Abstract
BACKGROUND
From the standpoint of normal embryologic development, the palpebral fissures are generally considered to be determined by and dependent on the underlying optic vesicles, outpouchings of the frontal area of the developing fetal brain. It has been suggested that short palpebral fissures are a reflection of an underlying defect in specific areas of forebrain development. Alternatively, short palpebral fissures, seen in a number of multiple malformation syndromes associated with small occipitofrontal circumference (OFC), such as the fetal alcohol syndrome (FAS), might be proportionally small as a reflection of the microcephaly. The purpose of this study was to examine whether short palpebral fissures are independent of or determined by the OFC.
METHODS
Age-specific palpebral fissure length (PFL) and OFC centiles were correlated in 273 children with FAS, 272 children with some features of FAS and 385 children with no structural features characteristic of FAS.
RESULTS
OFC and PFL centiles demonstrated a statistically significant but weak correlation in all three study groups. Among children with FAS, only 10.2 % of the total variation in PFL could be accounted for by OFC (p=0.0001). A similar pattern was observed for children with some features of FAS (r2 = 0.142; p=0.0001) and children with no structural features of FAS (r2 = 0.110; p=0.0001).
CONCLUSIONS
Palpebral fissure length is predominately independent of occipitofrontal circumference in children with and without features of FAS. Short palpebral fissures may well reflect a defect in forebrain development rather than being proportionally reduced in size as a reflection of microcephaly.
Keywords: palpebral fissure length, fetal alcohol syndrome (FAS), occipitofrontal circumference, forebrain development, microcephaly
INTRODUCTION
From the standpoint of normal embryologic development, the palpebral fissures are generally considered to be determined by and dependent on the underlying optic vesicles, outpouching of the frontal area of the developing fetal brain (Braddock et al., 1994; Hellstrom et al., 1997; Parnell et al., 2006). It has been suggested that short palpebral fissures in a mouse model of the fetal alcohol syndrome (FAS), and most likely in humans with FAS, are a reflection of an underlying defect in specific areas of forebrain development (Parnell et al., 2006). Alternatively, short palpebral fissures, seen in a number of multiple malformation syndromes associated with small occipitofrontal circumference (OFC) including FAS, might simply be proportionally small as a reflection of the associated microcephaly. The purpose of this study was to examine the extent to which short palpebral fissures are independent of or correlated with the OFC.
METHODS
Study Population
This study was part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). The CIFASD is an international consortium of basic science and clinical investigations sponsored by the U.S. National Institute of Alcohol Abuse and Alcoholism (NIAAA) and focused on addressing critical questions regarding the prenatal effects of alcohol. As part of CIFASD, a Dysmorphology Core was established to assure accurate and consistent diagnosis of FAS in children at all consortium sites through implementation of a standard protocol based on documentation of the clinical phenotype of FAS. Children at these sites were ascertained using a variety of methods including cross-sectional retrospective and prospective study designs.
By 2006, 930 children from six consortium sites (Buffalo, New York; San Diego, California; Rome, Italy; Moscow, Russia; Helsinki, Finland; and Cape Town, South Africa) were examined by at least one of us (HEH, LKR, MdelC, MAM and/or KLJ). The study has been approved by Human Subject Protection Programs at all participating clinical sites.
Dysmorphology Assessment
A structured protocol was used for assessment of specific dysmorphologic features that constitute FAS. Palpebral fissure length (PFL) was measured with a rigid ruler marked in millimeters. OFC was measured by a cloth measuring tape. Age-specific centiles for OFC and PFL were determined using previously published charts (Kuczmarski et al., 2000; Thomas et al., 1987; Tanner, 1978; Nelllhaus, 1968). The CDC 2000 growth charts (Kuczmarski et al., 2000) are limited to children 3 years of age and younger. For this reason, OFC percentiles for children over 3 years of age were determined using Nellhaus and Tanner growth charts.
Children were given a preliminary diagnosis solely on the basis of structural features of FAS (PFL ≤10th centile, a smooth philtrum and a thin vermilion border), microcephaly (OFC ≤ 10th centile) and growth deficiency height and/or weight ≤10th centile). In addition to the diagnosis of FAS, children could be classified in a “Deferred” group if they had evidence of growth deficiency and some structural features consistent with FAS, but insufficient to meet the specific diagnostic criteria. Children who had insufficient or no evidence of growth deficiency, microcephaly or any of the key structural features specific to FAS were classified into a “No FAS” group.
Statistical Analysis
Correlation analysis was conducted to determine the association between OFC and PFL centiles among children with FAS, children in the “Deferred” group, and children in the “No FAS” group. The sample correlation coefficient or Pearson correlation coefficient (r) and the square of the sample correlation coefficient (r2) were estimated. R-squared denotes the strength of the linear relationship between OFC centile and PFL centile and is interpreted as the proportion of variation in one of the variables (i.e., PFL centile) that can be explained by variation in the other variable (i.e., OFC centile). The significance of each correlation coefficient was tested and the p-value associated with the coefficient was estimated. Adjustment for age of the child was not incorporated since OFC and PFL centiles are already age- and /or age and gender specific. All statistical analyses were performed in SAS (Release 9.1, SAS Institute, Inc., Cary NC, 2002-2003).
