Abstract
Xeroderma pigmentosum is a rare, autosomal recessive disease caused by a defect in DNA repair. Patients with xeroderma pigmentosum often have cutaneous and ocular sun sensitivity, freckle-like skin pigmentation, multiple skin and eye cancers, and, in some patients, progressive neurodegeneration. Xeroderma pigmentosum predominantly affects the UV exposed ocular surface, resulting in eyelid atrophy and cancers, corneal dryness, exposure keratopathy, and conjunctival tumors. We report the clinical history and ocular pathology of two Caucasian women who had xeroderma pigmentosum with neurological degeneration: Case 1, who died at age 44 and Case 2, who died at age 45. Case 1 with mutations in the XPA gene, had more than 180 basal cell carcinomas of her skin and eyelids and died from complications of neurodegeneration. Case 2, with mutations in the XPD gene, was sun protected and had 3 skin cancers. She died from complications of neurodegeneration and pneumonia. Both patients had bilateral pinguecula, corneal pannus and exposure keratopathy. Case 1 had bilateral optic atrophy, while Case 2 had bilateral peripheral retinal pigmentary degeneration. Both patients developed retinal gliosis. The ophthalmic manifestations and pathology of xeroderma pigmentosum are discussed and reviewed with respect to this report and other cases in the literature. These cases illustrate the role of DNA repair in protection of the eyes from UV damage and neurodegeneration of the retina.
Keywords: ciliary body hamartoma, ocular pathology, optic atrophy xeroderma pigmentosum, pigmentary retinal degeneration
Introduction
Xeroderma pigmentosum (XP), first described by Hebra and Kaposi in 1874 presents in early childhood with photophobia, photosensitivity, cutaneous pigmentary changes, and a predisposition for malignancy in sun-exposed mucocutaneous areas and ocular structures.36 XP patients have a defect in DNA repair related to the nucleotide excision repair pathway15,17,20,75 or a bypass polymerase pathway.9 Mutations in XPA or XPC are present in approximately 50% of patients.55 The disease is extremely rare in North America and Europe (frequency 1/1,000,000),47 but is more common in areas of the world with increased consanguinity, including Japan (frequency 1/22,000),38 the Middle East, North Africa, and India.47,66
XP often presents with cutaneous manifestations within the first two years of life.73 Patients are predisposed to malignant skin neoplasms in the face, neck and upper trunk, the tip of the tongue, and the anterior eye surfaces. Ocular disease is evident in at least 40% of XP patients and often causes visual impairment.49 Clinically apparent ocular disease includes eyelid atrophy and tumors, corneal sicca and opacification, exposure keratitis, pterygium, and chronic conjunctival injection.24,31 Here, we report the pathology of autopsied eyes from two XP patients, in which we found classic findings of this disease and retinal degeneration.
Case Reports
CASE 1 (XP12BE)
Clinical History
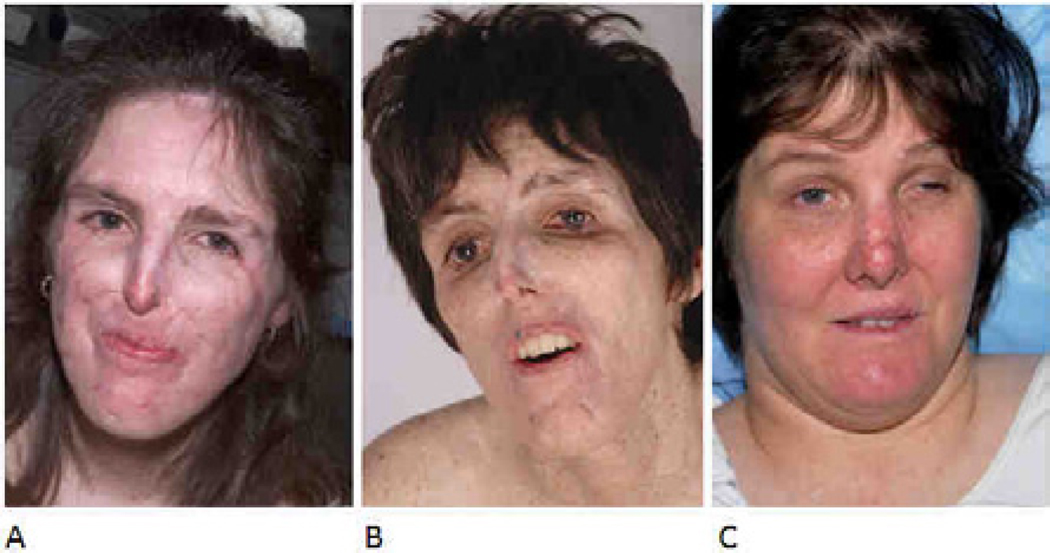
This 44 year-old Caucasian woman with a mutation in the XPA DNA repair gene75,76 was followed at the NIH Clinical Center since age 4 years. From 1976 to 1992, she had 166 histologically documented basal cell carcinomas, most of which were on her face (cheeks, lips, nose, eyelids, eyebrows, and canthus). She had no squamous cell carcinomas or melanomas (Fig. 1A). During treatment with oral 13-cis-retinoic acid, from 1984 to 1986 the frequency of new basal cell carcinomas was dramatically reduced, but increased after treatment was discontinued. Treatment was resumed in 1987 and continued to 2000. Over time, the frequency of new cancers decreased and, from 1993 to her death in 2009, she had only 17 additional basal cell carcinomas.50 She had progressive neurological degeneration beginning as absent deep tendon reflexes and progressing to loss of hearing, inability to walk, and eventually difficulty swallowing, necessitating G-tube placement (Fig. 1B). She had global cerebral atrophy on imaging. In July, 2009, she became progressively unresponsive and expired when supportive measures were withdrawn; autopsy was performed at the NIH.
Fig. 1.
Clinical images. A and B) Case 1, patient XP12BE, disease progression. A) At age 35 yrs, her face is thin with loss of subcutaneous tissue and scars from more than 100 surgical procedures for removal of skin cancers. Her eyes show mild conjunctival injection bilaterally. Her lips have cheilitis. B) At age 41 yrs, she had progressive loss of subcutaneous tissue with deep set eyes. Scarring of the eyelids, retraction of both lower eyelids, and a 4–6 mm lagophthalmos was present bilaterally without lash loss. C) Case 2, patient XP18BE, at age 40 yrs. Her face shows diffuse erythema and a surgical scar on her nose. There is mild conjunctival injection and ptosis of the left eyelid. Her lips have cheilitis. [The patients and their families provided permission to use these images.]
Ocular History
At age 4, the patient had a normal eye exam except for slight elevations of the nasal conjunctiva. At age 17, her visual acuity was 20/20 in both eyes with a myopic correction, and she had an early nasal corneal pannus in the right eye and two biopsy-proven eyelid basal cell carcinomas. She had reactive pupils, full ductions, and normal pattern reversal visual evoked responses. At age 19, there were no further lid tumors, but bilateral pinpoint cortical opacities; mild, unilateral, peripheral corneal scarring; an early, unilateral pterygium and unilateral segmental iris atrophy had developed. She had 8 basal cell carcinomas removed by age 27 from her lower eyelids, bilateral inner canthi, and eyebrows.
At age 41, she complained of extreme photophobia and had scarring of the eyelids, retraction of both lower eyelids, and a 4–6 mm lagophthalmos bilaterally without lash loss (Fig. 1B). Both eyes exhibited conjunctival injection, exposure keratopathy and pannus formation threatening the visual axis, with a score of 9.0 on the Oxford scale8, indicating corneal surface irregularity. She had an intermittent left exotropia. Pupils were 4 mm and minimally to nonreactive to light. Dilated fundus examination revealed mild optic disk pallor in both eyes and otherwise normal fundi. Soon after instillaltion of 1% tropicamide, she became less responsive and required medical attention. She returned to baseline within an hour, and her mental status change was attributed to an adverse reaction to the dilating drops. Three months later, the corneal pannus was stable, and meibomitis was observed bilaterally. The patient expired 2 years after her last eye examination. In summary, the XP-associated clinical ocular findings in case 1 were chronic conjunctival injection, pterygia, basal cell carcinomas of the eyelids and canthi, corneal pannus, exposure keratopathy, minimally reactive pupils, and optic nerve pallor.
Pathological Findings
Macroscopically, inferior corneal neovascularization and scarring were visible (Fig. 2A). Microscopically, the central corneal epithelium was irregularly thin with early keratinization and small focal bullous separations between the basal corneal epithelial cells and Bowman’s layer, consistent with bullous keratopathy. The limbal epithelium had mild focal parakeratosis and flattening. A layer of fibrovascular tissue interrupted the peripheral corneal epithelium and an irregular Bowman’s layer (Fig. 3). Neovascularization and inflammatory cellular infiltration (CD68+ (macrophages) cells > CD3+ (T lymphocytes) cells) were found superficially and in the stroma of the peripheral cornea and limbus. Focal thickening of Descemet's membrane, corneal guttata, prominence of Schwalbe’s ring, and corneal endothelial cell loss were also noted. Corneal findings were similar in both eyes. The anterior chamber angle, iris, lens, and sclera were unremarkable.
