Introduction: Workshop Goals & Objectives
The microbiota of the human vagina can affect the health of women, their fetuses, and newborns. A vaginal environment that is quantitatively dominated by hydrogen peroxide-producing Lactobacillus species has consistently been associated with healthy pregnancy outcomes, lack of abnormal vaginal symptoms, and reduced risk for several sexually transmitted pathogens, including HIV. Bacterial vaginosis (BV) represents a condition in which the normal protective lactobacilli are replaced by high quantities of commensal anaerobes, resulting in symptomatic vaginitis in many women. BV increases the risk of upper genital tract infection and adverse outcomes of pregnancy. While traditional cultivation has identified numerous BV-associated bacteria involved in these processes, recent advances in molecular biology have facilitated the detection and identification of bacteria without cultivation, some of which have not previously been described or well characterized. For instance, several novel bacteria in the Clostridiales order are highly specific indicators of bacterial vaginosis (BV), and bacteria related to Megasphaera, Leptotrichia, Atopobium, and Dialister species are commonly found in subjects with BV. These advances have notable implications for research related to the pathogenesis, natural history, diagnosis and adverse consequences of BV. A more complete understanding of vaginal microbial populations resulting from the adoption of molecular tools may lead to better strategies to maintain healthy vaginal microbial communities—thus enhancing women’s health—and will create opportunities to explore the role of novel bacteria in reproductive tract diseases.
On November 19-20, 2008, the NIH convened a workshop of experts in the field of research and clinical practice related to BV in order to discuss how these new advances should be interpreted and applied to research in progress and collaborations between relevant disciplines. The group discussed numerous questions. What do we know about pathogenesis, and what are the gaps in our knowledge (Table 1)? What are the consequences of BV, and what are the gaps in our understanding regarding how these complications arise? What interventions have and have not worked to prevent these consequences, and how do we leverage this knowledge to move forward with appropriate prevention strategies? How might advances in molecular biology contribute to an enhanced approach to diagnosing BV? Experts in the field summarized the current status of BV-related research in three main areas: epidemiology and adverse consequences of BV; diagnosis and treatment; and molecular advances in defining BV-related microbiology. Workgroups focused on these three main areas then met to deliberate about key challenges and questions. This paper summarizes the presentations of this workshop and outlines general recommendations arising from the related discussions.
Table 1.
Unanswered research questions in the field of BV
|
Background
Bacterial vaginosis is common, and consistently associated with adverse symptoms and reproductive tract outcomes
BV is the most prevalent form of vaginal infection in women of reproductive age, affecting 8% to 23%, and is the most common etiology of vaginal symptoms prompting women to seek medical care. 1 Symptomatic BV, which accounts for approximately 60% of all cases, typically causes abnormal vaginal discharge that is increased in amount and often malodorous. The discharge results in part from degradation of the normal vaginal mucin gel, which is efficiently performed by mucin-degrading enzymes produced by BV-associated bacteria (particularly Gram-negative anaerobes). 2 The odor, usually described as “fishy,” is derived from volatilization of the amines produced by the metabolism of anaerobic bacteria that characterize this disorder.
The etiology of BV is not known. At the microbiologic level, BV is a synergistic polymicrobial syndrome characterized by depletion of Lactobacillus species, especially those that produce H2O2, accompanied by intense overgrowth of commensal vaginal anaerobic bacteria (100 to 1000-fold above normal).3 Conventional cultivation of flora from vaginal fluid of women with BV typically yields a spectrum of primarily anaerobic commensals: Gardnerella vaginalis, Prevotella species, anaerobic gram-positive cocci, Mobiluncus species, Ureaplasma urealyticum, and Mycoplasma hominis. Critically, the initial event leading to this shift is unknown. BV occurs more frequently among women who report new or higher numbers of male sex partners, is common and highly concordant among female sex partners, and rarely occurs before sexual debut, patterns that invoke the epidemiology of a typical sexually transmitted infection (STI). 4-6,7, 8 Limited data also suggest that male condoms and circumcision may prevent BV and its recurrence. 9-11,12 Other risks for BV acquisition include douching for hygiene, 6 which likely acts to promote loss of hydrogen peroxide-producing lactobacilli, use of an intrauterine device for contraception, 11, 13 and black race. 14
BV is associated with serious sequelae related to the upper genital tract, increasing the risk of preterm delivery, 15-18 first trimester miscarriage in women undergoing in vitro fertilization, 19 amniotic fluid infections, 20 chorioamnionitis, 21 postpartum and postabortal endometritis, 22 and postabortal PID. 23 In non-pregnant women, 24 BV increases risk of post-hysterectomy infections 25, 26 and PID 17, 27, and risk of acquiring N. gonorrhoeae 4, 28, 29. BV itself may cause endocervical inflammation that manifests as mucopurulent cervicitis. 30, 31 Among HIV-infected women, the quantity of HIV shed in vaginal secretions from those with BV was increased nearly 6-fold increase relative to those without BV. 32,33 BV probably enhances women’s likelihood of sexual acquisition of HIV 28, 34-36, possibly through inducing reversible changes in the cervical or other mucosal immune environment.37 The exact means by which BV effects adverse reproductive tract sequelae is not clear. Possible explanations include loss of antimicrobial compounds produced by lactobacilli (lactic acid, H2O2); destruction of mucin gel coating the vaginal/cervical epithelium via inhibition of glycosidase-producing anaerobes; degradation of secretory leukocyte protease inhibitor (SLPI); induction of a cervical pro-inflammatory environment; and alteration in the immune cell environment of the cervix.
Available approaches to diagnosing BV are underutilized, and may not reflect the complexity of BV-associated microbiota as assessed by molecular methodology
In clinical practice, BV is typically diagnosed using the Amsel criteria, which include the presence of at least three of four findings: vaginal pH greater than 4.5, homogeneous vaginal discharge on examination, detection of fishy odor on addition of potassium hydroxide to vaginal fluid (positive “whiff test”), and presence of significant clue cells 38 (defined as >20% of the total vaginal epithelial cells seen on 100x magnification on saline microscopy). Other point-of-care diagnostic tests take advantage of immediate methods of detecting either high concentrations of G. vaginalis, a variety of the amines that are prominent, including sialidase, trimethylamine, and prolineaminopeptidase, or some combination of amines and abnormal pH. 39 Despite the ease of use of these tests, clinicians unfortunately do not regularly pursue a specific diagnosis of vulvovaginal complaints, and rely (usually inappropriately) on syndromic management to direct treatment. Moreover, accuracy of the Amsel criteria in clinical practice is likely limited by clinicians’ lack of or limited skill in using microscopy to detect clue cells and rule out other important findings, including trichomonads and yeast forms.
BV may also be diagnosed using a score applied to Gram stains of vaginal fluid, the Nugent criteria, which quantifies the number of lactobacilli relative to BV-associated bacterial morphotypes to create a scale of flora abnormality ranging from normal (score = 0-3) through intermediate (score = 4-6) to frank BV (score = 7-10).40 The Nugent score is widely regarded as the gold standard for the diagnosis of BV in research studies. Most recently, targeted qualitative and quantitative polymerase chain reaction (PCR) assays for detection of various BV-associated bacteria have been studied; this approach may offer some utility in the future, but has not been widely validated for diagnosis of BV in large, diverse populations of women and is costly.41, 42 Moreover, quantitative PCR has not yet been well studied for its ability to differentiate between women who have intermediate flora vs. frank BV as determined by the Nugent score; women with intermediate flora by Nugent score may have relatively high quantities of the BV-associated bacteria G. vaginalis and A. vaginae as determined by qPCR.42 The single commercially available panel using PCR offers detection of G. vaginalis, Bacteroides fragilis, Mobiluncus mulieris, and Mobilincus curtisii, and thus detects only a small proportion of the bacterial species that characterize BV. The optimal molecular methodology or criteria, both qualitatively and quantitatively, to serve as the next generation diagnostic modality has not been determined. Thus the current commercial tests require additional validation.