RESULTS
The mean age of study participants was 9.1 years with a range from 1.4 to 21.1 years of age (Table 1). Subjects were recruited from Italy (23.1%), the United States (24.0%), South Africa (28.2%), Finland (15.2%), and Russia (9.6%). Due to recruitment from a high-risk population (children in boarding schools and orphanages, children with permanent signs of CNS dysfunction or with known prenatal exposure to alcohol) 273 (29.4%) of the children were diagnosed with FAS, 272 (29.3%) were classified as Deferred, and 385 (41.4%) were classified as No FAS. Among children with a diagnosis of FAS 77.2% had PFL ≤ 10th centile and among those classified in the Deferred group 30.8% had PFL ≤ 10th centile. None of the children in the No FAS group had PFL ≤ 10th centile.
Table 1.
Description of the Sample (N=930)
| Characteristic | ||
|---|---|---|
| Mean±s.d | Range | |
| Child age (years) | 9.1±4.1 | 1.4-21.2 |
| N | (%) | |
| Clinical site: | ||
| Buffalo, NY | 78 | (8.4) |
| San Diego, CA | 94 | (10.1) |
| Rome, Italy | 215 | (23.1) |
| Plains Indians, US | 51 | (5.5) |
| Moscow, Russia | 89 | (9.6) |
| Helsinki, Finland | 141 | (15.2) |
| South Africa | 262 | (28.2) |
| Child sex: | ||
| Male | 481 | (51.7) |
| Female | 449 | (48.3) |
| FAS status: | ||
| Yes | 273 | (29.4) |
| No | 385 | (41.4) |
| Deferred | 272 | (29.3) |
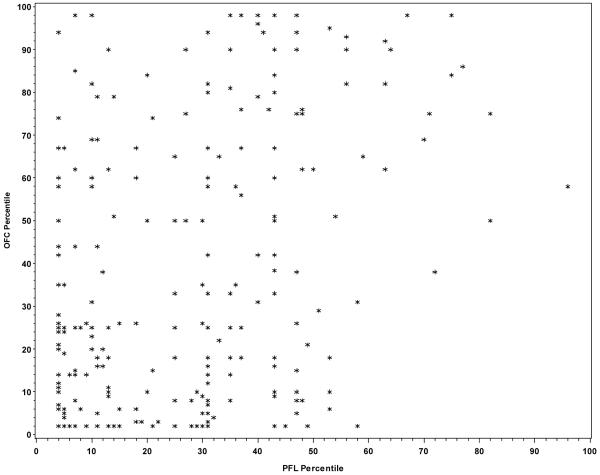
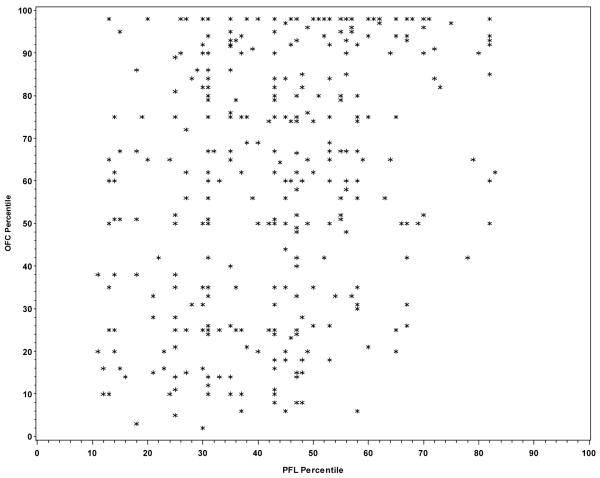
Among the 273 children with FAS, only 10.2% of the variation in PFL was explained by OFC (r2=0.102; p<0.001) indicating that 89.8% of the variation in PFL is due to factors other than OFC. From the graphical presentation of the correlation between the two variables (Figure 1), it does not seem that the lack of a stronger correlation was due to a nonlinear association between OFC and PFL centiles. Among children with some structural features of FAS (i.e., the “Deferred” group), 14.2% of the variation in PFL was explained by OFC (r2=0.142; p<0.0001) indicating that 85.8% of the variation in PFL is due to factors other than OFC and no obvious pattern was observed (Figure 2). As noted in Figure 3, similar results were observed among children with no structural features characteristic of FAS (r2=0.110; p<0.0001).
Figure 1.
Figure 2.
Figure 3.
DISCUSSION
This study examined the correlation between OFC centile and PFL centile in a sample of children with and without structural features of FAS. Although a statistically significant correlation between these two measures was found among all three groups (children with a diagnosis of FAS, those classified as Deferred, as well as those with no features compatible with FAS), the amount of variation accounted for by this covariance was low, ranging between 10 and 14.2%, and was similar regardless of group. In that the palpebral fissures are determined by the underlying optic vesicles which are outpouchings of the forebrain, an area that is particularly vulnerable to prenatal alcohol exposure, these data suggest that short palpebral fissures in FAS may well reflect a defect in specific areas of frontal brain development rather than simply reflecting a proportionate decrease in overall brain size.