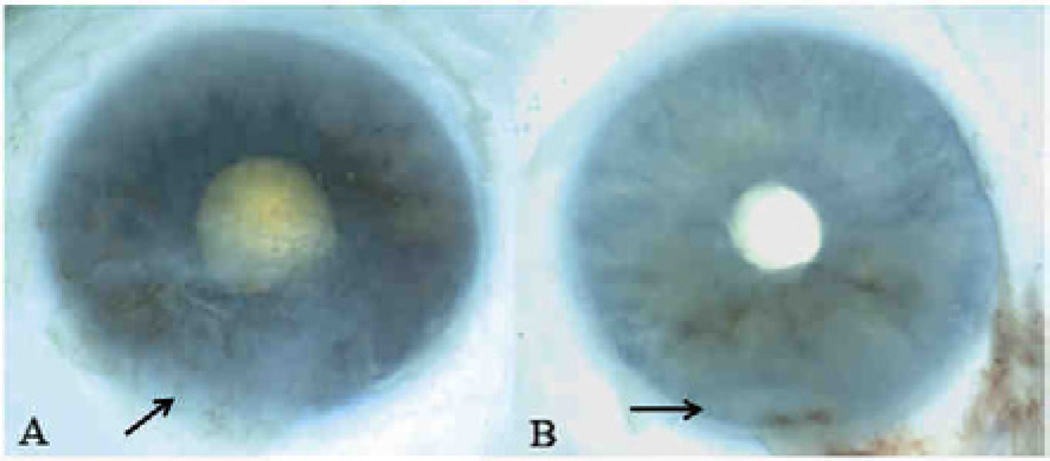
Fig. 2.
Macroscopic images of autopsy eyes of the XP patients. Both images show an inferior opacity (arrows), scar and neovascularization with pannus formation in the cornea that begins 2.0 mm from the center of cornea and measures 10.5 × 10.0mm. A) Case 1, XP12BE, right eye; B) Case 2, XP18BE, left eye
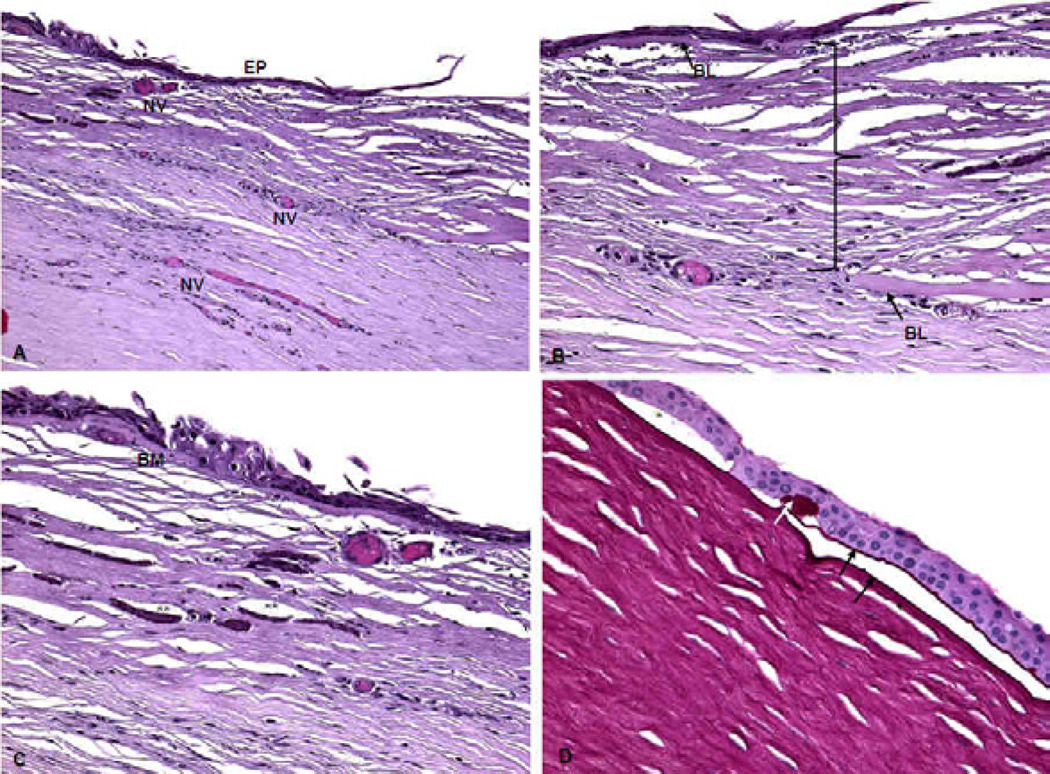
Fig. 3.
Microscopic images of the right cornea of Case 1, XP12BE. A) The central corneal epithelium (EP) is irregularly thin. There is early keratinization. Corneal neovascularization is present in the anterior stroma (NV). B) There is fibrovascular scar tissue between the peripheral corneal epithelium and Bowman’s layer extending into the mid corneal stroma (bracket). There is a break in Bowman’s layer (BL, two arrows). C) The epithelial basement membrane (BM) shows focal irregularities (*), is thickened and thinned in different locations, and has multiple layers. Calcifications (**) are noted. D) Bullous keratopathy is seen with focal small separations (*) between the basal corneal epithelial cells and Bowman’s layer, where a small amount of serous material is visible. Reduplication of the basement membrane (black arrows) and clumping of the basement membrane (white arrow) are evident. (A–C: Hematoxylin & Eosin, D: Periodic acid-Schiff; original magnifications, A ×100, B–D × 200)
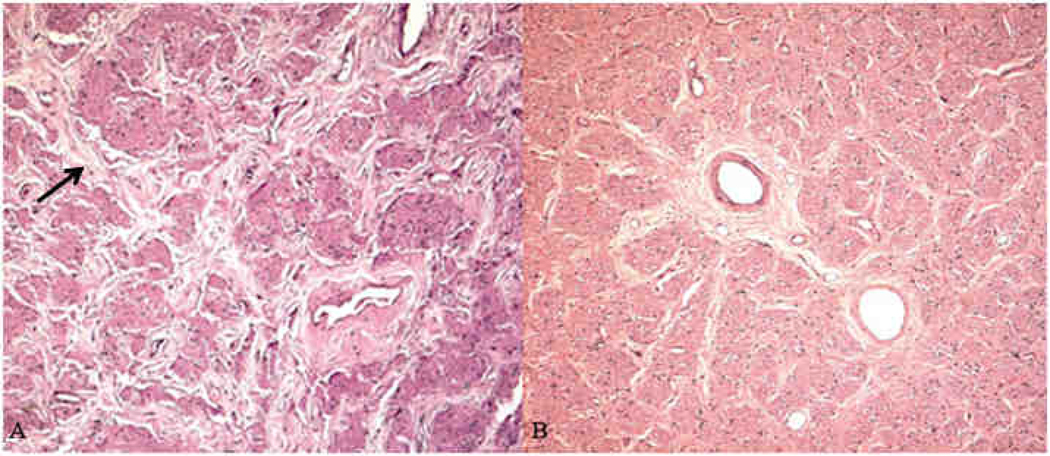
The ciliary body had mild hyalinization in both eyes. In the right eye, a cluster of pigmented epithelial cells was arranged in multiple layers in the temporal ciliary body (Fig. 4A and 4B). This lesion had a positive Fontana-Masson stain for melanin, a positive keratin stain (indicating epithelial keratin filaments) (Fig. 4C), and a negative S100 stain (A positive S100 would suggest melanoma.). This staining pattern is consistent with a ciliary pigmented epithelial hamartoma. A small retinal tuft composed of retinal and glial tissues was found in the peripheral retina of the left eye. There were scattered macrophages (CD68+) in the retina. In both eyes, the optic nerves had moderate to marked axonal loss with vacuolization and thickened septae, indicative of mild to moderate optic nerve atrophy (Fig. 5A). Moderate thickening of the optic artery vessel wall, indicative of optic artery sclerosis, was present in the right eye. The macula shows strong positive glial fibrillary acidic protein (GFAP) immunoreactivity indicating retinal gliosis and mild edematous change with separation of Henle’s fibers in the outer plexiform layer in the right eye (Fig. 6). In summary, ophthalmic pathology due to UV damage included corneal neovascularization, chronic keratitis, and pannus. Ocular lesions secondary to neurodegeneration include optic nerve atrophy, retinal degeneration, and possibly mild macular edema.
