Abstract
Adaptation to first-order (luminance defined) motion produces not only a motion aftereffect but also a position aftereffect, in which a target pattern’s perceived location is shifted opposite the direction of adaptation. These aftereffects can occur passively (when the direction of motion adaptation cannot be detected) and remotely (when the target is not at the site of adaptation). Although second-order (contrast defined) motion produces these aftereffects, it is unclear whether they can occur passively or remotely. To address these questions, we conducted two experiments. In the first, we used crowding to remove a local adapter’s second-order motion from awareness and still found a significant position aftereffect. In the second experiment, we found that the direction of motion in one region of a crowded array could produce a position aftereffect in an unadapted, spatially separated region of the crowded array. The results suggest that second-order motion influences perceived position over a large spatial range even without awareness.
Keywords: crowding, motion, awareness, second order, contrast defined, localization, mislocalization, motion aftereffect, MAE, global motion, attention
Introduction
Adaptation to first-order (luminance defined) motion produces not only a motion aftereffect (MAE) but also a shift in the perceived position of test patterns (McGraw, Whitaker, Skillen, & Chung, 2002; Nishida & Johnston, 1999; Snowden, 1998; Whitaker, McGraw, & Pearson, 1999; for a review, see Whitney, 2002). Both of these effects (the MAE and the position shift that accompanies it) occur locally and passively; following adaptation to a first-order moving pattern that is crowded out of awareness, there is a perceived MAE (Aghdaee, 2005; Aghdaee & Zandvakili, 2005) and a shift in the apparent location of the test pattern (Whitney, 2005, 2006). The perceived position of an object, therefore, depends on passively coded first-order motion.
The traditional first-order static MAE and the induced position shift that accompanies the MAE are usually thought of as local phenomena (Masland, 1969; Mather, Verstraten, & Anstis, 1998; Nishida & Johnston, 1999; Snowden, 1998; Whitaker et al., 1999; Wohlgemuth, 1911). However, there are a few more recent examples of global and remote first-order MAEs (Anstis & Reinhardt-Rutland, 1976; Ashida, Susami, & Osaka, 1996; Bex, Metha, & Makous, 1999; Bonnet & Pouthas, 1972; Culham, Verstraten, Ashida, & Cavanagh, 2000; Price, Greenwood, & Ibbotson, 2004; Snowden & Milne, 1997; Swanston & Wade, 1992; von Grünau & Dube, 1992; Wade, Spillmann, & Swanston, 1996; Weisstein, Maguire, & Berbaum, 1977; Zaidi & Sachtler, 1991). For example, first-order motion adaptation in one location can bias perceived motion (von Grünau & Dube, 1992) and perceived position (Whitney & Cavanagh, 2003) in unadapted locations, even several degrees from the adapted site. Common among most of these demonstrations is that visual motion is perceived in the unadapted as well as the adapted regions. Therefore, in addition to bottom–up motion processes, top–down mechanisms such as attentive tracking (Culham et al., 2000) or global feature-based attention (Boynton, Ciaramitaro, & Arman, 2006) may be involved in these remote first-order MAEs.
Similar to first-order motion, adaptation to second-order (contrast defined) motion produces a local MAE on dynamic test patterns (Ledgeway & Smith, 1994; McCarthy, 1993; Nishida, Ashida, & Sato, 1994; Nishida & Sato, 1995; van der Smagt, Verstraten, Vaessen, van Londen, & van de Grind, 1999; cf. Cropper & Hammett, 1997). Further, based on previous results using contrast-defined second-order motion (Bressler & Whitney, 2006), it is reasonable to expect that second-order motion adaptation produces not only an MAE but also a perceived shift in the position of the test pattern. This has yet to be tested though.
It also remains unclear whether the second-order MAE or the associated position shift occur at unadapted, remote locations. That is, is there spatial pooling or integration such that the second-order MAE at one location depends on the state of motion adaptation in broader regions of the visual field? If so, does this remote MAE depend on being aware of the motion that is present in the visual field? Because second-order motion is detected by both passive (Benton & Johnston, 2001; Benton, Johnston, & McOwan, 2000; Derrington, Allen, & Delicato, 2004; Lu & Sperling, 1995, 2001b; Nishida, Ledgeway, & Edwards, 1997; Whitney & Bressler, 2007) and active or attentive mechanisms (Allen & Ledgeway, 2003; Ashida, Seiffert, & Osaka, 2001; Del Vecchio, von Grünau, & Faubert, 2001; Ho, 1998; Lu, Liu, & Dosher, 2000; Seiffert & Cavanagh, 1998, 1999), it remains unclear what role awareness plays in coding second-order motion and the potential position shifts that follow motion adaptation.
We are therefore left with two outstanding questions. First, does second-order motion adaptation produce a perceived position shift, and does this position shift require awareness of motion direction? Second, is this effect entirely local or does it depend on spatial pooling of motion signals, and is this pooling dependent on the awareness of motion adaptation?
To address these questions, we conducted two experiments. In the first experiment, we used a crowded array of drifting second-order stimuli and measured the perceived shift in the positions of subsequently presented test patterns. We found that there was a significant shift in the perceived positions of the test patterns despite the inability of subjects to detect the direction of motion in the adaptation stimulus. In a second experiment, we found that the global direction of motion within the crowded array of second-order stimuli could influence the perceived positions of subsequently viewed second-order patterns located in a nonadapted location. This remote aftereffect of second-order motion adaptation occurred even when subjects could not discriminate the global direction of motion. The results suggest that second-order motion influences perceived position over a large spatial range even without awareness of the motion.
Methods
General methods
Three experienced psychophysical observers with normal or corrected-to-normal visual acuity participated in the experiments, one of whom was naïve as to the purpose of the study. Stimuli were presented on a Sony Multiscan G520 CRT (1,024 × 768, 120 Hz) with Vision Shell (visionshell.com) on an Apple G4 with OS9. The CRT was linearized with a gamma correction, and physical linearity was confirmed using a Minolta CS100A photometer. All experiments were conducted in a dark, soundproof room, with subjects seated and immobilized with a chin rest that was 57 cm from the monitor.
The experiment consisted of three stages. First, we measured each subject’s equiluminance point for the contrast-defined moving patterns. Second, we measured the perceived position shift following adaptation to a local second-order motion pattern under crowding and no-crowding conditions. Third, we measured the position shift following adaptation to global second-order motion patterns.