While oral or intravaginal antibiotic treatment usually relieves symptoms of BV in the short term, relapse is very common, and can be ameliorated by suppressive antibiotic treatment
Antimicrobial compounds with broad activity against most anaerobic bacteria are effective in relieving symptoms of BV. Metronidazole and clindamycin are the mainstays of therapy. The published studies have consistently reported resolution of BV in 71% to 89% or more of women one month after administration of these regimens.3 Intravaginal therapies have had efficacy similar to that of oral metronidazole regimens with fewer side-effects. One prospective study indicated that detection of any of several BV-associated bacteria at the onset of treatment with intravaginal metronidazole predicted treatment failure at 30 days.43 Clindamycin-resistant bacteria have been reported among women treated with vaginal clindamycin, although this was not associated with reduced cure rates.44 Tinidazole, a nitroimidazole with antiprotozoal and antibacterial activity, is approved for the treatment of BV in two oral dosing regimens (2 gram daily for 2 days or 1 gram given for 5 days).
Interpreting the results of treatment trials for BV requires an understanding of the current guidance—still in draft form—from the US Food and Drug Administration, which recommends that clinical cure be defined as the resolution of all four clinical signs (Amsel criteria) of BV.45 If this definition of cure is used, cure rates for all of the available therapies, whether administered orally or intravaginally, are usually about 50%. Using criteria that are more likely to reflect improvement in women’s vaginal symptoms (absence of BV by Amsel or Nugent criteria), most trials indicate that over 85% of women respond initially to currently recommended regimens. More perplexing is the high rate of early recurrence (30% at three months, 50% at six months) reflecting early relapse and more likely late reinfection, for which successful management has not been forthcoming. Although each symptomatic episode usually responds rapidly to conventional antibiotic treatment, rapid recurrence is frequently inevitable.
BV can be suppressed with ongoing antibiotic therapy. In a prospective study, women with current BV and at least two prior episodes of BV in the previous year were initially treated with 10 days of vaginal metronidazole gel then, if cured, randomly assigned to receive twice weekly metronidazole vaginal gel or placebo for 16 weeks.46 They were subsequently followed off therapy for 12 weeks. Probability for remaining cured during the 16-week treatment phase was 70% for metronidazole compared with 39% on placebo, which declined to 34% and 18%, respectively, by 28 weeks follow-up. Adverse effects were uncommon; however, secondary vaginal candidiasis occurred significantly more often in metronidazole-treated women (P = .02).
Several placebo-controlled trials have demonstrated that treatment of the male partner(s) does not improve the clinical outcome of treatment of BV, or reduce recurrence.3 The discrepancy between data suggesting sexual acquisition of BV, and the lack of benefit of treating the male partner, remains puzzling, but does not rule out a role for sexual transmission. Part of the issue may be that the selection or dosing of the antibiotics used in the trials done to date was not appropriate or adequate for eradicating a potential reservoir for BV-associated bacteria in men.
The role of vaginal repletion using Lactobacillus therapies remains unproven
Even though certain lactobacilli may play an important role in maintaining the normal vaginal flora, it remains to be determined whether the application of such lactobacilli is sufficient to restore the vaginal microflora. At least one human-derived strain of Lactobacillus crispatus (CTV-05) is currently under study as treatment for BV, but is not yet commercially available.47, 48 In a randomized, placebo-controlled trial of this product administered with oral metronidazole as treatment for BV, women randomized to the CTV-05 group were less likely to be colonized by endogenous strains of lactobacilli one month after treatment, suggesting that the L. crispatus contained in the CTV-05 disrupted vaginal colonization by endogenous lactobacilli, suggesting that probiotic strains of lactobacilli may disrupt colonization by endogenous strains of lactobacilli through competitive inhibition.49 However, a recent study of orally administered lactobacilli reported a high level of effectiveness among African women.50
The microbiota of BV is complex, and is characterized by a high degree of species richness and diversity, which includes many fastidious bacteria that are either uncultivated or have only recently been cultivated
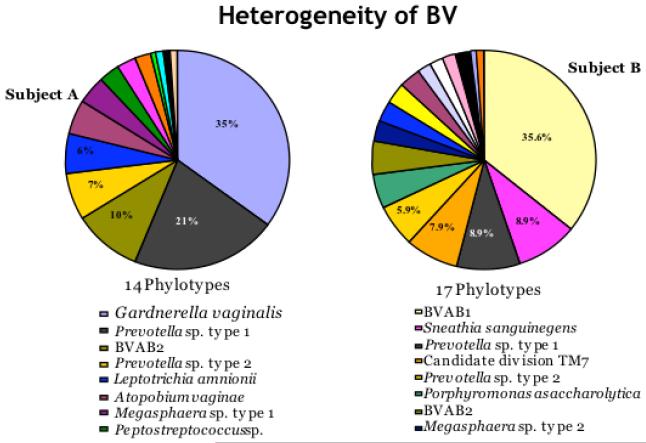
New tools in molecular biology have greatly expanded our understanding of microbial diversity in the human vagina, particularly by identifying fastidious or cultivation-resistant bacteria (Figure 1) 51-54. However, every technology has its strengths and limitations, and no method is free from bias51. A variety of molecular and cultivation methods can be used to build a model of the vaginal microbial ecosystem, allowing us to better understand how microbes interact with each other and the human host to promote health or facilitate disease.
Figure 1.
Molecular methods such as broad range 16S rRNA gene PCR with cloning and sequencing have demonstrated that the microbiology of BV is complex and different subjects may have very different vaginal bacterial communities as evidenced by these two subjects. The eight most abundant phylotypes (species level operational taxonomic units) are displayed for each subject.
Molecular vs. Cultivation Methods
Molecular methods have been widely adopted to study microbial populations because cultivation-based approaches do not detect or identify a large percentage of microbes present in most niches, including those associated with humans. Nevertheless, cultivation technology has some important advantages over molecular methods when detecting an organism that is capable of laboratory propagation. For example, cultivation methods can detect a single bacterium in a large volume of tissue or body fluid, offering unrivaled detection thresholds. For example, Escherichia coli and enterococci are not commonly detected in vaginal samples using molecular methods, but are commonly detected using cultivation approaches7, 55. This observation reflects the fact that some bacteria are easily propagated in the laboratory on selective media but are not the dominant bacteria based on cell concentration or bacterial DNA content. In addition, cultivation approaches are relatively inexpensive, have moderate throughput, and require less technical skill than molecular approaches making cultivation technology more accessible. Furthermore, cultivated isolates are valuable for studying microbial virulence factors, assessing metabolic capabilities of microbes, developing diagnostic tests, and assessing antibiotic susceptibility. Unfortunately, cultivation offers an incomplete view of microbial diversity because only those organisms capable of independent growth under the relatively artificial conditions found in the laboratory emerge to be counted. Even if propagated in pure culture, some novel microbes are not accurately identified using conventional phenotypic identification algorithms. In conclusion, cultivation is a very useful tool for the detection of cultivable microbes, but fails to detect many microbes such as several fastidious vaginal bacteria found in women with BV.
A Primer on Molecular Methods
A major advantage of molecular microbiological approaches is the ability to detect and identify microbes without cultivation, thereby overcoming cultivation bias. These strategies use genomic information to classify microbes, and there are several phylogenetically informative genes for this purpose including those coding for ribosomal RNA, heat shock proteins, RNA polymerase, and elongation factors. The sequences of these genes serve as molecular bar codes to identify microbes. For instance, the presence of unique Lactobacillus crispatus gene sequences in a vaginal fluid sample can be used to infer the presence of this bacterium without ever cultivating the organism. Several different molecular approaches have been used to characterize microbial communities, including:
Broad-Range PCR
Broad-range or consensus sequence PCR of ribosomal RNA genes is most commonly used to characterize the census of microbial communities. For example, primers that anneal with highly conserved regions of the bacterial 16S rRNA gene can be employed in a PCR to amplify segments of the gene from most bacterial species, and these amplification products typically contain intervening regions of sequence that are highly variable (or species/taxon specific) allowing for the identification of bacteria. Mixed bacterial communities yield mixed PCR products, and the sequences must be sorted in some way to distinguish between individual taxa. There are several widely used approaches for achieving this separation of PCR products for microbial classification, including:
Denaturing gradient gel electrophoresis (DGGE) with separation of PCR products into bands on a gel under denaturing conditions, with different PCR products (containing different sequences) having different mobility profiles in the gel56. Bands from the gel may be excised and sequenced to identify bacteria.