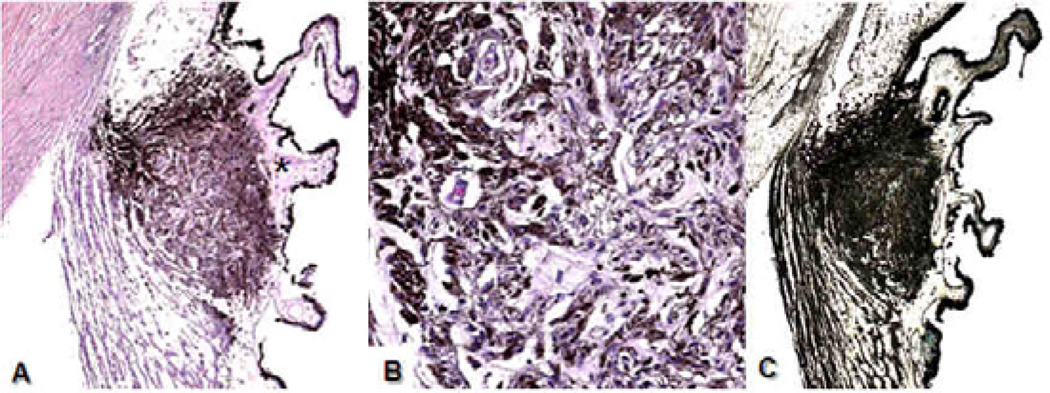
Fig. 4.
Microscopic image of the right ciliary body, Case 1, XP12BE. A) There is one cluster of pigmented granular cells in the temporal side of the ciliary body (arrow). Mild hyalinization (*) is also evident. B) The well-differentiated pigment epithelial cells are arranged in a linear glandular pattern. C) The hamartoma shows positive immunoreactivity against keratin (A and B: Hematoxylin & Eosin, C: avidin-biotin-complex immunochemistry with anti-keratin antibody; original magnifications, A and C: × 50, B × 200)
Fig. 5.
Microscopic images of the optic nerve A) Case 1, XP12BE. Mild-moderate optic atrophy with thickened septae (arrow), loss of neuronal elements, and vacuolization in the nerve bundles; B) Case 2 XP18BE. Normal optic nerve (A–B: Hematoxylin & Eosin, original magnifications × 100)
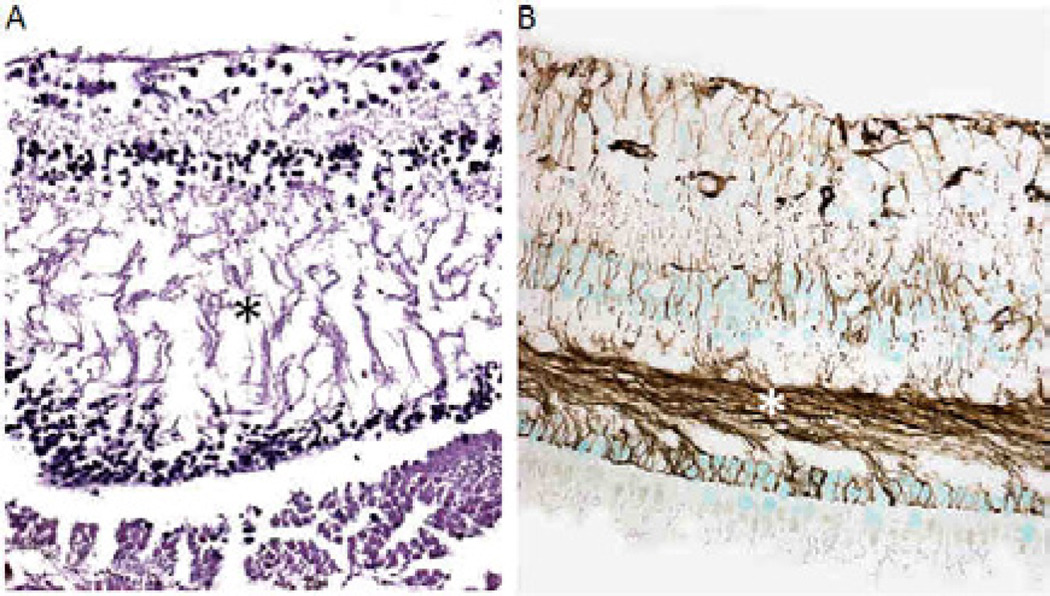
Fig. 6.
Microscopic images of the retina. A) Case 1, XP12BE. There is a loss of retinal neuronal cells and mild macular edema, visible by separation of Henle’s fibers (*) in the outerplexiform layer. B) Case 2, XP18BE. GFAP is strong positive in the retina, particularly the Henle’s fibers (*) (A: Hematoxylin & Eosin, B: avidin biotine peroxidase with GFAP as the primary antibody; original magnifications × 200)
CASE 2 (XP18BE)
Clinical History
Case 2 was a 45-year-old Caucasian woman with mutations in the XPD gene (Fig. 1C). She was diagnosed with developmental delay by age 7 and started special needs classes. The NIH Clinical Center began following her intermittently since age 12. By this time, she had photosensitivity with blistering, 3 skin tumors, and resection of several benign skin lesions. In contrast to Case 1, she had extensive early sun protection and did not have many skin cancers during her life; however, her neurologic deficits were significant. At age 31, neurologic examination revealed hearing loss, areflexia, and ataxia with a broad-based gait. She was wheelchair-bound by age 36. At age 40, she complained of decreased vision with diplopia. In April 2010, respiratory arrest in the context of multiple organ failure led to her death.
Ocular History
During her adolescence, the patient had a normal ophthalmic exam except for mild myopia for which she did not regularly wear glasses. At age 35, she developed exotropia, which progressed over the next 5 years. She complained of diplopia and severe photophobia, and on occasion would reach for things in the wrong places. At age 40, her vision was 20/200 OD and 20/60 OS. She a comitant exotropia at distance and near with full ductions and no gross pupillary abnormalities. Slit-lamp examination showed healthy anterior segments, lids, and conjunctiva. The corneas did not stain with fluorescein, and the tear films appeared normal. Dilated fundus exam was normal. Five years elapsed between her last eye examination and her death.
Pathological Findings
Macroscopic examination revealed an inferior opacity, scar, and neovascularization 1.0 mm from the margin of the cornea, measuring 10.5 mm × 10 mm in both eyes (Fig. 2B). In the peripheral fundus, patchy, clumped, pigmentary spicules were deposited in both eyes (Fig. 7). Otherwise, the anterior and posterior segments were grossly unremarkable. Microscopic examination of the cornea revealed thinning of the corneal epithelium, epithelial bullae and basement membrane abnormalities, absence of Bowman’s layer, and a superficial scar in both eyes inferiorly. Mild neovascularization and inflammatory cell infiltration was present in the superficial stroma of the peripheral cornea.
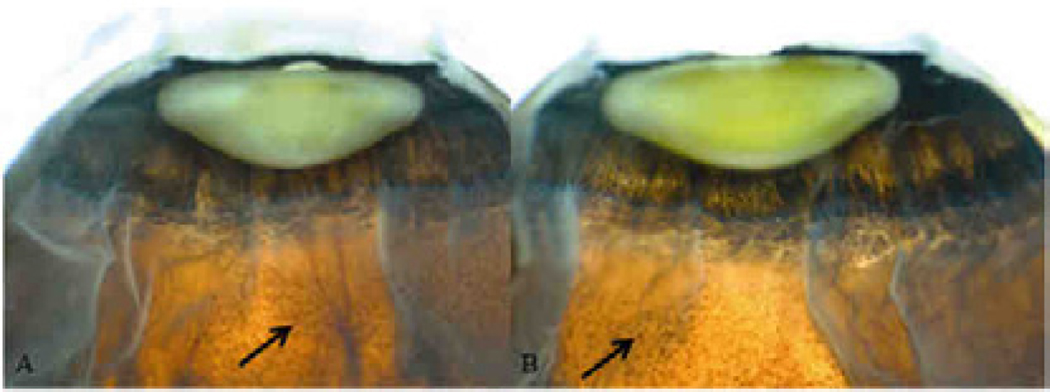
Fig. 7.
Macroscopic images of the retina, Case 2 XP18BE. Patchy pigmentary retinal degeneration (arrows) is visible in A) the right eye and B) the left eye
The iris had a mild lymphocytic infiltration. In the temporal mid-peripheral retina near the equator, several small retinal cysts spanned the ganglion cell layer to the inner nuclear layer, indicative of cystoid degeneration (Fig. 8A). Small focal chorioretinal adhesions with and without small drusen were present in both eyes. Mild focal lymphocytic infiltration was seen in choroid (Fig. 8B). RPE (retinal pigment epithelium) alterations with pigmentary degeneration and pigment clumps were noted in several areas in both peripheral retinas (Fig. 8B and 8C). The choroidal vessels were congested. The optic nerve had some corpora amylacea in both eyes, but no signs of optic atrophy (Fig. 5B). In the left eye, there was a small subconjunctival hemorrhage with mild conjunctival inflammatory cell infiltration and mild amorphous basophilic elastoid degeneration. Mild cataracts were also found in both eyes. In summary, ophthalmic pathology related to UV damage included conjunctival pinguecula, corneal scar, inflammation and neovascularization. Ophthalmic pathology related to neurodegeneration was peripheral retinal pigmentary degeneration. A comparison of the clinical and pathologic feature of Case 1 and 2 is presented in Table 1.