A study published by Parnell et al. in which ocular defects were used as a measure of effect in a mouse model of fetal alcohol spectrum disorder is particularly relevant to these observations in humans. In that study, compared to the normal control group, embryos treated with alcohol at 7-8 days gestation had reductions in the size of the forebrain, as well as size of the globe and palpebral fissures which were directly correlated with each other. These observations led them to conclude that in FAS, reduced PFL is reflective not only of reduced globe size, but is also indicative of forebrain damage.
Further support for the hypothesis that short palpebral fissures in FAS reflect alterations in frontal brain development is based on the observation that the optic nerve is often abnormal in FAS. In fact Stromland (1985) reported that forty-eight percent of such children have optic nerve hypoplasia. The optic vesicle, which subsequently forms the optic stalk, the immediate precursor of the optic nerve, initially possesses a cavity which is continuous with that of the prosencephalon. It therefore is not surprising that defects in forebrain development are associated with optic nerve hypoplasia.
In another study Wass et al. (2001) published data documenting various CNS markers of alcohol exposure using prenatal ultrasound. Four measurements of brain structure were made including transcerebellar distance, distance from the posterior margin of the thalamus to the inner calvarium, distance from the posterior margin of the cavum septum pellucidum to the inner surface of the calvarium (frontal lobe) and biparietal diameter. Regression analysis demonstrated that prenatal alcohol exposure was associated with reduction in the frontal lobe as measured by the distance from the posterior margin of the cavum septum pellucidum to the inner surface of the calvarium and to a lesser extent to the distance from the posterior margin of the thalamus to the inner calvarium. That other brain measurements were not affected indicates that there was a disproportionate effect of prenatal alcohol exposure on the frontal cortex as opposed to a global effect on brain structures.
The fact that 89.8% of the variation in PFL centile in the children with FAS in this study and 85.8% of the variation in those with some features of FAS were due to factors other than the OFC centile has implications relative to the mechanism whereby prenatal alcohol exposure impacts PFL. If the variation in the PFL is based exclusively on the size of the brain, a much stronger correlation coefficient between OFC centile and PFL centile would be expected and far less of the variation in PFL centile would be due to other factors. The fact that a similar percent of variation in PFL centile was evident in the children without features of FAS (the “No FAS” group) is not surprising. Parnell et al. indicated in their mouse model that palpebral fissure length was directly correlated with globe size not only in the ethanol-exposed group but also in the normal control group. Given the fact that reduced palpebral fissure length in their model of FAS reflects both reduced globe size and frontal brain size, it is understandable that PFL would primarily reflect size of the globe and forebrain as opposed to size of the OFC in normal children as well We speculate that a similar correlation exists in children with other multiple malformation syndromes associated with short palpebral fissures.
In conclusion, although a small OFC is predictive of short palpebral fissures in children with FAS, PFL is predominantly independent of head circumference. This suggests that short palpebral fissures may well reflect a defect in specific areas of frontal brain development rather than simply reflecting a proportionate decrease in overall brain size.
Acknowledgments
Grant Sponsor: National Institute on Alcohol Abuse and Alcoholism; Grant Number: 2U24AA014815-05
REFERENCES
- Braddock SR, Jones KL, Reynaldo D, Bejar R. The relationship between palpebral fissures and ocular size. Proceedings of the Greenwood Genetic Center. 1995;14:76. [Google Scholar]
- Hellstrom A, Svensson E, Stromland K. Eye size in healthy Swedish children and in children with fetal alcohol syndrome. Acta Ophthalmol Scand. 1997;75:423–428. doi: 10.1111/j.1600-0420.1997.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- Nelhaus G. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968;41:106–114. [PubMed] [Google Scholar]
- Parnell SE, Dehart DB, Wills TA, Chen S, Hodge CW, Besheer J, Waage-Baudet HG, Charness ME, Sulik KK. Maternal oral intake mouse model for fetal alcohol spectrum disorders: Ocular defects as a measure of effect. Alcohol Clin Exp Res. 2006;30:1791–1798. doi: 10.1111/j.1530-0277.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- Stromland K. Ocular abnormalities in the fetal alcohol syndrome. Acta Ophthalmol Suppl. 1985;171:1–50. [PubMed] [Google Scholar]
- Tanner JM. Physical growth and development. In: Forfar JO, Arneil GC, editors. Textbook of Pediatrics. Churchill Livingston, Edinburgh: 1978. pp. 253–303. [Google Scholar]
- Thomas LT, Gaitantzis YA, Frias JL. Palpebral fissure length from 29 weeks to 14 years. J Pediatr. 1987;111:267–268. doi: 10.1016/s0022-3476(87)80085-9. [DOI] [PubMed] [Google Scholar]
- Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. Am J Obstet Gynecol. 2001;185:737–42. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]