Equiluminance of contrast-defined motion
The measure of each subject’s equiluminance value was ascertained prior to the main experiment. Equiluminance values were determined using a minimum motion technique similar to that used by previous authors (Anstis & Cavanagh, 1983; Ledgeway & Smith, 1994; Lu & Sperling, 2001a; Nishida, Edwards, & Sato, 1997; Seiffert & Cavanagh, 1998). Subjects fixated on a point (0.35° diameter) 15.4° to the right of a circular aperture (center to center) that was 4.5° in diameter. Inside the aperture, a vertically oriented, luminance-defined sine wave (0.82 cycles/degree) was flickered in counterphase at 6 Hz. A second, contrast-defined (i.e., second order) grating was also presented in counterphase at 6 Hz. The contrast-defined grating consisted of a random-dot pattern (each dot was 0.12° × 0.12°) that is modulated by a contrast-defined sinusoid (also 0.82 cycles/degree). The luminance- and contrast-defined gratings were interleaved in a four-frame sequence such that each sine wave was shifted by 90° (i.e., quadrature phase, luminance grating presented in even frames, contrast-modulated grating presented in odd frames). The stimulus was presented for 1 s in each trial.
When the contrast-defined grating visibly deviated from equiluminance, the subject perceived unidirectional motion. If the contrast-defined grating was perfectly equiluminant, or if only the luminance-defined grating was visible, no directional motion was perceived. Whereas the minimum and maximum luminance values of the second-order patterns were kept constant in each trial, the luminance midpoint of the contrast-defined grating was randomly varied (one of nine values ranging from 29.8 to 39.8 cd/m2, centered on physical equiluminance). The equiluminance point was measured for second-order patterns with three different contrast modulation depths (0.31, 0.58, and 0.9). Subjects were asked to judge the direction of motion in the aperture (leftward/rightward). The point of equiluminance for each subject was the luminance midpoint (the relative luminance between the contrast-modulated segments of the second-order grating) that produced a percept of ambiguous motion or subjective equality (Anstis & Cavanagh, 1983; Ledgeway & Smith, 1994; Nishida, Edwards, et al., 1997; Seiffert & Cavanagh, 1998). Each subject participated in two sessions of 90 trials for each contrast, for a total of 180 trials per contrast. Data were averaged across sessions, and the proportion of rightward (leftward) responses was fitted to the logistic function:
| (1) |
where a indicates the slope of the function and b estimates the contrast modulation depth required to produce an ambiguous motion percept (the equiluminance point; Finney, 1971; McKee, Klein, & Teller, 1985). The equiluminance points for subjects T.H., S.A., and D.B. were 35.1, 34, and 34.5 cd/m2, respectively, for all contrasts. These values were used in the following experiments to ensure that the patterns were psychophysically equiluminant.
Second-order Gabor stimuli
The principal stimulus in all of the experiments below is what we call a second-order Gabor. Each second-order Gabor consisted of a dynamic random-dot pattern (0.12° square dots), modulated by a sinusoidal contrast-defined carrier (0.82 cycles/degree) that drifted either leftward or rightward, and a Gaussian contrast-modulated envelope (to blur the edges). The sinusoidal contrast-defined carrier (visible in Figure 1 as random dots alternating with gray bars) was the only moving component. The Gaussian contrast-modulated envelope was always static. The dynamic random-dot background was updated every frame and produced a broadband noise (e.g., TV snow). The Gabor in Figure 1 has a sinusoidal carrier with exaggerated contrast to reproduce in print; the actual Gabors had contrast modulation depths of 0.31, 0.58, or 0.90. Formally, each Gabor is described as
Figure 1.
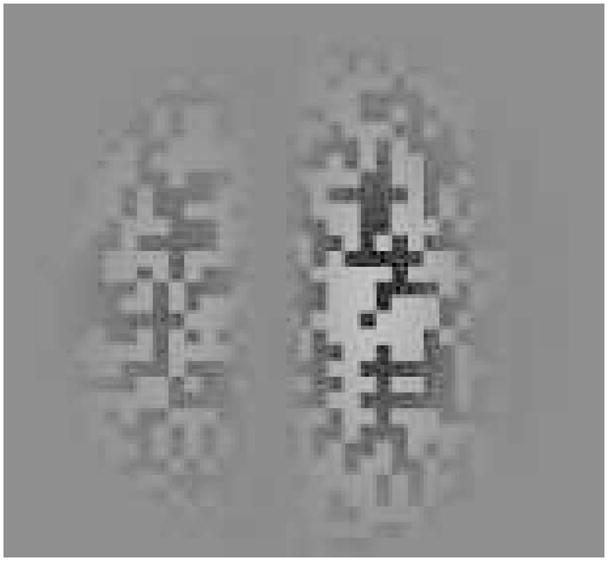
A second-order Gabor, used in each of the experiments. Each second-order Gabor was a dynamic random-dot background (only one frame is shown here) with a contrast-modulated sine wave and a Gaussian contrast envelope. The contrast modulation depth (the contrast between the dark and light random dots in the background) is exaggerated here; contrast was lower in the experiments.
| (2) |
where L(x,y,t) is the luminance at any point at time t, E is the physical equiluminance (mean luminance), V is the subject’s equiluminance value (see the Methods section), R(x,y,t) is a random-dot array in time, D is the depth of the contrast modulation (the incremental contrast above and below E), SF is the spatial frequency of the carrier (pixels per cycle), TF is the temporal frequency of the carrier (cycles per frame), r is the distance of (x,y) from the center of the Gabor, σ is the standard deviation of the static Gaussian contrast envelope, and M is the maximum radius of the Gaussian envelope. Because the monitor’s refresh was 120 Hz, t is defined in 8-ms increments.
Subjects also participated in an experiment to measure their direction discrimination threshold. Subjects fixated a dot and reported the direction of motion in a peripherally presented (15.3° center-to-center distance) second-order Gabor with a drifting contrast-modulated carrier. The location of the fixation point and second-order Gabor was identical to the positions of the stimuli in the main experiments below. The Gabor drifted in a random direction on each trial, and the Gabor’s contrast modulation depth was set to one of five values in each trial, ranging from 0.1 to 0.7 Michelson contrast. Each subject participated in a minimum of 400 trials (80 trials at each contrast, 0.8 s per trial, self-paced). Contrast depth required to discriminate direction of motion with 83% accuracy was measured by fitting a logistic psychometric function:
| (3) |
where a is the slope and b is the 83% threshold. Subject D.B.’s threshold was 0.23, S.A.’s threshold was 0.25, and T.H.’s threshold was 0.32, close to the lowest contrast tested in the main experiment.
In an additional control experiment (Whitney & Bressler, 2007), we confirmed that the second-order Gabors defined here contained no luminance artifacts. To do this, we measured whether there was any cross-adaptation between the second-order stimuli above and first-order (luminance defined) stimuli. In the experiment, subjects adapted to either a drifting second-order Gabor (described above) with a 0.67 contrast or a low-contrast (0.42%) drifting first-order Gabor (luminance sine wave with a Gaussian envelope). In the test period, we presented a first-order Gabor (0.04 contrast, drift-balanced motion) or a second-order Gabor (0.73 contrast). Consistent with Nishida, Ledgeway, et al. (1997), we found that there was no cross-adaptation between the first- and second-order Gabors. The second-order drifting patterns only generated an MAE on the second-order test patterns and not on the flickering low-contrast first-order patterns. Likewise, the low-contrast first-order pattern only generated an MAE on the first-order test pattern and not on the second-order test pattern. The lack of cross-adaptation confirms that our second-order Gabors were free of luminance artifacts.