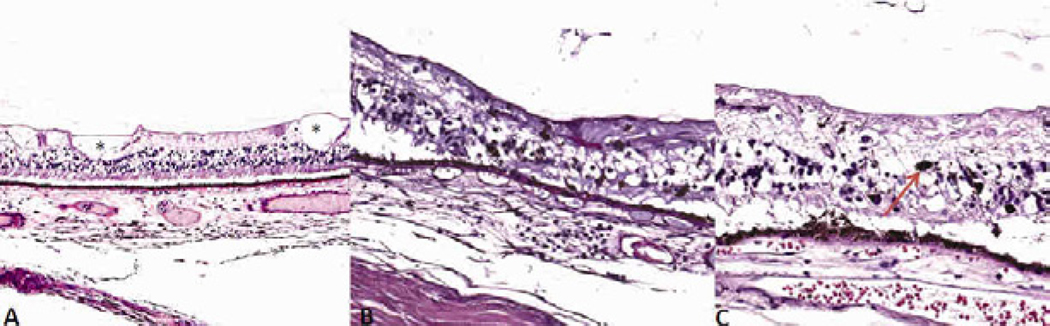
Fig. 8.
Microscopic images of the retina, Case 2 XP18BE. A) Typical peripheral cystoid degeneration of the retina (*). B–C) pigment migration to the inner retina (arrow) (Hematoxylin & Eosin, original magnifications, A × 100, B–C: × 200)
TABLE 1.
A Comparison of Case 1 and Case 2
| Case 1 XP12BE |
Case 2 XP18BE |
|
|---|---|---|
| Demographics | ||
| Age at death | 44 | 45 |
| Sex | Female | Female |
| Clinical information | ||
| Genetic mutation | XPA | XPD |
| Photosensitivity | ++ | ++ |
| Skin cancers | ++++ | + |
| Neurodegeneration | +++ | ++ |
| Sensorineural hearing loss | +++ | +++ |
| Clinical ocular symptoms | ||
| Photophobia | +++ | +++ |
| Conjunctivitis | +++ | − |
| Blepharitis | ++ | − |
| Blepharospasm | − | − |
| Dry eye | ++ | − |
| Diplopia | − | ++ |
| Eye exam findings | ||
| Ectropion | ++ | − |
| Lagophthalmos | ++ | − |
| Myopia | + | + |
| Exotropia | ++ | +++ |
| Exposure keratopathy and corneal pannus | +++ | +++ |
| Pupil abnormality | ++ | − |
| Optic nerve pallor | ++ | − |
| Pinguecula/pterygia | + | + |
| Conjunctival injection | ++ | − |
| Pathologic ocular findings | ||
| Conjunctiva | ||
| Conjunctivitis | ++ | + |
| Pinguecula | + | + |
| Cornea | ||
| Neovascularization | ++ | ++ |
| Epithelial irregularity | +++ | ++ |
| Band keratopathy | + | − |
| Epithelial bulbi | ++ | + |
| Bowman’s layer break | + | + |
| Keratitis | ++ | + |
| Ptyerygium | + | − |
| Lens | ||
| Cataract | − | + |
| Uvea | ||
| Ciliary body hamartoma | + | − |
| Iritis | − | + |
| Retina | ||
| Macular edema | + | − |
| Pigmentary retinal degeneration with pigment migration | − | ++ |
| Cystoid degeneration | + | + |
| Drusen | − | + |
| Gliosis | + | − |
| Focal chorioretinal adhesions | − | + |
| Optic nerve | ||
| Optic atrophy | ++ | − |
| Optic artery sclerosis | + | − |
− = not present; + = mild; ++ = moderate; +++ = severe.
Literature Review and Discussion
SYSTEMIC FEATURES AND GENETICS OF XP
XP is an autosomal recessive disease of defective DNA repair that affects males and females equally and is frequently symptomatic in childhood.15,24,49 Defects in nucleotide excision repair can lead to three diseases: XP, Cockayne syndrome, and trichothiodystrophy. XP and Cockayne syndrome both present with photosensitivity and progressive neurological degeneration.67 XP has a greatly increased risk of sun-induced cancers, and Cockayne patients have normal cancer risk. Retinitis pigmentosa retinopathy is well recognized in Cockayne syndrome, while retinal abnormalities are not common in XP patients.70 Cockayne syndrome patients may have poor vision with pupillary unresponsiveness, hypermetropia, nystagmus, hypoplastic irides, cataract, vitreous floaters, optic atrophy, and global progressive pigmentary retinal degeneration.20,70 While Cockayne syndrome also presents with photosensitivity and neurologic dysfunction, patients tend to have a “bird-like” face, microcephaly, premature aging, dwarfism, cachexia, sunken eyes, and usually die before age 30.10,54 Though the genetic defects are closely related, these diseases have a different systemic and ocular manifestations.20
Genetic defects in XP are heterogeneous, resulting from defects in 8 different genes (complementation groups).9,15,18,49 Seven of the complementation groups (XP complementation groups A through G) are deficient in nucleotide excision repair, and one group (XP variant) has defective post-replication repair.56 The prevalence of the complementation groups differs across the world, and complementation groups A, C, D, and variant are the most common in the United States and Europe.49 Patients with differing complementation groups have varied abilities to repair UV exposure- induced DNA damage, but overlapping clinical phenotypes.1 The two cases we presented have defects in different complementation groups, and case 1 XP12BE (group A) had many more cancers and a different ocular phenotype than case 2 XP18BE (group D).
Defects in nucleotide excision repair lead to premature sunlight-induced damage including hyperpigmentation, hypopigmentation, lentigos, telangectasias, actinic keratoses, and atrophy.48,49,52,53,75 Cutaneous symptoms usually present before 2 years of age, and the median age of first skin neoplasm is under 10 years.36,49 The frequency of non-melanoma skin cancer is increased 10,000-fold and melanoma is increased 2,000-fold in XP patients under age 20 years compared to the general US population.7,49,51,75
Progressive neurologic symptoms are present in about 25% of affected patients.49,55,76 XP complementation groups A, B, D, and G develop more central and peripheral nervous system abnormalities.32 Neurologic abnormalities include cognitive impairment, acquired microcephaly, abnormal motor activity, areflexia, sensorineural hearing loss, and abnormal speech.41,49,55,76 Studies suggest that neuronal degeneration in XP is a primary process, possibly caused by the inability to repair DNA that has been damaged by oxidative damage from endogenous metabolites.77
The majority of neoplasms in patients with XP occur in areas that are exposed to UV-radiation, including skin, anterior surfaces of the eye, and tip of the tongue.13,39,45,49,60 There is an increased frequency of central nervous system tumors.48,51 Internal neoplasms may be related to environmental carcinogen exposure that causes DNA damage, which, like UV-damage, is poorly repaired in XP patients.48 Overall, XP patients have a 70% probability of survival to 40 years of age, which is 28 years less than expected in the United States.49
CLINICAL OCULAR MANIFESTATIONS OF XP
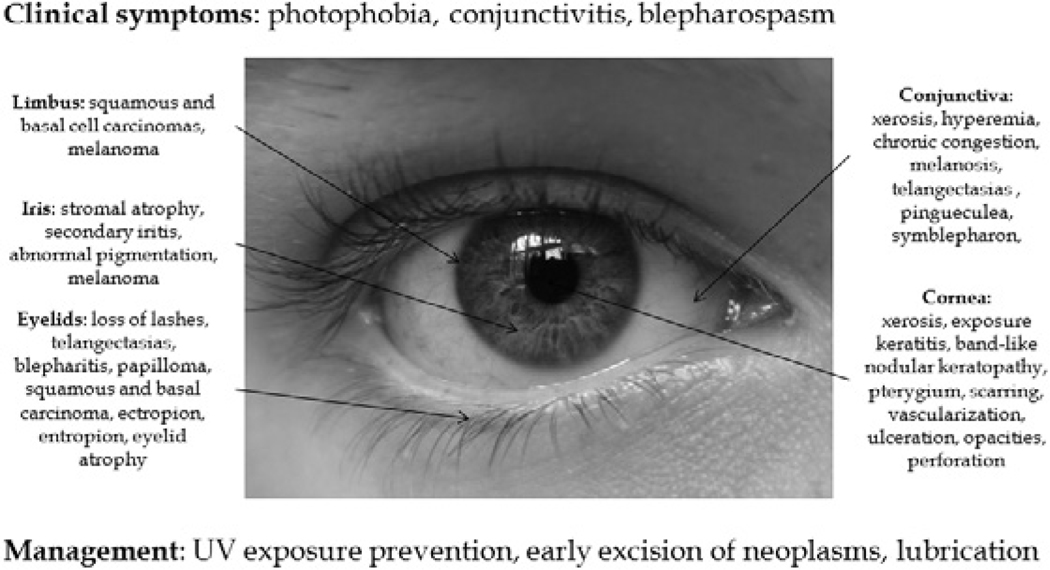
Ocular disease is evident in at least 40% of XP patients, and blepharospasm and photophobia are common symptoms.24,49,78 Eyelid skin changes reflect local skin changes, including usually erythema, pigmentation, atrophy, and malignant change.25,33,73 Telangectasias, loss of lashes, and chronic blepharitis are also seen.23,25,45 Atrophic scarred skin may cause ectropion of the lower eyelid and symblepharon.24,31,73 Lower lid loss may result in exposure keratitis, edema, and even corneal ulceration and perforation.6,88 Corneal opacification, neovascularization, pterygia, and band keratopathy are common, and band-shaped nodular dystrophy and squamous cell carcinomas have also been reported.24,26,28,29,30,34,41,43,44,49,92 Conjunctival involvement usually includes conjunctivitis, pinguecula, symblepharon, melanosis, and tumors developing from the interpalpebral zone of the limbus.3,14,21,23,25,37,39,43,45,62,69,79,86,93 Limbal tumors, especially pterygia, are common, and squamous cell carcinomas, malignant melanomas and limbal stem cell deficiency have been reported.24,27,31,44,64,86,87 The iris can be affected by iritis, stromal atrophy, pigment abnormalities, and, rarely, melanoma.40,41 Orbital tumors include basal cell carcinomas, squamous cell carcinomas, and melanomas.5,12,24,62,74,92 As the posterior segment is protected from UV damage by the cornea and lens, fundus abnormalities are not common; however, choroidal melanoma rarely develops.46,93,94 Unlike Cockayne syndrome, which is clinically associated with pigmentary retinal degeneration including retinitis pigmentosa, retinal abnormalities have not previously been reported in XP patients. Subclinical,histopathological retinal changes were found in these two patients (Table 1). Common clinical ocular manifestations of XP are summarized in Fig. 9.