Terminal restriction fragment length polymorphism (T-RFLP) analysis wherein PCR primers are fluorescently labeled and used to generate PCR products. The amplification products are cut with restriction enzymes and the sizes of the terminal fluorescently labeled PCR product fragments are determined on a gel using a fluorescence scanner and fluorescently labeled size standards run concurrently57. Amplification products with different sequences are cleaved into different size fragments with each unique bacterium tending to have a different size terminal fluorescent product. A database of terminal restriction fragment lengths from known bacteria is used to identify bacteria in the vaginal sample.
Cloning and sequencing, wherein broad-range PCR products are ligated into plasmids and each plasmid with a single 16S rRNA gene sequence is used to transform E. coli, turning each bacterium into a plasmid factory53. Individual colonies of E. coli are picked and the plasmid insert containing the 16S rRNA gene fragment is sequenced. The 16S rRNA gene sequence is compared to known bacterial sequences to identify the bacterium (in the case of a complete match), or to infer phylogenetic relationships (for an incomplete match). See Figure 1.
Cloning with amplified ribosomal DNA restriction analysis (ARDRA), wherein cloned 16S rRNA gene PCR products (plasmids or plasmid inserts) are cleaved with restriction enzymes and the fragments are analyzed by gel electrophoresis55. Different bacteria tend to have different ARDRA band patterns that can be linked to specific organisms by sequencing the full insert for selected clones. The ARDRA approach reduces the sequencing burden, but is no longer cost effective in the era of high throughput and low-cost sequencing.
Pyrosequencing using the 454 sequencing system. Although there are several high-throughput sequencing platforms that are commercially available and could be used to analyze PCR products, most produce short reads that are not optimal for phylogenetic analysis of complex microbial communities. In contrast, the Roche 454 platform generates read lengths (>250 bp) that are sufficiently long for meaningful phylogenetic analysis and identification of microbes58, 59. In this approach 16S rRNA gene PCR products are typically generated using fusion primer sequences that allow attachment of single sequences to microscopic beads. Emulsion PCR is performed on the beads, creating a molecular clone of a single sequence type on each bead.60 The emulsion is then broken and the beads are floated into wells on a picotiter plate where sequencing by synthesis occurs in each well. Photons of light corresponding to nucleotide incorporation events are recorded to produce a sequence read for each PCR product. Sequences are compared to known bacterial 16S rRNA gene sequences to identify organisms.
The advantage of broad-range PCR is the ability to assess microbial diversity without having an a priori hypothesis about the composition of the microbiota (in contrast, see taxon-directed PCR). Importantly, broad range PCR primers are not free from bias as not all species are amplified using so called “universal” primers, and not all taxa are amplified with the same amplification efficiency. Nevertheless, broad range PCR is capable of detecting greater bacterial diversity than cultivation methods. Broad range PCR with analysis of amplification products is also not very quantitative. The proportion of clones in a clone library provides a rough idea about the relative concentrations of 16S rRNA genes, which is roughly correlated with the number of bacteria, but does not provide data on the absolute concentrations of bacteria in a sample. Furthermore, a bacterium may be present in a vaginal sample but not detected in a broad range PCR clone library, DGGE gel, or T-RFLP analysis because it is a minority species. For example, a minority bacterium’s 16S rRNA gene sequence may be present in only 1 of 1000 clones, so analysis of 100 clones will likely fail to detect this bacterium in the community. The large number of “clones” or sequence reads produced by 454 pyrosequencing can mitigate this problem, though broad range PCR methods are still not as sensitive as directed PCR assays. Table 2 compares these broad-range PCR approaches.
Table 2.
Comparison of methods for analyzing broad-range PCR products for identification of microbes in vaginal samples*
|
Method Criterion |
DGGE | T-RFLP | Clone + ARDRA |
Clone + Sequence |
EM-PCR Pyrosequence |
|---|---|---|---|---|---|
| Diversity (species richness) | Low | Low | Low -Moderate | Moderate | High |
| Accuracy (correct identification) | Moderate − High |
Moderate | Moderate | High | Moderate |
| Phylogenetic resolution (ability to distinguish between closely related species) |
Low | Low | Moderate | High | Moderate |
| Throughput | High | High | Low | Low | High |
| Generation of reagents (clones) | No | No | Yes | Yes | No |
| Cost | Low | Low | Moderate | High | Moderate |
DGGE = denaturing gradient gel electrophoresis; T-RFLP = terminal restriction fragment length polymorphism; ARDRA = amplified ribosomal DNA restriction analysis; Em-PCR = emulsion PCR; Pyroseq = pyrosequencing using Roche 454 instrumen
Taxon-Directed PCR
An alternative molecular approach to broad range PCR is taxon-directed PCR wherein one designs primers to PCR amplify a specific microbe or microbial group41. Fluorescently labeled probes may also be used in a real time PCR format to increase the specificity of the assay and to generate quantitative data61. Design of the primers and probes can be used to tune the assay to target more selected or more inclusive taxonomic groups. For example, one could design a qPCR assays that detects all species in the Mobiluncus genus, or one could design an assay that is specific for Mobiluncus curtisii. The presence of the intended PCR products needs to be confirmed by sequencing or by probe hybridization—the mere presence of a PCR product of the expected size noted on gel electrophoresis is not sufficient evidence to establish identity. The advantage of taxon-directed PCR over broad-range PCR is the ability to detect low levels of bacterial DNA in a sample and assess for the presence of minority species. An optimized taxon-directed PCR assay is not affected by the presence of other bacteria and this allows one to determine if a specific bacterium is present or absent in a sample, at least down to the limit of detection of the assay. The disadvantage of this approach is the limited number of bacteria that can be practically assayed. If one runs 20 taxon-directed PCR assays on a vaginal sample and all assays are negative, it does not mean that the sample is free of bacteria, only that the bacteria assayed are not represented. Thus, this taxon-directed PCR approach provides a narrower view of microbial populations, but can detect lower levels of bacteria and can do this in a quantitative fashion with qPCR.
Fluorescence in situ hybridization (FISH)
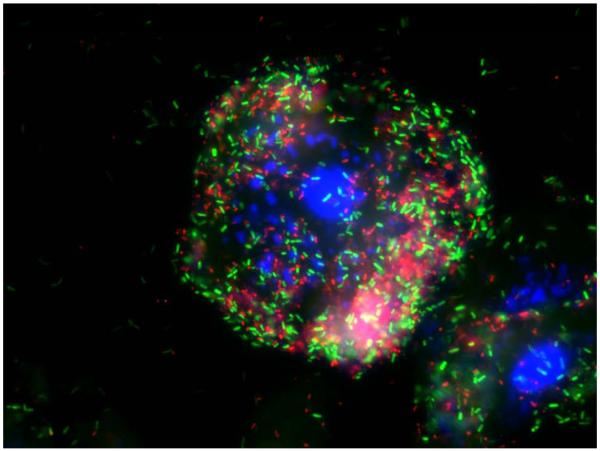
Fluorescently labeled oligonucleotide or peptide nucleic acid probes targeting bacterial ribosomes can be hybridized with fixed bacteria in tissues or body fluids to detect and identify bacteria by microscopy. The standard approach uses a panel of probes, including some that target all bacteria, and others that target specific taxonomic groups including species55. Bacteria have thousands of ribosomes per cell, and therefore bacteria have many binding sites for the probes that can lead to a strong fluorescence signal for visualization using fluorescence or confocal microscopy. For example, a FITC-labeled (green) probe targeting members of the Prevotella and Porphyromonas genera was hybridized with vaginal fluid from a subject with BV. After fixation of cells on a slide and hybridization of probe, unbound probe was washed away and bacteria were visualized by epifluorescence microscopy (Figure 2). FISH provides data on bacterial morphology and physical location in tissues that is not obtained using other molecular methods such as PCR. FISH also provides quantitative data by allowing for direct bacterial counts in tissues or body fluids. Limitations of FISH include high levels of background autofluorescence in some tissues after fixation, and the potential for hybridization of probes with non-target species or taxa leading to incorrect identification of bacteria. FISH is complementary to broad-range 16S rRNA gene PCR methods because the 16S rRNA gene sequences generated by PCR can be used to design FISH probes targeting bacterial ribosomes, thereby providing an independent method to confirm PCR results.