Experiment 1
Experiment 1 tested the position shift following adaptation to second-order motion in a crowded array of second-order Gabors (Figure 2).
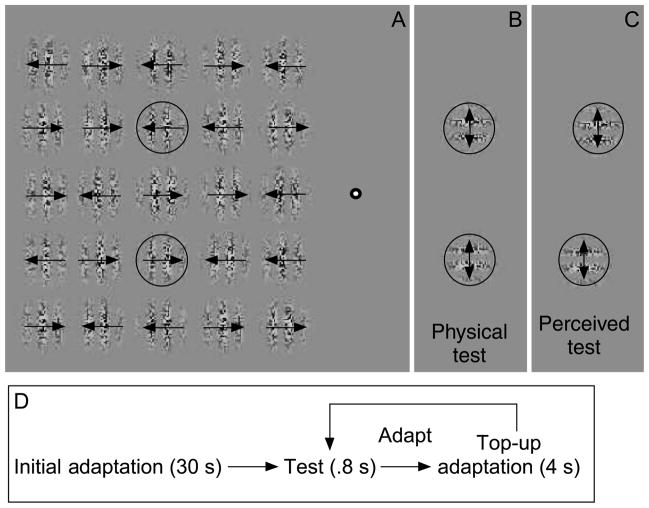
Figure 2.
The stimulus used in the first experiment. (A) An array of 25 second-order Gabor patches was presented during adaptation. Subjects fixated on the bull’s-eye to the right of the Gabors throughout each session. The motion of each crowder Gabor was randomly determined in the initial and top–up adaptation periods, whereas the motion of the adapting Gabors (circled) remained fixed throughout an experimental session. (B) The test period consisted of Gabors containing orthogonal drift-balanced motion. These Gabors were superimposed on the location of the adapting Gabors (circled). (C) After adapting to the stimuli, test Gabors appear to be shifted in the direction opposite that of the prior motion adaptation. The circles were not visible in the actual display. (D) The procedure in each session.
The stimuli consisted of an array of 25 second-order drifting Gabors (Figure 2). The diameter of each Gabor was 4.1° visual angle, and the vertical and horizontal separation between each Gabor was 4.4° (center to center). The direction of motion was randomly determined (leftward or rightward) on each trial, for each Gabor, except for the two central adapting Gabors that are circled in Figure 2 (the circles were not visible in the experiment). In these two adapting Gabors, the direction of motion was constant throughout each session. Subjects fixated on the bull’s-eye located 5.9° to the right of the rightmost row of Gabors (center-to-center distance) and 15.3° to the top target Gabor (center-to-center distance). The contrast modulation depth of each Gabor in the array (including the adapting Gabors) was either 0.31, 0.58, or 0.90, tested separately in different sessions. During the test period (Figure 2B), two drift-balanced second-order Gabors that had carriers orthogonal to the direction of motion adaptation were presented (McGraw et al., 2002; Whitney, 2005). These were identical to the adaptation Gabors except that they contained motion in both directions and were rotated 90°. The test Gabors were superimposed on the locations of the central adapting Gabors (circled in Figure 2). The contrast modulation depth of the test Gabors was fixed across all sessions at 0.58. This contrast was sufficient for all subjects to accurately guess the direction of motion under no-crowding conditions at least 85% of the time.
Each experimental session consisted of an initial adaptation period (30 s, Figures 2A and 2D) followed by repeated test (0.8 s, Figure 2B) and top–up adaptation periods (4 s) that were interleaved. The motion of the adapting Gabors remained constant throughout the session, but the motion of each crowder Gabor was randomly determined in each top–up adaptation period. Subjects made two judgments on each trial. First, using a binary choice method (left/right), they guessed the direction of motion in the central adapting Gabor at the top of the display (the top, circled Gabor in Figure 2A). They could make this response at any time during the adaptation period. This measured the effectiveness of crowding. Second, during the test period, subjects reported the relative alignment of the two test Gabors (top test Gabor, left or right of the bottom test Gabor; Figure 2B). The test Gabors could be aligned or misaligned by one of seven values that range from −0.45° to +0.45°. The proportion of responses that were opposite the direction of prior motion adaptation were plotted as a function of the misalignment between the Gabors, and a logistic psychometric function was fit to the data (identical to that described in the General methods section). The point of subjective equality (PSE) was measured as the physical misalignment between the test Gabors required to null any illusory misalignment observed (e.g., Figure 2C). Significance of the PSE was estimated using maximum likelihood procedures: the ratio likelihood test (ratio of −2 log likelihood with vs. without the PSE intercept, which is χ2 distributed with one degree of freedom). In each experimental session, there were seven possible misalignments between the test Gabors and six trials for each of these conditions. In separate sessions, the contrast modulation depth of the adapting Gabors was manipulated (0.31, 0.58, or 0.90 Michelson contrast). Subjects participated in a minimum of eight sessions for each of the three contrast modulation depths, for a total of at least 1,008 trials. α (significance) levels were Bonferroni corrected for multiple comparisons; because there were three tested contrast modulation depths, the α level was set at .0125.
In addition to measuring the position shift following motion adaptation under crowding conditions, the same procedure was used to measure the position shift following adaptation to motion without crowding. In these sessions, the procedure and stimuli were identical with the exception that there were no crowding Gabors present (only the adapting Gabors, circled in Figure 2, were presented). The test Gabors were identical to those described above.
Experiment 2
The second experiment measured the effect of global motion adaptation on the perceived location of the local test Gabors. The stimuli were very similar to those in the first experiment. The stimuli consisted of an array of 30 second-order drifting Gabors. The two central crowded adapting Gabors (circled in Figure 3A) did not contain directional motion. Rather, they contained drift-balanced motion in both directions; two contrast-modulated sine waves simultaneously drifted in opposite directions. There was, therefore, no net directional motion in these central crowded Gabors.
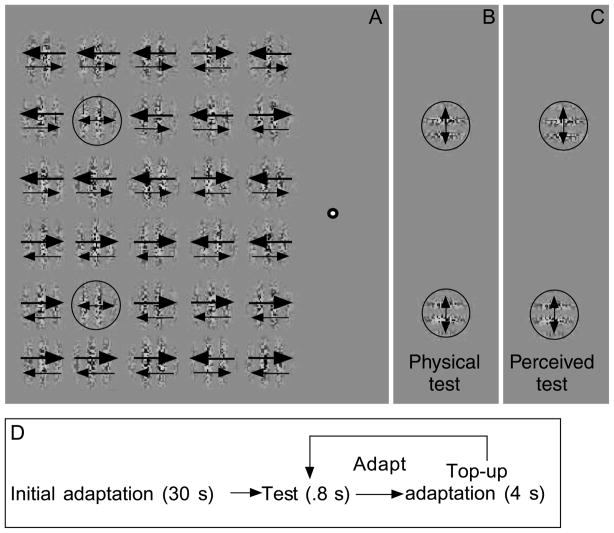
Figure 3.