Fig. 9.
A summary of clinical ocular findings in XP.
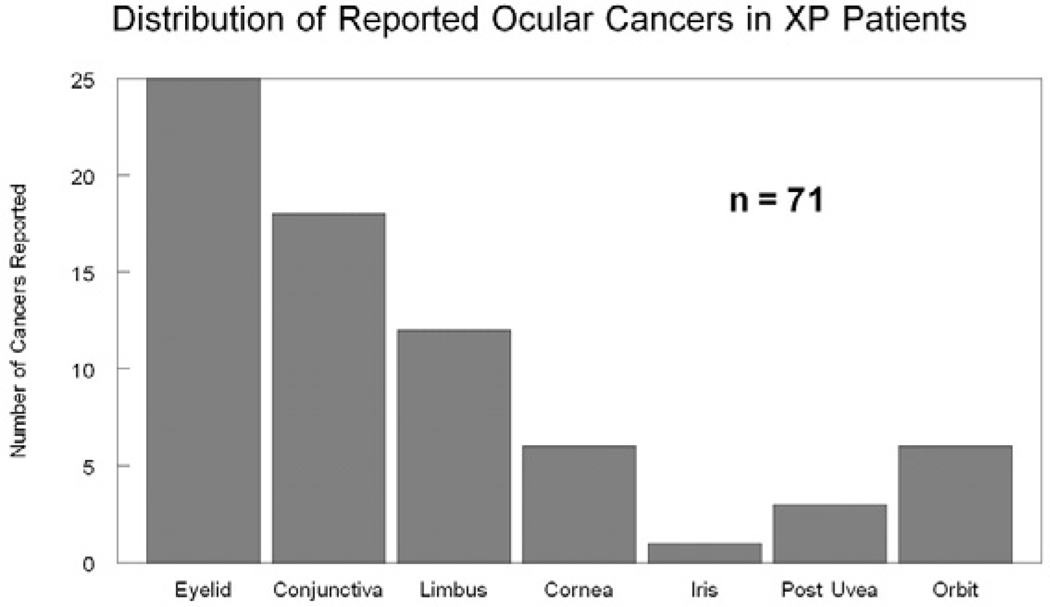
Clinical management of XP includes avoidance of sunlight, minimizing UV and cigarette smoke exposure, early excision of skin lesions, and genetic counseling.55 Oral 13-cis retinoic acid has been shown to reduce the incidence of new cancers in XP patients.50 Ophthalmic management includes UV-absorbing sunglasses with side shields, artificial tears, intermittent topical steroids, surveillance for ocular neoplasms, and management of complications.11 A summary of the reported ocular malignancies in XP patients and current management is presented (Table 2). Eyelid and conjunctival cancers are the most commonly reported (Fig. 10). Current management of eyelid tumors is complete resection using Mohs’ micrographic surgery, with or without reconstruction, or other tissue sparing techniques.16,90 Malignant conjunctival tumors that can be excised should be removed and treated with adjuvant cryotherapy/irradiation/topical chemotherapy.42,63,80,81,82,85 Some malignant limbal tumors can be removed by iridocyclectomy, while others may require enucleation.81 Corneal tumors have been managed with keratoplasty and topical chemotherapy.68 Iris tumors may be managed with local excision, plaque radiotherapy, or enucleation.83 Choroidal melanomas are commonly managed with plaque radiotherapy, but this has not been specifically studied in XP patients.84 If a tumor involves the orbit, imaging is required, and surgical excision with adjunctive radiation can be therapeutic.58 Despite their extreme sensitivity to UV light, XP patients can be treated with standard doses of radiation for treatment of neoplasms.19 Large or invasive ocular or orbital tumors may require enucleation and/or orbital exenteration.
TABLE 2.
A Summary of Reported Ocular Malignancies in Xeroderma Pigmentosum Cases
| Location | No. Cases | Mediana Age (years) |
Clinical Exam | Clinical Pathology | Current Management | Author (Year) |
|---|---|---|---|---|---|---|
| Eyelid | 16 | 8.3 | Thick red lid with ulceration, crusting, lid eversion; elevated mass; local invasion and spread involving nose and face | Squamous cell carcinoma | Complete surgical resection21 with modified Mohs micrographic surgery 12,16 and reconstructive therapy;102 photodynamic therapy if surgery not appropriate98 | El-Hefnawi and Mortada (1965)25 El-Hefnawi and Rasheed (1966)26 Hertle et al (1991)40 Calugaru et al (1992)11 Khatri et al (1992)48 Khatri et al (1999)49 Hadi et al (2000)35 Khatri et al (1999)49 |
| 2 | Rapidly growing solitary nodule with central crater | Keratoacanthoma | ||||
| 6 | Elevated mass with ulceration and clear margins | Basal cell carcinoma | El-Hefnawi and Mortada (1965)25 Khatri et al (1992)48 Khatri et al (1999)49 Gulati et al (1967)34 |
|||
| 1 | Flat pigmented lesion with irregular borders | Melanoma | ||||
| Conjunctiva | 8 | 4 | Squamous cell carcinoma | Early complete excision with adjuvant cryotherapy and/or irradiation, topical chemotherapy, and orbital exenteration for more invasive disease46,68,86,87,89,92 | Siegelman and Sutow (1965)93 Hertle et al (1991)40 Jacyk (1999)42 Chowdhury et al (2005)14 |
|
| 1 | Pale pink glistening swelling | Keratoacanthoma | El-Hefnawi and Mortada (1965)25 | |||
| 2 | Intraepithelial carcinoma | |||||
| 7 | 14.5 | Pigmented conjunctival lesion | Melanoma | Jensen (1962)43 El-Hefnawi and Mortada (1965)25 Vivian et al (1993)101 Aoyagi et al (1993)3 Mackiewicz et al (1994)64 Mehta et al (1996)67 |
||
| Limbus | 9 | 7 | Non-nodular, elevated, vascular, diffuse, pigmented tumor | Squamous cell carcinoma | Local resection (iridocyclectomy), enucleation or modified exenteration88 | Siegelman and Sutow (1965)93 Sivasubramaniam and Hoole (1952)94 Khatri et al (1992)48 Goyal et al (1994)32 Zafar et al (1997)104 Khatri et al (1999)49 Nalgirkar et al (2000)69 El-Hefnawi and Mortada (1965)25 Saebo et al (1978)83 Wolff-Rouendaal et al (1976)103 Giller and Kaufmann (1959)31 El-Hefnawi and Mortada (1965)25 Varghese et al (1997)100 Johnson et al (1989)44 |
| 2 | 15 | Pigmented inner limbal tumor | Melanoma | |||
| 1 | 20 | Leiomyosarcoma | ||||
| Cornea | 6 | 17.5 | Elevated pearly vascular proliferative mass | Squamous cell carcinoma | Local excision,88 topical chemotherapy,73 enucleation | |
| Iris | 1 | 31 | Large pigmented mass arising from the iris with anterior chamber blood | Melanoma | Local excision if circumscribed; enucleation if more than half of the iris and TM involved or secondary glaucoma; plaque radiotherapy in some cases90 | |
| Posterior Uvea | 3 | 24.5 | B-scan with apparent ciliary body mass; Incidental finding after enucleation for other tumor | Choroidal melanoma | Plaque radiotherapy Small: laser/ TTT; Large: local resection, enucleation, rare orbital exenteration91 | Wolff-Rouendaal et al (1976)103 Kitagawa et al (1981)50 Vivian et al (1993)101 |
| Orbit | 3 | 14 | Proptosis with infiltration of the eyeball | Squamous cell carcinoma | CT/MRI; surgical excision: if negative margins possible: Mohs62 or en face21; If orbital invasion: orbital exenteration with adjunct radiation97 | Calugaru et al (1992)11 Varghese et al (1997)100 |
| 2 | 11 | Large orbital mass; proptosis | Melanoma | El-Hefnawi and Mortada (1965)25 Rizvi et al (2008)79 Bellows et al (1974)5 |
||
| 1 | 6 | Large fungating vascular mass | Angiosarcoma |
CT = computed tomography; MRI = magnetic resonance imaging; TM = trabecular meshwork; TTT = transpupillary thermotherapy.
