxin. Eur J Biochem. 1990 Feb 22;188(1):39–45. doi: 10.1111/j.1432-1033.1990.tb15368.x. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Poulain B., Weller U., Habermann E., Tauc L. Light chain of tetanus toxin intracellularly inhibits acetylcholine release at neuro-neuronal synapses, and its internalization is mediated by heavy chain. FEBS Lett. 1989 Aug 14;253(1-2):47–51. doi: 10.1016/0014-5793(89)80926-3. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Brunner J., Duflot E., Boquet P., Roa M. Tetanus toxin is labeled with photoactivatable phospholipids at low pH. Biochemistry. 1986 Feb 25;25(4):919–924. doi: 10.1021/bi00352a027. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Gao Z., Bauerlein E., Boquet P., DasGupta B. R. Interaction of botulinum and tetanus toxins with the lipid bilayer surface. Biochem J. 1988 Apr 15;251(2):379–383. doi: 10.1042/bj2510379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaug J. O., Sandvig K., Olsnes S. Cell-mediated reduction of the interfragment disulfide in nicked diphtheria toxin. A new system to study toxin entry at low pH. J Biol Chem. 1987 Jul 25;262(21):10339–10345. [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Burns D. L., Hsia J. A., Yost D. A., Myers G. A., Hewlett E. L. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J Biol Chem. 1983 Oct 10;258(19):11879–11882. [PubMed] [Google Scholar]
- Ozutsumi K., Sugimoto N., Matsuda M. Rapid, simplified method for production and purification of tetanus toxin. Appl Environ Microbiol. 1985 Apr;49(4):939–943. doi: 10.1128/aem.49.4.939-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce E. J., Davison M. D., Parton R. G., Habig W. H., Critchley D. R. Characterization of tetanus toxin binding to rat brain membranes. Evidence for a high-affinity proteinase-sensitive receptor. Biochem J. 1986 Jun 15;236(3):845–852. doi: 10.1042/bj2360845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi H. O., Ludwig D. S., Mercer K. L., Schoolnik G. K., Kornberg R. D. Three-dimensional structure of cholera toxin penetrating a lipid membrane. Science. 1988 Mar 11;239(4845):1272–1276. doi: 10.1126/science.3344432. [DOI] [PubMed] [Google Scholar]
- Roa M., Boquet P. Interaction of tetanus toxin with lipid vesicles at low pH. Protection of specific polypeptides against proteolysis. J Biol Chem. 1985 Jun 10;260(11):6827–6835. [PubMed] [Google Scholar]
- Simpson L. L. Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol. 1986;26:427–453. doi: 10.1146/annurev.pa.26.040186.002235. [DOI] [PubMed] [Google Scholar]
- Tomasi M., Montecucco C. Lipid insertion of cholera toxin after binding to GM1-containing liposomes. J Biol Chem. 1981 Nov 10;256(21):11177–11181. [PubMed] [Google Scholar]
- Weller U., Mauler F., Habermann E. Tetanus toxin: biochemical and pharmacological comparison between its protoxin and some isotoxins obtained by limited proteolysis. Naunyn Schmiedebergs Arch Pharmacol. 1988 Aug;338(2):99–106. doi: 10.1007/BF00174855. [DOI] [PubMed] [Google Scholar]
- Wellhöner N. H. Tetanus neurotoxin. Rev Physiol Biochem Pharmacol. 1982;93:1–68. doi: 10.1007/BFb0032668. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]
- Wright H. T., Marston A. W., Goldstein D. J. A functional role for cysteine disulfides in the transmembrane transport of diphtheria toxin. J Biol Chem. 1984 Feb 10;259(3):1649–1654. [PubMed] [Google Scholar]
- Wright H. T., Robertus J. D. The intersubunit disulfide bridge of ricin is essential for cytotoxicity. Arch Biochem Biophys. 1987 Jul;256(1):280–284. doi: 10.1016/0003-9861(87)90447-4. [DOI] [PubMed] [Google Scholar]