Stimuli used in the second experiment. (A) An array of 30 Gabor patches was presented during adaptation. The two adapted locations (circled) contained drift-balanced motion (no net directional motion). The eight Gabors immediately surrounding the adapted locations also contained drifting sine wave contrast modulations in both directions simultaneously, but the contrast of one direction was increased (to 0.79 Michelson contrast), whereas the contrast of the oppositely drifting component was reduced (to 0.37 Michelson contrast). The size of the arrows in the Gabors surrounding the adapted locations therefore indicate an imbalanced motion signal. The Gabors in the two columns closest to the fixation point contained randomized directions on each trial to increase the effectiveness of crowding and to prevent discrimination of global motion direction in the top or bottom halves of the display. (B) The test Gabors were drift-balanced orthogonal versions of the adapted Gabors in Panel A. (C) Perceived misalignment between the test Gabors following motion adaptation in the surrounding region. The circles were not visible in the actual experiment.
The surrounding crowder Gabors were divided into halves: a top half (surrounding the top adaptor Gabor) and a bottom half (surrounding the bottom adaptor Gabor). Each surrounding crowder Gabor contained two oppositely drifting sine wave contrast modulations. Unlike the first experiment, therefore, the crowder Gabors contained motion in both directions. The motion in each direction was not drift balanced (indicated by the arrows in Figure 3). Rather, in each session, the modulation depth of one of the contrast-defined sine waves was increased to 79% (Michelson contrast), whereas the contrast depth of the oppositely drifting sine wave was reduced to 37%; the term “net motion” will refer to the fact that one direction had a higher contrast modulation depth. In the top half of the array of Gabors, all of the crowder Gabors contained net motion in one direction (i.e., 79% motion in one direction and 37% motion in the opposite direction). In the bottom half of the array, the reverse was true. The top and bottom halves of the Gabor array therefore contained net motion in opposite directions. The Gabors in the two vertical columns closest to the fixation point also contained two oppositely drifting sine wave contrast modulations (one at 79% Michelson contrast and the other at 37%) to ensure that crowding was still effective. However, the direction of the dominant contrast carrier’s motion for each of these Gabors was determined randomly on each trial. Ideally, this stimulus was intended to produce crowding such that the local central adaptor Gabor (circled in Figure 3A) and the net motion direction in both the top and bottom halves of the display would be crowded and, therefore, indiscriminable. The Gabors in the test period were identical to those in the first experiment, except that they were separated vertically by 12.12°, and the range of the physical horizontal offsets was from −1.13° to +1.13°. The adapting and test Gabors occupied overlapping locations (indicated by the circled regions in Figure 3).
The procedure and task were identical to that in the first experiment except that only one contrast modulation depth was tested (0.58 Michelson contrast). Subjects always fixated at the bull’s-eye. During each trial, subjects made two judgments. First, they judged the net direction of motion in the top half of the Gabor array (which contained either net motion leftward or net motion rightward). The two nonnaïve subjects knew that the top and bottom motion fields were opposing, and thus, if these subjects perceived either the top or the bottom, they could correctly report the net direction of motion in the top half of the display. The naïve subject was asked to judge only the direction of motion in the bottom half of the array. Second, subjects judged the relative position of the two test Gabors, just as in the first experiment. Subjects participated in eight sessions of 42 trials for a total of 336 trials.
Results
Experiment 1
The purpose of Experiment 1 was to determine the position aftereffect following adaptation to local motion that was crowded out of awareness. Subjects performed a vernier alignment task (Figure 2B) after adapting to a dense array of drifting Gabor patterns (Figure 2A). When the test patterns were physically aligned, they appeared to be misaligned opposite the direction of motion adaptation (Figure 2C). In a control experiment, subjects performed the same task following adaptation to a noncrowded display (only the two adapting Gabors were presented).
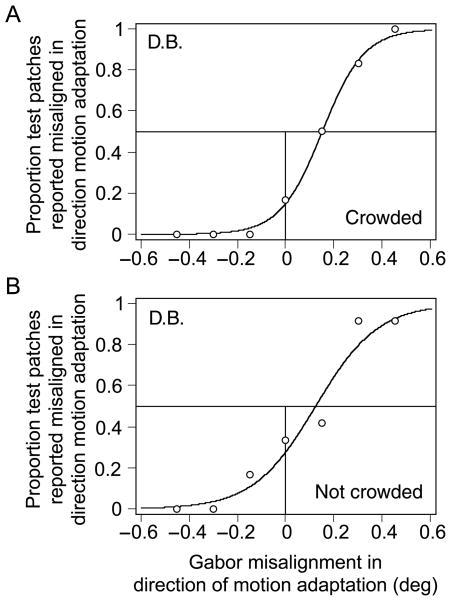
Figure 4A shows a representative psychometric function for subject D.B. following crowded adaptation, when the contrast modulation depth of the adapting Gabors was 0.31. The PSE was 0.15°, indicating that for the test patterns to be perceived as aligned, they had to be physically misaligned horizontally by 0.15°. Although this distance is quite small, the PSE for this subject was significant, χ2(1) = 48.6, p < .001.
Figure 4.
Representative psychometric functions in the first experiment for one subject. (A) Representative results for the crowded condition, in which the adaptation Gabors had a contrast modulation depth of 0.31. Positive values along the abscissa indicate that the Gabors were misaligned in the direction of adaptation (positive values therefore indicate the presence of an aftereffect). The subject’s PSE for this condition was 0.15°, χ2(1) = 48.6, p < .001. (B) Representative results for the noncrowded condition, in which the adaptation Gabors are presented, but no other crowders were present. The format is the same as in Panel A. The contrast modulation depth of the adapting Gabors was 0.58. The PSE for this condition was 0.123°, χ2(1) = 8.02, p < .01.