Median age and sex distribution were calculated from cases that reported demographic data with pathology, not all cases.
Fig. 10.
A summary of the reported ocular neoplasms in XP.
OPHTHALMIC PATHOLOGY OF XP
In case 1, the patient had chronic conjunctival injection with an irregular surface that grew onto the corneal pannus, bilateral early nasal pterygia, and conjunctival melanosis. In case 2, there was a small subconjunctival hemorrhage, mild inflammation, and pingueculae. Conjunctival pathology in XP patients includes telangiectasia, xerosis, chronic congestion, pigmentation, and pingueculea.78 Keratoacanthoma of the conjunctiva14 and malignant melanoma of the conjunctiva have also been reported.3,21,62 In XP patients, conjunctival cells have also been shown to have defective repair of DNA damage.65
In both cases, corneal pathology included exposure keratopathy, bullous keratopathy, pannus formation, keratitis, neovascularization and scar. Corneal pathology, occurring in 17–40% of XP cases, includes dryness, exposure keratitis, hazyness, band-like nodular keratopathy, scarring, ulceration, occasional perforation, and corneal opacity with vascularization.78,91 Squamous cell carcinoma of the cornea, though rare, has been reported.24,30,92 Corneal neovascularization is often present, and keratoplasty has variable success in XP.6,29 In an animal model of XP, corneal neovascularization has been shown to be caused by DNA damage from chronic UV radiation-induced pyrimidine dimer formation.4
In case 1, basal cell carcinomas of bilateral eyelids, canthi, and eyebrows were resected, leading to ectropion and lagophthalmos. Only three skin tumors not involving the lids were observed in case 2. Eyelid pathology, reported in 16% of XP cases, is similar that of the skin.49,78 Atrophic, scarred and retracted eyelids, coupled with a DNA damage repair defect, predispose XP patients to UV-damage and associated ocular surface tumors, both benign (pinguecula and pterygia) and malignant (squamous cell carcinomas, basal cell carcinomas, and melanomas).
In the right ciliary body of case 1, a glandular pattern of ciliary pigmented epithelial cells (keratin+, melanin+, S100−) clustered and formed a hamartoma (Fig. 4). Ciliary body hamartomas are reported in patients with tuberous sclerosis complex, and choroidal melanomas are reported in XP.22,93,94 This benign ciliary pigment epithelial hamartoma, which may be incidental, has not been previously reported in an XP patient. In contrast, ocular melanomas, present in 1% of XP patients, and cutaneous malignant melanomas, present in 36% of 106 XP patients seen at the NIH,7 are more widely reported.49,89
As in other XP patients with defects in XPA and XPD, our two patients had severe, progressive neurologic disease.66,76 Case 1 had global cerebral atrophy, minimally to non-reactive pupils, and optic disk pallor. Optic atrophy with loss of neuronal elements, vacuolization in the nerve bundles, and thickening of septae were visible histologically, as was mild ophthalmic artery sclerosis. These findings were not seen in case 2, who also had significant neurologic disease. Optic atrophy was previously described neuropathologically in a XP/Cockayne syndrome patient with severe neurologic degeneration.59 While clinical findings of systemic neurologic impairment have been widely documented, optic atrophy has not been reported pathologically in XP patients.20,49,71,76 In case 1, the optic atrophy may be related to the relatively advanced age (44) of the patient with a neurodegenerative disease, primary neurodegeneration, or secondary descending neurodegeneration. For unclear reasons, the patient with the XPA mutation had optic atrophy, while the patient with the XPD mutation did not. The neuropathophysiology of XP is still being elucidated, and increased oxidative stress is one possible mechanism for progressive neurodegeneration.35,61,72
The retinal findings in our cases are new associations for XP. Mild macular edema, seen in case 1 with prominent gliosis and loss of retinal neuronal cells, was previously reported as a secondary finding in an XP patient with a iris melanoma.40 Case 1 had no other known causes for macular edema,2 and it is also possible that the mild macular edema was post-mortem artifact. Case 2 had XP with peripheral pigmentary retinal degeneration, focal chorioretinal adhesions, cystoid degeneration of the retina near the equator, and retinal gliosis. The significance of the chorioretinal adhesions is unclear, and cystoid degeneration of the retina is an innocuous common finding in autopsy eyes.67 The pigment deposition seen in inner retinal layers of the peripheral retina, however, was in a pattern similar to that found in Cockayne syndrome.57 Investigation of the neurodegenerative process in this disease is warranted and hopefully will lead to the development of neuroprotective strategies for XP patients. Both UV protection and neuroprotection may become important in management.
Methods of Literature Search
The literature selection for this review included a Medline database search from 1965 to June 2010 of publications in the English language. Searches were conducted with the headings xeroderma pigmentosum, ocular pathology, ocular tumor, melanoma, carcinoma, eyelid, ciliary body, cornea, iris, conjunctiva, and treatment. Articles populated under ‘Related citations’ from these articles were reviewed. Additional articles and textbooks were selected from the bibliographies of the references.
Acknowledgments
We acknowledge our patients and their families for their contributions to our research. Drs. Jinping Lai and Yen-Chun Liu of the Laboratory of Pathology, NCI performed the autopsies of the XP patients under supervision of Drs. David Kleiner and Carl Oberholtzer. We thank the NIH library staff for making this type of comprehensive literature search possible. This research was supported by the Howard Hughes Medical Institute (HLR) and the Intramural Research Programs of the National Eye Institute, and the Center for Cancer Research of the National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Andrews AD, Barrett SF, Robbins JH. Xeroderma pigmentosum neurological abnormalities correlate with colony-forming ability after ultraviolet radiation. Proc Natl Acad Sci U S A. 1978;75:1984–1988. doi: 10.1073/pnas.75.4.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anttinen A, Koulu L, Nikoskelainen E, et al. Neurological symptoms and natural course of xeroderma pigmentosum. Brain. 2008;131:1979–1989. doi: 10.1093/brain/awn126. [DOI] [PubMed] [Google Scholar]
- 3.Aoyagi M, Morishima N, Yoshino Y, et al. Conjunctival malignant melanoma with xeroderma pigmentosum. Ophthalmologica. 1993;206:162–167. doi: 10.1159/000310384. [DOI] [PubMed] [Google Scholar]
- 4.Applegate LA, Ley RD. DNA damage is involved in the induction of opacification and neovascularization of the cornea by ultraviolet radiation. Exp Eye Res. 1991;52:493–497. doi: 10.1016/0014-4835(91)90047-i. [DOI] [PubMed] [Google Scholar]
- 5.Bellows RA, Lahav M, Lepreau FJ, Albert DM. Ocular manifestations of xeroderma pigmentosum in a black family. Arch Ophthalmol. 1974;92:113–117. doi: 10.1001/archopht.1974.01010010119007. [DOI] [PubMed] [Google Scholar]
- 6.Blanksma LJ, Donders PC, van Voorst Vader PC. Xeroderma pigmentosum and keratoconus. Doc Ophthalmol. 1986;64:97–103. doi: 10.1007/BF00166690. [DOI] [PubMed] [Google Scholar]
- 7.Bradford PT, Goldstein AM, Tamura D, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2010 doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Broughton BC, Cordonnier A, Kleijer WJ, et al. Molecular analysis of mutations in DNA polymerase eta in xeroderma pigmentosum-variant patients. Proc Natl Acad Sci U S A. 2002;99:815–820. doi: 10.1073/pnas.022473899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brumback RA, Yoder FW, Andrews AD, et al. Normal pressure hydrocephalus. Recognition and relationship to neurological abnormalities in Cockayne's syndrome. Arch Neurol. 1978;35:337–345. doi: 10.1001/archneur.1978.00500300011002. [DOI] [PubMed] [Google Scholar]