Figure 2.
Fluorescence micrograph showing a vaginal epithelial cell coated with bacteria from a subject with BV. A FITC labeled probe (green) targets Prevotella/Porphyromonas species and a Cy3 labeled probe (red) targets Atopobium/Eggerthella species. DAPI stains cell nuclei blue in this overlay image.
Phyloarrays
Microarray platforms using either solid supports (glass slides) or beads can be employed to characterize microbial communities without cultivation62. A typical phyloarray contains thousands of target 16S rRNA gene sequences at defined locations. The query sample (bacterial rRNA or rDNA) is labeled with a fluorescent reporter and this probe mix is hybridized to the array to determine if sequences in the sample are complementary to specific targets as reflected by fluorescence signal. Microarrays can be used to identify bacterial sequences generated by broad-range 16S rRNA gene PCR, or can be used to identify rRNA sequences directly isolated from clinical samples without PCR amplification. The advantages of phyloarrays include their ability to detect thousands of different microbial taxa in a single sample using a moderate throughput format at a reasonable cost. The disadvantages of phyloarrays include their failure to identify truly novel species (since these are not represented on the chip), the potential for cross-hybridization events that lead to misidentification of closely related taxa, and the higher detection thresholds compared to qPCR (i.e. worse analytical sensitivity).
In conclusion, cultivation-independent molecular methods have been extremely useful in understanding the diversity of bacteria associated with BV and these methods complement cultivation-based studies. Broad range PCR with cloning and Sanger sequencing provides the most complete assessment of bacterial phylogeny based on analysis of large regions of the 16S rRNA gene. High-throughput pyrosequencing allows for deep sampling of the microbial community and the detection of minority species. DGGE and T-RFLP are methods that provide rapid assessment of multiple samples at low cost to assess the dominant species. Quantitative PCR provides data on concentrations of particular species and how they change over time and under different influences. FISH provides information on bacterial morphology and localization in tissues. Phyloarrays allow for the detection of thousands of known bacteria by probe hybridization in a single sample. Each of these methods is useful, but all are subject to practical and scientific limitations; all methods have well-documented biases.
Deliberations of the Workshop Working Groups (Table 3)
Table 3.
Goals and questions for the Working Groups
|
Diagnosis and Clinical Management Working Group
As summarized in Table 3, the goals of this working group included a discussion of two main related areas. The first was to work towards consensus definitions for clinical and laboratory outcomes that are pivotal to the study of BV’s natural history, including therapeutic trials. The second area was the current diagnostic standards for BV, particularly as they relate to inclusion criteria for clinical trials that aim to follow women’s risks for or response to treatment of BV throughout the lifespan, and how molecular approaches to defining BV might best be studied and validated.
Optimizing definitions and clinical endpoints
In reviewing the BV literature, it became clear that across studies there was often a lack of standardization and consistency in reporting on several key areas, including timing of sampling in prospective studies, lack of adequate characterization of the women enrolled (particularly for status of vaginal symptoms associated with BV), and participants’ recent use of products that might alter the vaginal microbiota, including vaginal products and systemic antibiotics. The group agreed that several features could be used to encourage inclusion of appropriate target participants and to improve the generalizability and reliability of vaginal findings. These included: (1) a clear statement of whether sampling was being performed at baseline and, if women were followed prospectively, what interval time periods were used; (2) participants’ report of vaginal symptoms (see below for discussion of potential definition), in addition to report of the objective sign of vaginal discharge compatible with BV; (3) enrollment of reproductive age women as a priority, recognizing the need to expand study into pre-pubertal and peri-/post-menopausal women; (4) use of Nugent score as a gold standard, despite limitations discussed below; (5) use of modified Amsel criteria (including all but the KOH (“whiff”) test, whose subjectivity was emphasized; (6) encouragement of vaginal specimen collection in ideal situations, including lack of prior antibiotic (30 days) and any intravaginal product in the previous 24 hours; and (7) collection of vaginal fluid using swabs applied to a standard anatomic site (lateral vaginal wall). Discussion of specific questions is reviewed below.
Which criteria should be used in research studies to define BV and normal vaginal microbiota, and should asymptomatic subjects be considered “normal” regardless of their vaginal microbiota?
The group proposed a framework for defining the vaginal microbiota as described below.
○ BV is present when Nugent score ≥7 and modified Amsel criteria are positive, regardless of symptoms. However, whether symptoms are present should be clearly conveyed. A definition of abnormal symptoms was proposed to include, at a minimum, participant report of increased, malodorous vaginal discharge.
○ Vaginal microbiota is normal when Lactobacillus is the predominant species, as defined by Nugent score <4.
○ Vaginal microbiota is intermediate when sampling reveals no days of normal microbiota, and no days of BV, as defined above.
How should cure of BV be defined? How should we distinguish between BV persistence, relapse, and recurrence?
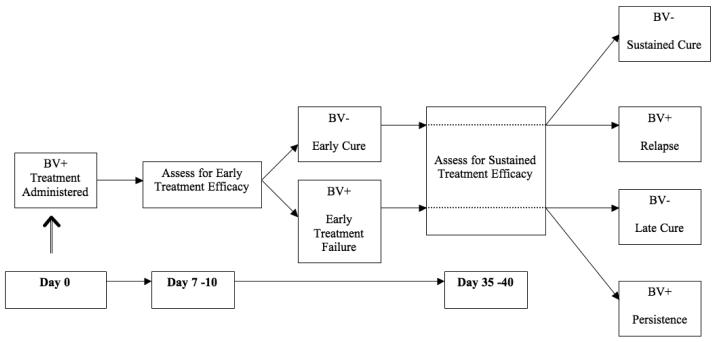
Therapeutic studies of BV have used different schedules to define outcomes post-therapy. The group discussed several schemes for sequential sampling to detect BV using the above criteria, with the consensus being that at least two samples should be obtained after the initiation of treatment; these should reflect that while some clinical features of BV improve rapidly with treatment (for example, loss of abundant clue cells), others take considerably longer (for example, normalization of vaginal pH). Ideally, the timeframe over which these two samples are collected should span at least one menstrual cycle if relevant. The first assessment should be undertaken at 7-10 days post-initiation of treatment, and would afford an estimate of early treatment efficacy (cure). At this assessment, cure should be defined by the absence of significant number of clue cells (<20% of all epithelial cells on saline microscopy at 100X magnification) and attainment of Nugent score ≤6; improvement of symptoms should be included, but would constitute a separate component of treatment response. The second assessment should occur 35-40 days post-initiation of treatment, and would afford an estimate of three possible outcomes, depending on the result of the assessment at 7-10 days: long-term efficacy, persistence, or relapse (Figure 3). At this point, cure should be defined as absence of significant number of clue cells, normalization of pH (≤4.5), and Nugent score ≤3 (normal flora).
Figure 3.
Proposed timeline and terminology for describing response to treatment of bacterial vaginosis
Of critical importance is the collection and report of participant’s subjective symptoms at follow up; questions regarding abnormal symptoms should be standard and should include status of abnormal vaginal discharge and odor. By this standard, women with a Nugent score of >3 would be categorized as treatment failures, whether or not their Nugent score is determined to be in the intermediate or BV range. Moreover, utilization of the Nugent score for this purpose does not rely on speciation of the Lactobacillus involved, a procedure that is well beyond the capacity of the majority of laboratories involved in clinical trials related to BV.