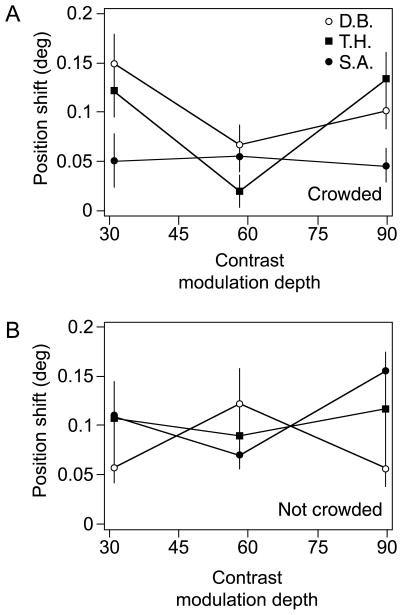
Figure 5 shows the results for three subjects across several adaptation contrasts. There was an overall effect of motion adaptation on perceived position, F(1,6) = 40.3, p < .01. Within each subject, each condition yielded a significant position shift aftereffect, except for subject T. H.’s 0.58 contrast condition; the next least significant effect was subject D.B.’s 0.58 contrast condition, χ2(1) = 8.81, p < .01. The lowest tested Michelson contrast was 0.31, which was close to the motion discrimination threshold for subject T.H. and slightly higher than the threshold for subjects D.B. and S.A. (to discriminate motion direction with 83% accuracy, subjects T.H., D.B., and S.A. required contrast modulation depths of 0.32, 0.23, and 0.25, respectively).
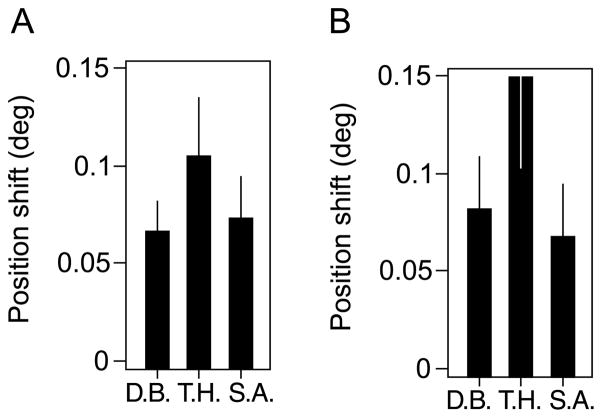
Figure 5.
Results of the first experiment. (A) The graph shows the second-order motion-induced position shift at three contrast modulation depths when crowders were present. Subject T.H. did not show a significant PSE at the midcontrast (0.58), but all other PSEs were significantly above zero; the least of the significant PSEs was for D.B., at the 0.58 contrast condition, χ2(1) = 8.81, p < .01. (B) The second-order motion-induced position shift without crowding. Each PSE was significant; the least significant of which was for subject T.H. in the 0.31 contrast condition, χ2(1) = 6.87, p < .01. There was no significant difference in the PSEs with and without crowding, F(1,2) = 0.37, p = .61. Averaged across both conditions, the perceived misalignment was ~0.10°, which is just above threshold vernier discrimination at the tested eccentricity and separation (Klein & Levi, 1987; Levi & Klein, 1990; Levi, Klein, & Aitsebaomo, 1985). Error bars denote ±SE of nonlinear regression.
Figure 5B shows the results for all three subjects for the control experiment in which there was no crowding (only the two adaptation Gabors were presented during the adaptation period). There was a significant overall effect of motion adaptation on perceived position, F(1,6) = 81.3, p < .01. Within each subject, each contrast condition yielded a significant position aftereffect (lowest significance was for subject T.H. in the 0.31 contrast condition), χ2(1) = 6.87, p < .01. The position aftereffect was slightly larger without crowding, but the difference (0.015°) was not significant, F(1,2) = 0.37, p = .61 (repeated measures ANOVA).
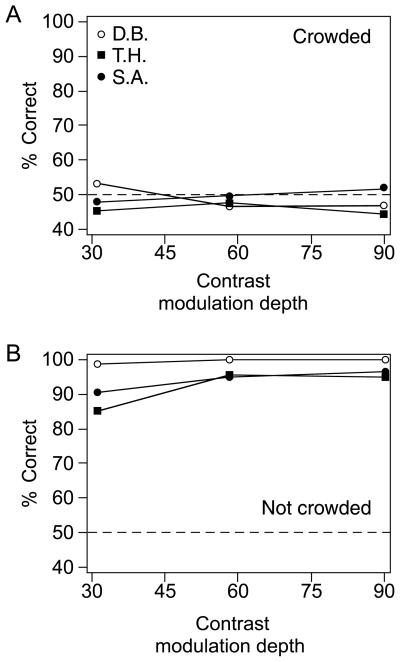
Figure 6 shows the effectiveness of crowding, measured as the accuracy with which subjects guessed motion direction in the adaptation Gabors with crowding (Figure 6A) and without crowding (Figure 6B). Subjects performed at chance during crowding runs, but in the no-crowding condition, they were able to reliably detect direction of adaptation at all tested contrasts (>85% accuracy in all conditions). These results are consistent with previous experiments on crowding of second-order motion (Whitney & Bressler, 2007). The accuracy with which subjects guessed motion direction under crowding remained constant as a function of Gabor contrast modulation depth, indicating that the effectiveness of crowding did not vary with contrast. Although subjects were at chance level in detecting the direction of adaptation in the crowding condition, the position after-effect remained significant, demonstrating that awareness of motion adaptation is not necessary for the second-order position aftereffect. The results in Figure 6 could be replotted as a multiple of contrast threshold required to discriminate motion direction. However, the aftereffect is invariant with respect to both physical and threshold contrast, F(2,4) = 2.0, p = .25.
Figure 6.
Effectiveness of crowding in the first experiment. (A) In the first experiment, during crowded adaptation, subjects were instructed to report the direction of motion in the adapting Gabor (top, circled Gabor in Figure 2). Response accuracy is plotted as a function of adapting Gabor contrast. All three subjects were at chance in all conditions. (B) Response accuracy when there was no crowding (only the adapting Gabors were present). All subjects responded with more than 85% accuracy.
Experiment 2
The purpose of Experiment 2 was to measure whether there are local aftereffects that result from global adaptation to the surrounding crowders in crowded displays. The stimulus in this experiment is shown in Figure 3. Because the two central adapting Gabors contained no directional motion during adaptation, there should have been no motion adaptation and, therefore, no motion-induced position shift in the test period. However, Figure 7 shows that there was a perceived shift in the position of the test Gabors following adaptation to global motion, even in the absence of any net local motion at the tested location. The direction of the position shift in the test period was opposite that of the net directional motion presented in the surrounding crowder Gabors during adaptation (i.e., consistent with a remote MAE). Therefore, global motion adaptation in crowded displays influences local judgments, even at a nonadapted site.
Figure 7.