- 11.Calonge M, Foster CS, Rice BA, Baer JC. Management of corneal complications in xeroderma pigmentosum. Cornea. 1992;11:173–182. doi: 10.1097/00003226-199203000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Calugaru M, Barsu M. Exuberant epibulbar tumor penetrating into the orbit in xeroderma pigmentosum. Graefes Arch Clin Exp Ophthalmol. 1992;230:352–357. doi: 10.1007/BF00165944. [DOI] [PubMed] [Google Scholar]
- 13.Chidzonga MM, Mahomva L, Makunike-Mutasa R, Masanganise R. Xeroderma pigmentosum: a retrospective case series in Zimbabwe. J Oral Maxillofac Surg. 2009;67:22–31. doi: 10.1016/j.joms.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury RK, Padhi T, Das GS. Keratoacanthoma of the conjunctiva complicating xeroderma pigmentosum. Indian J Dermatol Venereol Leprol. 2005;71:430–431. doi: 10.4103/0378-6323.18954. [DOI] [PubMed] [Google Scholar]
- 15.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 16.Cook BE, Jr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108:2088–2098. doi: 10.1016/s0161-6420(01)00796-5. quiz 2099-100, 121. [DOI] [PubMed] [Google Scholar]
- 17.Day RS. Xeroderma pigmentosum variants have decreased repair of ultraviolet-damaged DNA. Nature. 1975;253:748–749. doi: 10.1038/253748a0. [DOI] [PubMed] [Google Scholar]
- 18.De Weerd-Kastelein EA, Keijzer W, Bootsma D. Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat New Biol. 1972;238:80–83. doi: 10.1038/newbio238080a0. [DOI] [PubMed] [Google Scholar]
- 19.DiGiovanna JJ, Patronas N, Katz D, et al. Xeroderma pigmentosum: spinal cord astrocytoma with 9-year survival after radiation and isotretinoin therapy. J Cutan Med Surg. 1998;2:153–158. doi: 10.1177/120347549800200308. [DOI] [PubMed] [Google Scholar]
- 20.Dollfus H, Porto F, Caussade P, et al. Ocular manifestations in the inherited DNA repair disorders. Surv Ophthalmol. 2003;48:107–122. doi: 10.1016/s0039-6257(02)00400-9. [DOI] [PubMed] [Google Scholar]
- 21.Duke-Elder S. St. Louis: C V Mosby; 1974. [Google Scholar]
- 22.Eagle RC, Jr, Shields JA, Shields CL, Wood MG. Hamartomas of the iris and ciliary epithelium in tuberous sclerosis complex. Arch Ophthalmol. 2000;118:711–715. doi: 10.1001/archopht.118.5.711. [DOI] [PubMed] [Google Scholar]
- 23.El-Hefnawi H, El-Nabawi M, Rasheed A. Xeroderma pigmentosum. I. A clinical study of 12 Egyptian cases. Br J Dermatol. 1962;74:201–213. doi: 10.1111/j.1365-2133.1962.tb13492.x. [DOI] [PubMed] [Google Scholar]
- 24.El-Hefnawi H, Mortada A. OCULAR MANIFESTATIONS OF XERODERMA PIGMENTOSUM. Br J Dermatol. 1965;77:261–276. doi: 10.1111/j.1365-2133.1965.tb14641.x. [DOI] [PubMed] [Google Scholar]
- 25.el Hefnawi H, Rasheed A. Xeroderma pigmentosum. A report of further six cases. J Egypt Med Assoc. 1966;49:419–434. [PubMed] [Google Scholar]
- 26.Etzine S, Kaufmann JC. Band-Shaped Nodular Dystrophy of the Cornea. Am J Ophthalmol. 1964;57:760–763. doi: 10.1016/0002-9394(64)92222-6. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes M, Sangwan VS, Vemuganti GK. Limbal stem cell deficiency and xeroderma pigmentosum: a case report. Eye (Lond) 2004;18:741–743. doi: 10.1038/sj.eye.6700717. [DOI] [PubMed] [Google Scholar]
- 28.Freedman J. Xeroderma pigmentosum and band-shaped nodular corneal dystrophy. Br J Ophthalmol. 1977;61:96–100. doi: 10.1136/bjo.61.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman J. Corneal transplantation with associated histopathologic description in xeroderma pigmentosum occurring in a black family. Ann Ophthalmol. 1979;11:445–448. [PubMed] [Google Scholar]
- 30.Giller H, Kaufmann WC. Ocular lesions in xeroderma pigmentosum; a case report of carcinoma of the cornea. AMA Arch Ophthalmol. 1959;62:130–133. doi: 10.1001/archopht.1959.04220010134016. [DOI] [PubMed] [Google Scholar]
- 31.Goyal JL, Rao VA, Srinivasan R, Agrawal K. Oculocutaneous manifestations in xeroderma pigmentosa. Br J Ophthalmol. 1994;78:295–297. doi: 10.1136/bjo.78.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grewal RP. Neurons and DNA repair: neurologic involvement in xeroderma pigmentosa. Med Hypotheses. 1991;34:171–173. doi: 10.1016/0306-9877(91)90188-5. [DOI] [PubMed] [Google Scholar]
- 33.Hadi U, Tohmeh H, Maalouf R. Squamous cell carcinoma of the lower lid in a 19-month-old girl with xeroderma pigmentosum. Eur Arch Otorhinolaryngol. 200;257:77–79. doi: 10.1007/pl00007514. [DOI] [PubMed] [Google Scholar]
- 34.Harris LC, Keet MP. Xeroderma pigmentosum. A report on 6 cases in South African Bantu children. J Pediatr. 1960;57:759–768. doi: 10.1016/s0022-3476(60)80171-0. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi M. Roles of oxidative stress in xeroderma pigmentosum. Adv Exp Med Biol. 2008;637:120–127. doi: 10.1007/978-0-387-09599-8_13. [DOI] [PubMed] [Google Scholar]
- 36.Hebra F, Kaposi M. On diseases of the skin including exanthemata. New Synddenham Soc. 1874;61:252–258. [Google Scholar]
- 37.Hertle RW, Durso F, Metzler JP, Varsa EW. Epibulbar squamous cell carcinomas in brothers with Xeroderma pigmentosa. J Pediatr Ophthalmol Strabismus. 1991;28:350–353. doi: 10.3928/0191-3913-19911101-15. [DOI] [PubMed] [Google Scholar]
- 38.Hirai Y, Kodama Y, Moriwaki S, et al. Heterozygous individuals bearing a founder mutation in the XPA DNA repair gene comprise nearly 1% of the Japanese population. Mutat Res. 2006;601:171–178. doi: 10.1016/j.mrfmmm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Jacyk WK. Xeroderma pigmentosum in black South Africans. Int J Dermatol. 1999;38:511–514. doi: 10.1046/j.1365-4362.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- 40.Johnson MW, Skuta GL, Kincaid MC, et al. Malignant melanoma of the iris in xeroderma pigmentosum. Arch Ophthalmol. 1989;107:402–407. doi: 10.1001/archopht.1989.01070010412036. [DOI] [PubMed] [Google Scholar]
- 41.Jung EG. Xeroderma pigmentosum. Int J Dermatol. 1986;25:629–633. doi: 10.1111/j.1365-4362.1986.tb04522.x. [DOI] [PubMed] [Google Scholar]
- 42.Kearsley JH, Fitchew RS, Taylor RG. Adjunctive radiotherapy with strontium-90 in the treatment of conjunctival squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 1988;14:435–443. doi: 10.1016/0360-3016(88)90257-x. [DOI] [PubMed] [Google Scholar]
- 43.Khanna VN, Garg KC, Bisaria KK. Ocular manifestations of Xeroderma pigmentosum. J Indian Med Assoc. 1965;45:550–553. [PubMed] [Google Scholar]
- 44.Khatri ML, Shafi M, Mashina A. Xeroderma pigmentosum. A clinical study of 24 Libyan cases. J Am Acad Dermatol. 1992;26:75–78. doi: 10.1016/0190-9622(92)70010-d. [DOI] [PubMed] [Google Scholar]
- 45.Khatri ML, Bemghazil M, Shafi M, Machina A. Xeroderma pigmentosum in Libya. Int J Dermatol. 1999;38:520–524. doi: 10.1046/j.1365-4362.1999.00751.x. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa KOT, Inoue M. Choroidal malignant melanoma occurring in a patient with xeroderma pigmentosum. Folia Ophthalmol Jpn. 1981;32:657–663. [Google Scholar]
- 47.Kleijer WJ, Laugel V, Berneburg M, et al. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744–750. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 49.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 50.Kraemer KH, DiGiovanna JJ, Moshell AN, et al. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N Engl J Med. 1988;318:1633–1637. doi: 10.1056/NEJM198806233182501. [DOI] [PubMed] [Google Scholar]