Recognizing the dynamic nature of vaginal microbiota, the value of daily, prospective collection (including self-collection) of vaginal samples for characterization of the microbiota, following women who began with BV, with normal microbiota, and with intermediate microbiota, was emphasized; this approach should constitute a research effort in itself, apart from therapeutic clinical trials.
The role of cultivation or molecular microbiological criteria in the diagnosis of BV
The group agreed that normal vaginal microbiota could be characterized by the presence or absence of specific bacteria, but that further work was needed to define the performance characteristics of assays that would do this. For example, different BV-associated bacteria are likely to have different predictive values for detection of BV, and combining some of these bacteria might enhance these values. Gardnerella vaginalis, which appears to be ubiquitous in women with BV and characterizes the BV-associated biofilm in studies that have specifically looked for this bacterium, is also present in the vagina of up to 70% of women with normal microbiota.41 Other BV-associated bacteria, including Atopobium vaginae, BVAB-1, BVAB-3, and Megasphaera type-2, are highly specific for BV, each with a negative predictive value for BV >92% when used as a single assay and, in the case of BVAB-2 and Megasphaera type-2, >95% when used in combination. However, the group acknowledged that approaches using bacterium-specific assays to diagnose BV require validation in larger and more diverse groups of women, as the studies to date have been small or have focused on epidemiologically distinct groups with limited data collected on subjects. An additional objective of such studies should be to consider whether quantitative thresholds of BV-associated bacteria—for example, with quantitative PCR—might offer additional enhancements to diagnostic performance. Finally, while species-specific PCR assays for the Lactobacillus species most predictive of vaginal health (namely, L. crispatus and L. jensenii) might be useful markers for resolution of BV, the group determined that validation of this approach, particularly as it relates to the standard use of Nugent criteria, was required.
Additional priorities for BV-related research
Group members emphatically noted the need for research in several additional areas. First, recognizing the limited rates of long term cure afforded by current treatment regimens, development of alternative treatment approaches should be emphasized. Second, given the role of unprotected sex in subsets of women with BV and the observation that male circumcision has been associated with reduced incidence of BV,12 the role of male sex partners, particularly as a reservoir for BV-associated bacteria, in BV pathogenesis and response to treatment should be clarified. Third, the contribution of host immunogenetics to BV is not understood and needs to be studied. Fourth, the natural history of vaginal Lactobacillus acquisition (apart from their involvement in the natural history of BV) is not understood, and should be clarified through prospective studies of women throughout the life span. The group noted that organized establishment of specimen repositories across institutions could advance work towards these goals by providing access to collections of samples from diverse groups of women with clearly defined demographic, clinical, and microbiologic characteristics.
Critically, the group agreed that a key outcome of the workshop should be the communication of these discussions with FDA and a dialogue initiated on the current draft guidance (which date from 1998) for definition of clinical trial endpoints,45 and because the definition of new terms (recurrence, persistence) could provide clarity and critical information on new aspects of therapeutic outcomes.
Working Group on Molecular Methods in BV
What is the state of knowledge in the field?
Members of the Molecular Working Group have used a wide variety of methods to characterize the vaginal microbiota in women with and without BV, and conclude that most major vaginal bacterial species have likely been detected using these methods, though it is possible that low abundance species remain to be discovered. The relevance and role of minority species (the “rare biosphere”) in the vaginal microbial ecosystem remains to be determined. Approaches for detecting these rare species include use of:
Taxon-limited PCR primers with sequence analysis of the PCR products, such as with broad range Prevotella genus primers.
Blocking primers to suppress amplification of known dominant bacterial species in order to enrich for novel or low abundance species in broad range PCR.
Deep 454 pyrosequencing methods to detect rare broad range PCR products.
Phyloarrays (microarrays) that can simultaneously detect thousands of microbes including those present at relatively low abundance.
It is also possible that some populations of women have novel bacterial communities yet to be discovered; this question can only be resolved by analyzing culturally and geographically diverse populations of women.
Is there value in harmonizing or standardizing molecular approaches across groups?
Different research groups have formed slightly different conclusions about the composition of the vaginal microbiota. Many factors can influence results of molecular surveys, including the precise site of sampling, the collection method (swab, scrape, lavage), the DNA extraction method, the primers used in PCR and subsequent conditions (e.g. annealing temperature), and the method used to characterize the amplicons. Do differences in studies reflect true differences related to variation in the human vaginal microbiota, or methodological biases due to use of different methods? For instance, should all groups use the same DNA extraction methods and broad range 16S rRNA gene PCR primers to achieve better consensus? Members of the group concurred that achieving consensus on methods is unlikely. Furthermore, some felt that there was an advantage to using multiple approaches to characterize the vaginal microbiota as this strategy would help compensate for the biases of individual methods and lead to a more accurate assessment of the true microbial ecology of the human vagina. On the other hand, there was universal agreement in the value of sample repositories. Access to aliquoted samples such as cervicovaginal fluid or vaginal swabs from well-characterized individuals would allow different labs to compare results and distinguish between methodological and biological differences. Furthermore, banked samples would allow investigators to apply new technologies to these samples in the future. The group concurred that creation of a sample repository would have great scientific value for the research community, but declined to suggest any guidelines for standardizing molecular methods.
The nomenclature problem: how should bacteria be named when one only has a 16S rRNA gene sequence and there are no close phylogenetic affinities for the organism?
The challenges associated with naming uncultivated bacteria are not unique to the vaginal niche. A formal genus and species designation cannot be minted for a newly described bacterium without having a cultivated isolate, though a provisional Candidatus status is allowed when in situ hydridization images demonstrate the microbe in its natural state.63 The problem with the Candidatus status is that phenotypic data (metabolism, spore formation, Gram stain characteristics) discovered during cultivation may significantly change what is known about the organism and hence its most appropriate taxonomic placement. Although there was wide agreement that having multiple names for the same organism would lead to confusion, there was no consensus on how to avoid this problem. The Working Group suggested engaging the NIH Human Microbiome Project Working Groups, the American Society for Microbiology, and taxonomists to help create standards for naming bacteria detected in molecular surveys.
What are the optimal targets for the molecular diagnosis of BV?
A few groups have investigated the use of bacterium-specific PCR assays for the diagnosis of BV 41, 42, 64. Although several bacterial targets appear promising, the optimal targets remain to be defined and additional studies are needed. In particular, the Molecular Working Group highlighted the importance of studies that compare PCR assay results to both Amsel and Nugent criteria for the diagnosis of BV, as well as comparing PCR to point-of-care diagnostic tests for BV. An important unresolved issue is how to handle Gram stain results with Nugent scores of 4-6 indicating intermediate flora. Some investigators have tended to ignore this group, focusing on subjects with Nugent scores of 0-3 indicating normal flora and 7-10 indicating BV, thereby artificially increasing reported diagnostic test performance42. Unfortunately this is not a tenable approach to analysis when all subjects must be considered when evaluating a diagnostic test. Another unresolved question is what to do with a positive molecular diagnostic test for BV in a pregnant woman. There is ongoing debate about when BV should be treated to reduce the incidence of preterm birth, or whether there is any value to treatment of BV in pregnancy. There is even less information about which vaginal bacterial species may impart elevated risks of preterm birth.65 Ongoing studies should help resolve some of these questions.
What are the current research gaps in the field?
There are several outstanding questions that warrant further investigation. The most basic question is, what is normal for the human vagina? Normality can be assessed on several levels. What is normal clinically, microbiologically, and immunologically? A separate but related question is, what is healthy for the human vagina? A condition may be considered normal, or within the normal range, yet not be optimal in reference to health outcomes. Which bacteria or bacterial community types are associated with BV, and what accounts for the heterogeneity in microbiology found in BV? A major limitation of several studies of the vaginal microbiota is their failure to use any objective method such as Amsel or Nugent criteria to assess for BV. Since about half of women with BV are asymptomatic, assessing for symptoms is not a reliable approach for excluding subjects with BV. How does the vaginal microbiota change with menstruation, sex, age, hygiene practices, and antibiotics? Do particular vaginal bacteria or bacterial communities impart a higher risk of preterm birth, HIV infection, pelvic inflammatory disease, or post-surgical infection?