Results of the second experiment. (A) Following adaptation to the stimulus in Figure 3, in which the crowded adapting Gabors had no directional motion, but the surrounding Gabors had a net directional motion signal, the test Gabors appeared shifted in position. The PSE was 0.067° for D.B., χ2(1) = 7.17, p < .01, 0.11° for T.H., χ2(1) = 8.0, p < .01, and 0.092 for S.A., χ2(1) = 8.9, p < .01. The results indicate that there was a significant, directionally specific aftereffect at a location that was not adapted to directional motion; the motion in the surrounding region therefore generated a remote aftereffect. (B) The position aftereffect for missed trials. The same analysis was conducted as in Panel A but only for those trials in which the subject incorrectly reported the direction of motion adaptation. There was no significant difference between correct and incorrect trials, t(2) = 1.1, p > .05. Error bars denote ±SE of nonlinear regression.
One might worry that subjects were aware of the net directional motion in the surrounding crowder Gabors. Subjects, however, were not able to guess the direction of motion in either (top or bottom) half of the crowded array (accuracy was 51% for D.B., 50% for T.H., and 48% for S. A.). Although there were several coherently drifting Gabors with net directional motion, the two nearest columns of Gabors contained random motion directions. This was evidently sufficient to eliminate each subject’s ability to discriminate global motion direction.
There are cases in which unambiguous motion in one region can disambiguate or bias motion judgments in other locations (e.g., motion capture, induced motion, and other similar effects; Fang & He, 2004; Murakami & Shimojo, 1993; Ramachandran & Anstis, 1983; Reinhardt-Rutland, 1988). If subjects were able to detect the net motion in the surrounding crowder Gabors, this could have influenced either the percept of the target Gabor’s motion or the stage at which adaptation occurred. To test whether this was possible, we separately analyzed trials in which subjects correctly or incorrectly guessed the direction of motion in the array (Figure 7B). There was no difference in the aftereffect, suggesting that even trials that are perceived incorrectly produce a comparable position aftereffect.
General discussion
The results of the first experiment showed that exposure to second-order motion under crowded conditions can produce shifts in the perceived positions of test objects. The aftereffect was approximately constant, with and without crowding, and crowding was highly effective at preventing discrimination of motion direction at all tested contrasts. The results of the second experiment demonstrated that the net global motion that is present in a crowded display can produce a local aftereffect even at an unadapted location. This result suggests that the perceived position of second-order patterns depends on second-order motion collected over a relatively large region and that this spatial summation does not depend on awareness of motion direction.
The goal of the first experiment was to determine if perceiving the position of a second-order pattern requires top–down attention or awareness. It is known that second-order motion contributes to perceived position (Bressler & Whitney, 2006), and there are models of motion detection based on feature or attentive tracking that could mediate motion perception (Cavanagh, 1992). The results here, however, revealed that even in the absence of awareness, local second-order motion adaptation shifted the perceived position of test objects. That subjects could not attentively track the motion in the adapting stimulus yet perceived an aftereffect suggests that updating the positions of contrast-defined patterns can occur passively.
Although most studies of the MAE have focused on local adaptation and aftereffects, there are a number of experiments that show that global or remote MAEs also exist (Anstis & Reinhardt-Rutland, 1976; Ashida et al., 1996; Bex et al., 1999; Bonnet & Pouthas, 1972; Culham et al., 2000; Price et al., 2004; Snowden & Milne, 1997; Swanston & Wade, 1992; von Grünau & Dube, 1992; Wade et al., 1996; Weisstein et al., 1977; Zaidi & Sachtler, 1991). Two broad types of explanation have emerged for these global and remote MAEs. One class of explanation is based largely on passive properties of motion detectors, such as summation or integration fields, and receptive field properties of motion-sensitive units in MT and MST or specialized relative motion detectors (Ashida et al., 1996; Bex et al., 1999; Snowden & Milne, 1997). The second explanation revolves around top–down processes such as feature-based attention or attentive tracking (Boynton et al., 2006; Culham et al., 2000). It has remained an open question whether attention, or top–down modulation, was required to produce a global or remote MAE (or the associated position shift that accompanies the MAE).
Experiment 2 addressed this issue. The results showed that second-order motion adaptation in one region of the visual field influenced the perceived positions of stimuli in nonadapted locations, even when the direction of motion adaptation was not perceived (crowded out of awareness). This could be the result of global motion detectors, spatial pooling of motion signals, or specialized second-order motion detectors, but the mechanism involved must not rely on feature- or location-based attentional processes. Rather, there must be passive second-order motion processes that operate over relatively large distances.
If there is spatial summation of second-order motion information that influences local judgments of position, then it is somewhat surprising that we found no difference in the aftereffect with and without crowding in Experiment 1. One possible reason for this is that the contrast imbalance (directional motion) was consistent and strong over a large region of space only in the second experiment. If there is a contrast threshold (or nonlinearity) for the global adaptation, this could explain why the surrounding crowders influenced the local after effect only in Experiment 2, whereas there was little effect of the surrounding crowders in Experiment 1.
A more likely explanation is that there are separate local and global aftereffects tapped by Experiments 1 and 2, respectively. In the first experiment, the local directional adaptation was only slightly mitigated by the surrounding nondirectional motion. In the second experiment, there was no local directional motion, only directional surround motion, which was sufficient to trump the ambiguous local signal. Because local adaptation more strongly influences the local aftereffect, this would explain the pattern of results in both experiments and would also explain why the global effect in Experiment 2 was slightly weaker, overall, than the effect in Experiment 1. Future experiments that pit local against global adaptation could directly address this issue. In any case, the results of the two experiments suggest that there can be an aftereffect that arises from either local or global processes and that crowding does not prevent these effects.
Recently, there has been a debate over the origin of aftereffects following crowded adaptation (Blake, Tadin, Sobel, Raissian, & Chong, 2006; He, Cavanagh, & Intriligator, 1996; Intriligator & Cavanagh, 2001). Our results suggest that following crowded adaptation (of any stimulus), the measured local aftereffect could be the result of both local adaptation and global (or remote) adaptation from surrounding patterns. This is important because it could cause an underestimation (or overestimation) of the magnitude of the local aftereffect in previous studies (Aghdaee, 2005; Aghdaee & Zandvakili, 2005; Blake et al., 2006; He et al., 1996; He, Cavanagh, & Intriligator, 1997; Montaser-Kouhsari & Rajimehr, 2005; Rajimehr, 2004a, 2004b; Rajimehr, Montaser-Kouhsari, & Afraz, 2003; Rajimehr, Vaziri-Pashkam, Afraz, & Esteky, 2004; Whitney, 2005, 2006). In motion crowding studies, for example, the random directions of motion in the crowder stimuli usually average to zero but could serve as a global dynamic noise pattern, effectively raising thresholds across all directions and reducing the measured aftereffect. The same logic (or other types of global adaptation effects) could hold for other features, such as orientation or form. Further, the extent of spatial pooling or global adaptation might be contrast dependent or even stimulus specific (e.g., only within the second-order motion pathway in these experiments). For example, for first-order stimuli, the extent of spatial pooling increases with decreasing stimulus contrast (Sceniak, Ringach, Hawken, & Shapley, 1999; Tadin, Lappin, Gilroy, & Blake, 2003). Global first-order adaptation effects might therefore manifest themselves disproportionately at lower stimulus contrasts.