- 51.Kraemer KH, DiGiovanna JJ, Peck GL. Chemoprevention of skin cancer in xeroderma pigmentosum. J Dermatol. 1992;19:715–718. doi: 10.1111/j.1346-8138.1992.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 52.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 53.Kraemer KH. Sunlight and skin cancer: another link revealed. Proc Natl Acad Sci U S A. 1997;94:11–14. doi: 10.1073/pnas.94.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraemer KH, Patronas NJ, Schiffmann R, et al. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–1396. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraemer KH. In: Xeroderma Pigmentosum. Pagon RA, Bird TC, Dolan CR, Stephens K, editors. Seattle, WA: University of Washington, Seattle; 2008. GeneReviews[Internet] [PubMed] [Google Scholar]
- 56.Lambert WC, Lambert MW. DNA and chromosomal instability. In: Alper JC, editor. Genetic disorders of the skin. St Louis: Mosby; 1991. pp. 320–358. [Google Scholar]
- 57.Levin PS, Green WR, Victor DI, MacLean AL. Histopathology of the eye in Cockayne's syndrome. Arch Ophthalmol. 1983;101:1093–1097. doi: 10.1001/archopht.1983.01040020095016. [DOI] [PubMed] [Google Scholar]
- 58.Limawararut V, Leibovitch I, Sullivan T, Selva D. Periocular squamous cell carcinoma. Clin Experiment Ophthalmol. 2007;35:174–185. doi: 10.1111/j.1442-9071.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 59.Lindenbaum Y, Dickson D, Rosenbaum P, et al. Xeroderma pigmentosum/cockayne syndrome complex: first neuropathological study and review of eight other cases. Eur J Paediatr Neurol. 2001;5:225–242. doi: 10.1053/ejpn.2001.0523. [DOI] [PubMed] [Google Scholar]
- 60.Mahindra P, DiGiovanna JJ, Tamura D, et al. Skin cancers, blindness, and anterior tongue mass in African brothers. J Am Acad Dermatol. 2008;59:881–886. doi: 10.1016/j.jaad.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKinnon PJ. DNA repair deficiency and neurological disease. Nat Rev Neurosci. 2009;10:100–112. doi: 10.1038/nrn2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehta C, Gupta CN, Krishnaswamy M. Malignant melanoma of conjunctiva with xeroderma pigmentosa--a case report. Indian J Ophthalmol. 1996;44:165–166. [PubMed] [Google Scholar]
- 63.Mehta M, Fay A. Squamous cell carcinoma of the eyelid and conjunctiva. Int Ophthalmol Clin. 2009;49:111–121. doi: 10.1097/IIO.0b013e3181928fb9. [DOI] [PubMed] [Google Scholar]
- 64.Nalgirkar AR, Borkar SS, Nalgirkar SA. Xeroderma pigmentosum with multiple malignancies. Indian Pediatr. 2000;37:1377–1379. [PubMed] [Google Scholar]
- 65.Newsome DA, Kraemer KH, Robbins JH. Repair of DNA in xeroderma pigmentosum conjunctiva. Arch Ophthalmol. 1975;93:660–662. doi: 10.1001/archopht.1975.01010020626012. [DOI] [PubMed] [Google Scholar]
- 66.Nishigori C, Moriwaki S, Takebe H, et al. Gene alterations and clinical characteristics of xeroderma pigmentosum group A patients in Japan. Arch Dermatol. 1994;130:191–197. [PubMed] [Google Scholar]
- 67.O'Malley PF, Allen RA. Peripheral cystoid degeneration of the retina. Incidence and distribution in 1,000 autopsy eyes. Arch Ophthalmol. 1967;77:769–776. doi: 10.1001/archopht.1967.00980020771010. [DOI] [PubMed] [Google Scholar]
- 68.Panda A, Bajaj MS, Balasubramanya R, Prakash G. Topical mitomycin C for conjunctival-corneal squamous cell carcinoma. Am J Ophthalmol. 2003;135:122–123. doi: 10.1016/s0002-9394(02)01861-5. author reply 123-4. [DOI] [PubMed] [Google Scholar]
- 69.Paridaens AD, McCartney AC, Hungerford JL. Premalignant melanosis of the conjunctiva and the cornea in xeroderma pigmentosum. Br J Ophthalmol. 1992;76:120–122. doi: 10.1136/bjo.76.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pearce WG. Ocular and genetic features of Cockayne's syndrome. Can J Ophthalmol. 1972;7:435–444. [PubMed] [Google Scholar]
- 71.Rapin I, Lindenbaum Y, Dickson DW, et al. Cockayne syndrome and xeroderma pigmentosum. Neurology. 2000;55:1442–1449. doi: 10.1212/wnl.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reardon JT, Bessho T, Kung HC, et al. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc Natl Acad Sci U S A. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reese AB, Wilber IE. The eye manifestations of xeroderma pigmentosum. Am J Ophthalmol. 1943;26:901–911. [Google Scholar]
- 74.Rizvi SA, Amitava AK, Mehdi G, et al. Orbital amelanotic melanoma in xeroderma pigmentosum: a rare association. Indian J Ophthalmol. 2008;56:421–423. doi: 10.4103/0301-4738.42423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robbins JH, Kraemer KH, Lutzner MA, et al. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974;80:221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- 76.Robbins JH, Brumback RA, Mendiones M, et al. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991;114(Pt 3):1335–1361. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]
- 77.Robbins JH, Kraemer KH, Merchant SN, Brumback RA. Adult-onset xeroderma pigmentosum neurological disease--observations in an autopsy case. Clin Neuropathol. 2002;21:18–23. [PubMed] [Google Scholar]
- 78.Sevel D. Xeroderma Pigmentosum with Ocular Complications. Br J Ophthalmol. 1963;47 doi: 10.1136/bjo.47.11.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao L, Newell B, Quintanilla N. Atypical fibroxanthoma and squamous cell carcinoma of the conjunctiva in xeroderma pigmentosum. Pediatr Dev Pathol. 2007;10:149–152. doi: 10.2350/06-06-0103.1. [DOI] [PubMed] [Google Scholar]
- 80.Shields CL, Shields JA, Armstrong T. Management of conjunctival and corneal melanoma with surgical excision, amniotic membrane allograft, and topical chemotherapy. Am J Ophthalmol. 2001;132:576–578. doi: 10.1016/s0002-9394(01)01085-6. [DOI] [PubMed] [Google Scholar]
- 81.Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol. 2004;49:3–24. doi: 10.1016/j.survophthal.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 82.Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan Lecture. Arch Ophthalmol. 1997;115:808–815. doi: 10.1001/archopht.1997.01100150810025. [DOI] [PubMed] [Google Scholar]
- 83.Shields JAaSCL. Melanocytic tumors of the iris stroma: Intraocular tumors: atlas and textbook. 2nd. Chapter 2. Lippincott Williams & Wilkins; 2008. p. 22. [Google Scholar]
- 84.Shields JAaSCL. Posterior Uveal Melanoma: Management: Intraocular tumors: atlas and textbook. 2nd. Chapter 10. Lippincott Williams & Wilkins; 2008. pp. 140–141. [Google Scholar]
- 85.Shildkrot Y, Wilson MW. Conjunctival melanoma: pitfalls and dilemmas in management. Curr Opin Ophthalmol. 2010 doi: 10.1097/ICU.0b013e32833b7aab. [DOI] [PubMed] [Google Scholar]
- 86.Siegelman MH, Sutow WW. Xeroderma pigmentosum. Report of 3 cases. J Pediatr. 1965;67:625–630. doi: 10.1016/s0022-3476(65)80434-6. [DOI] [PubMed] [Google Scholar]
- 87.Sivasubramaniam P, Hoole T. Xeroderma pigmentosum; a report of seven cases with ocular complications. Ceylon Med J. 1952;1:75–79. [PubMed] [Google Scholar]
- 88.Stenson S. Ocular findings in xeroderma pigmentosum: report of two cases. Ann Ophthalmol. 1982;14:580–585. [PubMed] [Google Scholar]
- 89.Stern JB. Nomenclature for flat lesions of melanoma: a response. Am J Dermatopathol. 1992;14:487–489. doi: 10.1097/00000372-199210000-00017. [DOI] [PubMed] [Google Scholar]
- 90.Togsverd-Bo K, Haedersdal M, Wulf HC. Photodynamic therapy for tumors on the eyelid margins. Arch Dermatol. 2009;145:944–947. doi: 10.1001/archdermatol.2009.157. [DOI] [PubMed] [Google Scholar]
- 91.Touzri RA, Mohamed Z, Khalil E, et al. Ocular malignancies of xeroderma pigmentosum: clinical and therapeutic features. Ann Dermatol Venereol. 2008;135:99–104. doi: 10.1016/j.annder.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Varghese R, Raghuveer CV. Oculocutaneous malignancies in xeroderma pigmentosum. Indian J Cancer. 1997;34:12–15. [PubMed] [Google Scholar]
- 93.Vivian AJ, Ellison DW, McGill JI. Ocular melanomas in xeroderma pigmentosum. Br J Ophthalmol. 1993;77:597–598. doi: 10.1136/bjo.77.9.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolff-Rouendaal D. Xeroderma pigmentosum with ophthalmological symptoms. Ophthalmologica. 1976;173:290–291. doi: 10.1159/000307912. [DOI] [PubMed] [Google Scholar]