The importance of longitudinal studies
There was wide agreement that the data collected from well-designed longitudinal studies will be critical for answering some of the unresolved questions stated above. Ideally, studies will collect vaginal samples, such as swabs, on a daily basis from hundreds of women to determine how the vaginal microbiota changes on short time scales in response to hormonal and behavioral factors. Women with diverse ages, races, sexual orientations, sexual practices, geographic representation, and underlying health status should be enrolled. For instance, there is a paucity of data on pre-adolescent women. Twin studies would help illuminate the role of genetics in vaginal bacterial colonization. Metadata should be collected along with the vaginal swabs, including data on sexual practices, vaginal pH, antibiotic use, and periodic Gram stains of vaginal fluid. These studies will shed light on the changes in vaginal microbiota that correlate with BV and the dynamic microbial ecology of the human vagina. Are there keystone species in BV that form the initial wave of bacteria that facilitate colonization with other species, or does the entire community of BV-associated bacteria become established simultaneously? What changes in the vaginal environment precede the development of BV?
Experimental controls for molecular studies, or when should you believe PCR results?
PCR is the workhorse of molecular studies, but this powerful technique is subject to some common problems, including false positive and false negative results. Interpreting PCR studies therefore requires the use of appropriate experimental controls. No-template controls (NTC) consisting of all necessary PCR reagents but lacking template DNA are used to monitor for false positive results at the PCR assembly stage. False positive NTCs may result from microbial contamination of PCR reagents or carry-over contamination from previously amplified PCR products. DNA extraction (or digest) controls are used to monitor for false positive results arising during the DNA extraction procedure and should be included with every assay. False negative results can be assessed using several types of assays. For instance, self-collected vaginal swabs are occasionally mislabeled or not actually used to collect vaginal fluid. Use of a human gene PCR assay, such as one targeting the 18S rRNA or beta-globin gene, or use of a common bacterial target (broad range 16S rRNA gene PCR) can be used to determine if the swab was used to collect vaginal fluid. Swabs without human DNA have not contacted a human surface or have degraded material. It is also important to assess for PCR inhibitors in the extracted DNA from vaginal samples. Use of an internal amplification control PCR using exogenously added template at known concentration, along with primers and probe, is the most direct approach for monitoring PCR inhibition as this second PCR takes place in the same tube as the bacterial PCR assay. Failure or delay in amplification of the exogenous template (e.g. jellyfish aequorin gene) provides evidence that a PCR inhibitor is present leading to false negative results.66 Molecular studies should rigorously test for false positive and false negative results using appropriate assays so that results are interpreted with the most accuracy.
Conclusions
Molecular microbiological methods have been extremely useful in describing the true extent of microbial diversity found in the human vagina and the complex microbiology of BV. Different molecular approaches provide slightly different perspectives that are best interpreted by understanding the strengths and limitations of each method. Molecular and cultivation-based methods are complementary, and data from all approaches should be synthesized to create the most accurate models of the vaginal microbial ecosystem. The use of shared definitions, sample repositories, and appropriate experimental controls will help create scientific consensus and advance the field.
Working Group on Complications of BV
BV as defined by Amsel and/ or Nugent criteria is associated with significant morbidity in women including poor pregnancy outcomes, PID, HIV acquisition, other sexually transmitted infections and post gynecologic surgery wound infections.3 However, failure of antibiotic treatment to prevent some of these complications67 and an observed lack of association of BV with PID in one prospective study68 have raised doubt about the causal role of the syndrome in these conditions.68 The BV Complications Workgroup agreed that while the Nugent and Amsel criteria have been useful research tools over the last several decades, further advances of our understanding of how vaginal bacteria affect women’s health will be dependent upon current research focused on the vaginal microbiome. This research has the potential to provide more precise classification of genitourinary tract microbiota into distinct categories. With this categorization, future studies of BV complications can determine if specific combinations of organisms are more pathogenic than others, and causally associated with different morbid events. Determination of causality will be dependent not only on more precise categorization of the vaginal microbiota, but also on variations in the host environment that may be associated with changes in bacterial communities over time. 69-71 Continued development of the transcriptomic and metabolomic technology will be of critical importance to the success of these endeavors.
The group also recognized that vaginal microbiota varies significantly over time. Natural history studies of the vaginal microenvironment as facilitated by the human microbiome project will be important in designing future studies of BV complications, as it seems likely that only persistent vaginal bacterial community types will predict disease. Natural history studies should focus on understanding what drives shifts in the vaginal microbiota. Such studies will be important in designing future prevention strategies. Specific points related to the major complications of BV are addressed below.
Abnormal pregnancy outcome
One of the problems contributing to the difficulty of establishing a direct link between BV and preterm delivery (PTD) and preterm premature rupture of membranes (PPROM) is the fact that most studies have focused on late term events. The later the onset of these events, the greater the number of potential etiologies that may confound associations between specific changes in the vaginal microbiota and poor pregnancy outcome.67 The group agreed that such studies should be initiated earlier in pregnancy and that sequential specimens should be collected at relatively short intervals in order to clearly establish that putative pathogenic microbial communities are persistent. As early PTD and PPROM are less common than that later events, cases control study designs will be necessary in order to limit the number of patients that must be prospectively followed for the development of these relatively rare events. Otherwise the cost of such studies will be prohibitive. As noted above, a key to such studies is a better understanding of the extent of variation in vaginal bacterial communities so that women can be selected for inclusion in research protocols. In this context, focusing on women with prior PTD may be the best initial approach. PTD has also been associated with gingivitis,72 and it may be important to determine if there are links between the microbiota of the mouth and the vagina. Finally, previous studies of the effect of BV treatment on pregnancy outcome were hampered by the relatively poor sustained efficacy of current BV treatment. Within a few months following treatment with any currently recommended treatment, nearly 50% of women have a recurrence of symptomatic or asymptomatic BV.73, 74 Therefore, the advisability of future treatment studies of BV in pregnancy heavily depends on the availability of more effective therapy.
Association between BV and HIV infection
The association of BV and HIV infection has been well established.75 Moreover, studies have demonstrated that incident HIV infection is associated with BV.75, 76 If better BV treatment strategies can be devised and these can be shown to lower the risk of HIV acquisition, the public health benefits would be enormous. However, as in the case of pregnancy outcomes, it may be critical to improve upon current treatment approaches before design of such studies can be optimized. It may be that longer duration treatment or intermittent suppressive treatment will be necessary unless new, more effective antimicrobials become available. Additionally, as noted above, better data are needed on the differences in microbial communities and on how the vaginal microbiota influence the local host immune milieu, especially with regard to the recruitment to the vaginal tissues of target cells for HIV infection. Subjects enrolled into the placebo arms of ongoing or planned trials of topical microbicides could be used to evaluate the effect of the vaginal microbiota on the host immune system, as these studies include assessment of many if not all of the host response pathways affected by BV. The importance of this issue is such that, once more effective therapeutic approaches have been identified, this research should be initiated as soon as possible. Specimens can be collected and stored while the fine points of vaginal microbiota variations and the host response are being worked out.
Pelvic inflammatory disease
PID is difficult to study as the disease is relatively uncommon, and the clinical diagnosis is highly non-specific. Achieving adequate case numbers usually requires a multicenter approach or a long study duration. Laparoscopy for diagnosis is no longer the standard of care so it is difficult to justify direct fallopian tube sampling in order to determine which vaginal microorganisms are present at the infection site where the greatest damage is done. Endometrial biopsies are possible and have been used in a number of relatively recent PID studies. 77-79 Simultaneous sampling of the vagina and endometrium in adequate numbers of patients should inform us as to which microorganisms at least have the capability of ascending into the upper genital tract as well as establishing the relationship to histological endometritis. Quantitative NAAT assays may be particularly important in this setting as it is difficult to avoid some degree of contamination of the biopsy instrument as it is passed through the endocervix into the uterine cavity.