All of these considerations serve as a reminder that although crowding may result in spatial pooling of signals (Experiment 2) as well as in an effective contrast suppression of the crowded target (Blake et al., 2006), this does not mean that the phenomenon of crowding—the inability to scrutinize, characterize, or identify a crowded item—is caused by pooling or contrast suppression. Indeed, there are potentially interacting influences of local and global adaptation. Under the right circumstances, these might be additive, but under most circumstances, any global adaptation could serve to reduce the locally measured aftereffect. Therefore, measuring aftereffect strength as a ratio of the aftereffect with versus without crowding is questionable; it could lead to a pattern of results that appears to be a contrast gain modulation but is really an interaction of distinct and potentially contrast-dependent local and global adaptations. Future studies on crowded adaptation should consider using alternative control stimuli to measure the baseline local adaptation effect.
Conclusions
Contrast-defined second-order motion that is crowded out of awareness can produce a significant shift in the perceived positions of test stimuli, indicating that the positions of second-order patterns are coded passively, without a necessary top–down attentional component. Further, the results suggest that adapting to a crowded display can produce independent and possibly even conflicting aftereffects from both local and remote locations within the crowded display.
Acknowledgments
Thanks to two anonymous reviewers for helpful comments.
Footnotes
Commercial relationships: none.
Contributor Information
Thomas D. Harp, Department of Psychology, University of California, Davis, CA, USA
David W. Bressler, Center for Mind and Brain, University of California, Davis, CA, USA
David Whitney, Center for Mind and Brain, and Department of Psychology, University of California, Davis, CA, USA.
References
- Aghdaee SM. Adaptation to spiral motion in crowding condition. Perception. 2005;34:155–162. doi: 10.1068/p5298. [DOI] [PubMed] [Google Scholar]
- Aghdaee SM, Zandvakili A. Adaptation to spiral motion: Global but not local motion detectors are modulated by attention. Vision Research. 2005;45:1099–1105. doi: 10.1016/j.visres.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Allen HA, Ledgeway T. Attentional modulation of threshold sensitivity to first-order motion and second-order motion patterns. Vision Research. 2003;43:2927–2936. doi: 10.1016/j.visres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Anstis SM, Cavanagh P. A minimum motion technique for judging equiluminance. In: Mollon J, Sharpe RT, editors. Color vision: Physiology and psychophysics. London: Academic Press; 1983. pp. 155–166. [Google Scholar]
- Anstis SM, Reinhardt-Rutland AH. Interactions between motion aftereffects and induced movement. Vision Research. 1976;16:1391–1394. doi: 10.1016/0042-6989(76)90157-7. [DOI] [PubMed] [Google Scholar]
- Ashida H, Seiffert AE, Osaka N. Inefficient visual search for second-order motion. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2001;18:2255–2266. doi: 10.1364/josaa.18.002255. [DOI] [PubMed] [Google Scholar]
- Ashida H, Susami K, Osaka N. Re-evaluation of local adaptation for motion aftereffect. Perception. 1996;25:1065–1072. doi: 10.1068/p251065. [DOI] [PubMed] [Google Scholar]
- Benton CP, Johnston A. A new approach to analysing texture-defined motion. Proceedings of the Royal Society B: Biological Sciences. 2001;268:2435–2443. doi: 10.1098/rspb.2001.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CP, Johnston A, McOwan PW. Computational modelling of interleaved first- and second-order motion sequences and translating 3f+4f beat patterns. Vision Research. 2000;40:1135–1142. doi: 10.1016/s0042-6989(00)00026-2. [DOI] [PubMed] [Google Scholar]
- Bex PJ, Metha AB, Makous W. Enhanced motion aftereffect for complex motions. Vision Research. 1999;39:2229–2238. doi: 10.1016/s0042-6989(98)00329-0. [DOI] [PubMed] [Google Scholar]
- Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet C, Pouthas V. Apparent size and duration of a movement after-effect. Quarterly Journal of Experimental Psychology. 1972;24:275–281. doi: 10.1080/14640747208400281. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Ciaramitaro VM, Arman AC. Effects of feature-based attention on the motion aftereffect at remote locations. Vision Research. 2006;46:2968–2976. doi: 10.1016/j.visres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bressler DW, Whitney D. Second-order motion shifts perceived position. Vision Research. 2006;46:1120–1128. doi: 10.1016/j.visres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Attention-based motion perception. Science. 1992;257:1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- Cropper S, Hammett S. Adaptation to motion of a second-order pattern: The motion aftereffect is not a general result. Vision Research. 1997;37:2247–2259. doi: 10.1016/s0042-6989(97)00028-x. [DOI] [PubMed] [Google Scholar]
- Culham JC, Verstraten FA, Ashida H, Cavanagh P. Independent aftereffects of attention and motion. Neuron. 2000;28:607–615. doi: 10.1016/s0896-6273(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Del Vecchio AS, von Grünau MW, Faubert J. Attentional selection of first- and second-order motion stimuli [Abstract] Journal of Vision. 2001;1(3):88, 88. doi: 10.1167/1.3.88. http://journalofvision.org/1/3/88/ [DOI] [Google Scholar]
- Derrington AM, Allen HA, Delicato LS. Visual mechanisms of motion analysis and motion perception. Annual Review of Psychology. 2004;55:181–205. doi: 10.1146/annurev.psych.55.090902.141903. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Stabilized structure from motion without disparity induces disparity adaptation. Current Biology. 2004;14:247–251. doi: 10.1016/j.cub.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. 3. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution. Trends in Cognitive Sciences. 1997;1:115–121. doi: 10.1016/S1364-6613(97)89058-4. [DOI] [PubMed] [Google Scholar]
- Ho CE. Letter recognition reveals pathways of second-order and third-order motion. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:400–404. doi: 10.1073/pnas.95.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intriligator J, Cavanagh P. The spatial resolution of visual attention. Cognitive Psychology. 2001;43:171–216. doi: 10.1006/cogp.2001.0755. [DOI] [PubMed] [Google Scholar]
- Klein SA, Levi DM. Position sense of the peripheral retina. Journal of the Optical Society of America A, Optics and Image Science. 1987;4:1543–1553. doi: 10.1364/josaa.4.001543. [DOI] [PubMed] [Google Scholar]