Post procedure infections
BV has been associated with post partum endometritis80, vaginal cuff cellulitis following abdominal hysterectomy26, 81, and post abortion PID82. Assessment of preoperative vaginal microflora in conjunction with post operative molecular assessment of wound infection sites will be important to understanding more clearly the role of specific organisms in these infections. Preoperative treatment may be necessary in only a subset of patients. The possible effects of vaginal microbiota thought to be “high risk” on the host immune response also should be investigated. These studies could be nested within treatment studies, though better treatment options will be needed before such studies are undertaken. As emphasized by the Diagnosis and Clinical Management Working Group, careful case and outcome definitions are needed for these studies.
The most important conclusion of this working group was that a great deal of groundwork is necessary before undertaking further studies of the role of vaginal microbiota in health and disease in the female genitourinary tract. Better diagnostic methods and more efficacious treatment strategies are needed before large scale trials are undertaken to evaluate prevention of complications of BV such as preterm birth.
Conclusions & Future Directions
Despite considerable research effort and recent advances, BV remains an enigmatic condition. Efforts to link BV to a single cultivated bacterial pathogen, such as Gardnerella vaginalis, have been unconvincing. Molecular tools have revealed the complex microbiology of BV, but again have not found a credible single causal pathogen. BV is most likely a heterogeneous syndrome caused by different communities of vaginal bacteria, as has been described for the syndrome of periodontitis linked to changes in oral microbial communities 83. If this view is correct, BV can be considered a dysbiotic condition caused not by a single pathogen but by a change in microbial composition and community structure. A woman’s individual risk for a acquiring a particular etiologic vaginal bacterial community might depend on specific practices, such as unprotected sex or douching. Although the Nugent and Amsel criteria have been useful research tools, research focused on the vaginal microbiome may provide more precise classification of vaginal microbiota into distinct categories. Future studies of BV and its associated adverse outcomes should determine if specific combinations of organisms are more pathogenic than others, and causally associated with different adverse events. Moreover, determination of causality will depend not only on more precise categorization of the vaginal microbiota, but also on variations in the host environment that may be associated with changes in bacterial communities over time. In this report, we offer suggestions and recommendations that we hope will facilitate conduct of consistent approaches to collaborative efforts towards advancing our understanding of the vaginal microbiota and its impact on human health.
Acknowledgments
Financial support: National Institute of Allergy and Infectious Diseases
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sobel J. Current Concepts: Vaginitis. N Engl J Med. 1997;337:1896–903. doi: 10.1056/NEJM199712253372607. [DOI] [PubMed] [Google Scholar]
- 2.Olmsted SS, Meyn LA, Rohan LC, Hillier SL. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex Transm Dis. 2003;30:257–61. doi: 10.1097/00007435-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Hillier SL, Marrazzo JM, Holmes KK. Bacterial vaginosis. In: Holmes KK, Sparling PF, Mardh P-A, et al., editors. Sexually Transmitted Diseases. 4th ed McGraw-Hill; New York: 2008. pp. 737–68. [Google Scholar]
- 4.Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–63. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 5.Barbone F, Austin H, Louv WC, Alexander WJ. A follow-up study of methods of contraception, sexual activity, and rates of trichomoniasis, candidiasis, and bacterial vaginosis. Am J Obstet Gynecol. 1990;163:510–4. doi: 10.1016/0002-9378(90)91186-g. [DOI] [PubMed] [Google Scholar]
- 6.Avonts D, Sercu M, Heyerick P, Vandermeeren I, Meheus A, Piot P. Incidence of uncomplicated genital infections in women using oral contraception or an intrauterine device: a prospective study. Sex Transm Dis. 1990;17:23–9. [PubMed] [Google Scholar]
- 7.Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, Stine K, Hillier SL. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–13. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 8.Berger BJ, Kolton S, Zenilman JM, Cummings MC, Feldman J, McCormack WM. Bacterial vaginosis in lesbians: a sexually transmitted disease. Clin Infect Dis. 1995;21:1402–5. doi: 10.1093/clinids/21.6.1402. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–5. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 10.Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80:58–62. doi: 10.1136/sti.2003.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoubnikova M, Hellberg D, Nilsson S, Mardh PA. Contraceptive use in women with bacterial vaginosis. Contraception. 1997;55:355–8. doi: 10.1016/s0010-7824(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 12.Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200:42, e1–7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calzolari E, Masciangelo R, Milite V, Verteramo R. Bacterial vaginosis and contraceptive methods. Int J Gynaecol Obstet. 2000;70:341–6. doi: 10.1016/s0020-7292(00)00217-4. [DOI] [PubMed] [Google Scholar]
- 14.Royce RA, Jackson TP, Thorp JM, Jr., et al. Race/ethnicity, vaginal flora patterns, and pH during pregnancy. Sex Transm Dis. 1999;26:96–102. doi: 10.1097/00007435-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 16.Meis PJGR, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams JD, Thom E, Andrews WW. The preterm prediction study: significance of vaginal infections. Am J Obstet Gynecol. 1995;173:1231–5. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 17.Hillier SL, Kiviat NB, Hawes SE, et al. Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecol. 1996;175:435–41. doi: 10.1016/s0002-9378(96)70158-8. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg RL, Thom E, Moawad AH, Johnson F, Roberts J, Caritis SN. The preterm prediction study: fetal fibronectin, bacterial vaginosis, and peripartum infection. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996;87:656–60. doi: 10.1016/0029-7844(96)00034-8. [DOI] [PubMed] [Google Scholar]
- 19.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. Bmj. 1999;319:220–3. doi: 10.1136/bmj.319.7204.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver HM, Sperling RS, St Clair PJ, Gibbs RS. Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol. 1989;161:808–12. doi: 10.1016/0002-9378(89)90406-7. [DOI] [PubMed] [Google Scholar]
- 21.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 22.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990;75:52–8. [PubMed] [Google Scholar]
- 23.Larsson PG, Platz-Christensen JJ, Thejls H, Forsum U, Pahlson C. Incidence of pelvic inflammatory disease after first-trimester legal abortion in women with bacterial vaginosis after treatment with metronidazole: a double-blind, randomized study. Am J Obstet Gynecol. 1992;166:100–3. doi: 10.1016/0002-9378(92)91838-2. [DOI] [PubMed] [Google Scholar]
- 24.Sweet RL. Gynecologic conditions and bacterial vaginosis: implications for the non-pregnant patient. Infect Dis Obstet Gynecol. 2000;8:184–90. doi: 10.1155/S1064744900000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson PG, Platz-Christensen JJ, Sundstrom E. Is bacterial vaginosis a sexually transmitted disease? Int J STD AIDS. 1991;2:362–4. doi: 10.1177/095646249100200511. [DOI] [PubMed] [Google Scholar]
- 26.Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1016–21. doi: 10.1016/0002-9378(90)91115-s. discussion 21-3. [DOI] [PubMed] [Google Scholar]
- 27.Wiesenfeld H, Hillier S, Krohn M, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100:456–63. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 28.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 29.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663–8. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 30.Schwebke JR, Schulien MB, Zajackowski M. Pilot study to evaluate the appropriate management of patients with coexistent bacterial vaginosis and cervicitis. Infect Dis Obstet Gynecol. 1995;3:199–22. doi: 10.1155/S1064744995000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwebke JR, Weiss HL. Interrelationships of bacterial vaginosis and cervical inflammation. Sex Transm Dis. 2002;29:59–64. doi: 10.1097/00007435-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Cu-Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CC. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33:894–6. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 33.Seck K, Samb N, Tempesta S, et al. Prevalence and risk factors of cervicovaginal HIV shedding among HIV-1 and HIV-2 infected women in Dakar, Senegal. Sex Transm Inf. 2001;77:190–3. doi: 10.1136/sti.77.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid G, Markowitz L, Joesoef R, Koumans E. Bacterial vaginosis and HIV infection. Sex Transm Infect. 2000;76:3–4. doi: 10.1136/sti.76.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS (London, England) 1998;12:1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353:525–35. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 37.Rebbapragada A, Howe K, Wachihi C, et al. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J Acquir Immune Defic Syndr. 2008;49:520–2. doi: 10.1097/QAI.0b013e318189a7ca. [DOI] [PubMed] [Google Scholar]
- 38.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 39.Myziuk L, Romanowski B, Johnson SC. BVBlue Test for Diagnosis of Bacterial Vaginosis. J Clin Microbiol. 2003;41:1925–8. doi: 10.1128/JCM.41.5.1925-1928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007;45:3270–6. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menard JP, Fenollar F, Henry M, Bretelle F, Raoult D. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis. 2008;47:33–43. doi: 10.1086/588661. [DOI] [PubMed] [Google Scholar]
- 43.Marrazzo JM, Thomas KK, Fiedler TL, Ringwood K, Fredricks DN. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann Intern Med. 2008;149:20–8. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beigi RH, Austin MN, Meyn LA, Krohn MA, Hillier SL. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol. 2004;191:1124–9. doi: 10.1016/j.ajog.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) [Retrieved July 30, 2010];Guidance for industry: bacterial vaginosis—developing antimicrobial drugs for treatment. Draft guidance. 1998 July; Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guid ances/ucm070969.pdf.
- 46.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283–9. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 47.Antonio MA, Hillier SL. DNA fingerprinting of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J Clin Microbiol. 2003;41:1881–7. doi: 10.1128/JCM.41.5.1881-1887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patton DL, Sweeney YT Cosgrove, Antonio MA, Rabe LK, Hillier SL. Lactobacillus crispatus capsules: single-use safety study in the Macaca nemestrina model. Sex Transm Dis. 2003;30:568–70. doi: 10.1097/00007435-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Antonio MA, Hillier S. Endogenous Vaginal Lactobacilli are Suppressed by Administration of Probiotic Lactobacilli Following Treatment of Bacterial Vaginosis. Annual Scientific Meeting of the Infectious Diseases Society for Obstetrics and Gynecology; Montreal, Canada. August 2009.2009. [Google Scholar]
- 50.Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006;8:2772–6. doi: 10.1016/j.micinf.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 52.Zozaya-Hinchliffe M, Lillis R, Ferris M, Taylor S, Martin DH. Carriage of bacterial vaginosis-associated species by male sexual partners (abstract no. C-161). American Society of Microbiology 108th General Meeting; Boston, MA. 2008.2008. [Google Scholar]
- 53.Verhelst R, Verstraelen H, Claeys G, et al. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 2004;4:16. doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burton JP, Reid G. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J Infect Dis. 2002;186:1770–80. doi: 10.1086/345761. [DOI] [PubMed] [Google Scholar]
- 55.Fredricks DN, Marrazzo JM. Molecular methodology in determining vaginal flora in health and disease: its time has come. Curr Infect Dis Rep. 2005;7:463–70. doi: 10.1007/s11908-005-0049-2. [DOI] [PubMed] [Google Scholar]
- 56.Burton JP, Dixon JL, Reid G. Detection of Bifidobacterium species and Gardnerella vaginalis in the vagina using PCR and denaturing gradient gel electrophoresis (DGGE) Int J Gynaecol Obstet. 2003;81:61–3. doi: 10.1016/s0020-7292(02)00408-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150:2565–73. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 58.Spear GT, Sikaroodi M, Zariffard MR, Landay AL, French AL, Gillevet PM. Comparison of the Diversity of the Vaginal Microbiota in HIV-Infected and HIV-Uninfected Women with or without Bacterial Vaginosis. J Infect Dis. 2008 doi: 10.1086/591942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravel J, Gajer P, Abdo Z, et al. Microbes and Health Sackler Colloquium: Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huse SM, Dethlefsen L, Huber JA, Welch D Mark, Relman DA, Sogin ML. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet. 2008;4:e1000255. doi: 10.1371/journal.pgen.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol. 2009;47:721–6. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yergeau E, Arbour M, Brousseau R, et al. Microarray and real-time PCR analyses of the responses of high-arctic soil bacteria to hydrocarbon pollution and bioremediation treatments. Appl Environ Microbiol. 2009;75:6258–67. doi: 10.1128/AEM.01029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murray RG, Stackebrandt E. Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. Int J Syst Bacteriol. 1995;45:186–7. doi: 10.1099/00207713-45-1-186. [DOI] [PubMed] [Google Scholar]
- 64.Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol. 2005;43:4607–12. doi: 10.1128/JCM.43.9.4607-4612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelson DB, Hanlon A, Hassan S, et al. Preterm labor and bacterial vaginosis-associated bacteria among urban women. J Perinat Med. 2009;37:130–4. doi: 10.1515/JPM.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell CM, Hitti JE, Agnew KJ, Fredricks DN. Comparison of oral and vaginal metronidazole for treatment of bacterial vaginosis in pregnancy: impact on fastidious bacteria. BMC Infect Dis. 2009;9:89. doi: 10.1186/1471-2334-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD000262.pub3. CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104:761–9. doi: 10.1097/01.AOG.0000139512.37582.17. [DOI] [PubMed] [Google Scholar]
- 69.Schwebke J, Morgan S, Weiss H. The use of sequential self-obtained vaginal smears for detecting changes in the vaginal flora. Sex Transm Dis. 1997;24:236–9. doi: 10.1097/00007435-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Keane FE, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS. 1997;8:489–94. doi: 10.1258/0956462971920631. [DOI] [PubMed] [Google Scholar]
- 71.Priestley CJ, Jones BM, Dhar J, Goodwin L. What is normal vaginal flora? Genitourin Med. 1997;73:23–8. doi: 10.1136/sti.73.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bobetsis YA, Barros SP. Offenbacher S. Exploring the relationship between periodontal disease and pregnancy complications. J Am Dent Assoc. 2006;137(Suppl):7S–13S. doi: 10.14219/jada.archive.2006.0403. [DOI] [PubMed] [Google Scholar]
- 73.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–86. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 74.Koumans EH, Markowitz LE, Hogan V. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: a synthesis of data. Clin Infect Dis. 2002;35:S152–72. doi: 10.1086/342103. [DOI] [PubMed] [Google Scholar]
- 75.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS (London, England) 2008;22:1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr., Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 77.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195:803–8. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 78.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162:585–90. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 79.Yudin MH, Hillier SL, Wiesenfeld HC, Krohn MA, Amortegui AA, Sweet RL. Vaginal polymorphonuclear leukocytes and bacterial vaginosis as markers for histologic endometritis among women without symptoms of pelvic inflammatory disease. Am J Obstet Gynecol. 2003;188:318–23. doi: 10.1067/mob.2003.105. [DOI] [PubMed] [Google Scholar]
- 80.Newton E, Prihoda TJ, Gibbs RS. A clinical and microbiologic analysis of risk factors for puerperal endometritis. Obstet Gynecol. 1990;75:402. [PubMed] [Google Scholar]
- 81.Larsson PG, Platz-Christensen JJ. Bacterial vaginosis and the vaginal leucocyte/epithelial cell ratio in women attending an outpatient gynaecology clinic. Eur J Obstet Gynecol Reprod Biol. 1991;42:217–20. doi: 10.1016/0028-2243(91)90223-8. [DOI] [PubMed] [Google Scholar]
- 82.Larsson PG, Bergman B, Forsum U, Platz-Christensen JJ, Pahlson C. Mobiluncus and clue cells as predictors of PID after first-trimester abortion. Acta obstetricia et gynecologica Scandinavica. 1989;68:217–20. doi: 10.3109/00016348909020992. [DOI] [PubMed] [Google Scholar]
- 83.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]