- Ledgeway T, Smith AT. Evidence for separate motion-detecting mechanisms for first- and second-order motion in human vision. Vision Research. 1994;34:2727–2740. doi: 10.1016/0042-6989(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. The role of separation and eccentricity in encoding position. Vision Research. 1990;30:557–585. doi: 10.1016/0042-6989(90)90068-v. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Research. 1985;25:963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Liu CQ, Dosher BA. Attention mechanisms for multi-location first- and second-order motion perception. Vision Research. 2000;40:173–186. doi: 10.1016/s0042-6989(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. The functional architecture of human visual motion perception. Vision Research. 1995;35:2697–2722. doi: 10.1016/0042-6989(95)00025-u. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Sensitive calibration and measurement procedures based on the amplification principle in motion perception. Vision Research. 2001a;41:2355–2374. doi: 10.1016/s0042-6989(01)00106-7. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Three-systems theory of human visual motion perception: Review and update. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2001b;18:2331–2370. doi: 10.1364/josaa.18.002331. [DOI] [PubMed] [Google Scholar]
- Masland RH. Visual motion perception: Experimental modification. Science. 1969;165:819–821. doi: 10.1126/science.165.3895.819. [DOI] [PubMed] [Google Scholar]
- Mather G, Verstraten F, Anstis S. The motion aftereffect: A modern perspective. Cambridge, MA: MIT Press; 1998. [DOI] [PubMed] [Google Scholar]
- McCarthy JE. Directional adaptation effects with contrast modulated stimuli. Vision Research. 1993;33:2653–2662. doi: 10.1016/0042-6989(93)90225-l. [DOI] [PubMed] [Google Scholar]
- McGraw PV, Whitaker D, Skillen J, Chung ST. Motion adaptation distorts perceived visual position. Current Biology. 2002;12:2042–2047. doi: 10.1016/s0960-9822(02)01354-4. [DOI] [PubMed] [Google Scholar]
- McKee SP, Klein SA, Teller DY. Statistical properties of forced-choice psychometric functions: Implications of probit analysis. Perception & Psychophysics. 1985;37:286–298. doi: 10.3758/bf03211350. [DOI] [PubMed] [Google Scholar]
- Montaser-Kouhsari L, Rajimehr R. Subliminal attentional modulation in crowding condition. Vision Research. 2005;45:839–844. doi: 10.1016/j.visres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Murakami I, Shimojo S. Motion capture changes to induced motion at higher luminance contrasts, smaller eccentricities, and larger inducer sizes. Vision Research. 1993;33:2091–2107. doi: 10.1016/0042-6989(93)90008-k. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ashida H, Sato T. Complete interocular transfer of motion aftereffect with flickering test. Vision Research. 1994;34:2707–2716. doi: 10.1016/0042-6989(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Nishida S, Edwards M, Sato T. Simultaneous motion contrast across space: Involvement of second-order motion? Vision Research. 1997;37:199–214. doi: 10.1016/s0042-6989(96)00112-5. [DOI] [PubMed] [Google Scholar]
- Nishida S, Johnston A. Influence of motion signals on the perceived position of spatial pattern. Nature. 1999;397:610–612. doi: 10.1038/17600. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ledgeway T, Edwards M. Dual multiple-scale processing for motion in the human visual system. Vision Research. 1997;37:2685–2698. doi: 10.1016/s0042-6989(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Nishida S, Sato T. Motion aftereffect with flickering test patterns reveals higher stages of motion processing. Vision Research. 1995;35:477–490. doi: 10.1016/0042-6989(94)00144-b. [DOI] [PubMed] [Google Scholar]
- Price NS, Greenwood JA, Ibbotson MR. Tuning properties of radial phantom motion after-effects. Vision Research. 2004;44:1971–1979. doi: 10.1016/j.visres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Rajimehr R. Static motion aftereffect does not modulate positional representations in early visual areas. Cognitive Brain Research. 2004a;20:323–327. doi: 10.1016/j.cogbrainres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Rajimehr R. Unconscious orientation processing. Neuron. 2004b;41:663–673. doi: 10.1016/s0896-6273(04)00041-8. [DOI] [PubMed] [Google Scholar]
- Rajimehr R, Montaser-Kouhsari L, Afraz SR. Orientation-selective adaptation to crowded illusory lines. Perception. 2003;32:1199–1210. doi: 10.1068/p5076. [DOI] [PubMed] [Google Scholar]
- Rajimehr R, Vaziri-Pashkam M, Afraz SR, Esteky H. Adaptation to apparent motion in crowding condition. Vision Research. 2004;44:925–931. doi: 10.1016/j.visres.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Anstis SM. Displacement thresholds for coherent apparent motion in random dot-patterns. Vision Research. 1983;23:1719–1724. doi: 10.1016/0042-6989(83)90188-8. [DOI] [PubMed] [Google Scholar]
- Reinhardt-Rutland AH. Induced movement in the visual modality: An overview. Psychological Bulletin. 1988;103:57–71. doi: 10.1037/0033-2909.103.1.57. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast’s effect on spatial summation by macaque V1 neurons. Nature Neuroscience. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position displacement, not velocity, is the cue to motion detection of second-order stimuli. Vision Research. 1998;38:3569–3582. doi: 10.1016/s0042-6989(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position-based motion perception for color and texture stimuli: Effects of contrast and speed. Vision Research. 1999;39:4172–4185. doi: 10.1016/s0042-6989(99)00129-7. [DOI] [PubMed] [Google Scholar]
- Snowden RJ. Shifts in perceived position following adaptation to visual motion. Current Biology. 1998;8:1343–1345. doi: 10.1016/s0960-9822(07)00567-2. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Milne AB. Phantom motion after effects—Evidence of detectors for the analysis of optic flow. Current Biology. 1997;7:717–722. doi: 10.1016/s0960-9822(06)00329-0. [DOI] [PubMed] [Google Scholar]
- Swanston MT, Wade NJ. Motion over the retina and the motion aftereffect. Perception. 1992;21:569–582. doi: 10.1068/p210569. [DOI] [PubMed] [Google Scholar]
- Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature. 2003;424:312–315. doi: 10.1038/nature01800. [DOI] [PubMed] [Google Scholar]
- van der Smagt MJ, Verstraten FA, Vaessen EB, van Londen T, van de Grind WA. Motion aftereffect of combined first-order and second-order motion. Perception. 1999;28:1397–1411. doi: 10.1068/p2899. [DOI] [PubMed] [Google Scholar]
- von Grünau M, Dube? S. Comparing local and remote motion aftereffects. Spatial Vision. 1992;6:303–314. doi: 10.1163/156856892x00145. [DOI] [PubMed] [Google Scholar]
- Wade NJ, Spillmann L, Swanston MT. Visual motion aftereffects: Critical adaptation and test conditions. Vision Research. 1996;36:2167–2175. doi: 10.1016/0042-6989(95)00266-9. [DOI] [PubMed] [Google Scholar]
- Weisstein N, Maguire W, Berbaum K. A phantom-motion aftereffect. Science. 1977;198:955–958. doi: 10.1126/science.929181. [DOI] [PubMed] [Google Scholar]
- Whitaker D, McGraw PV, Pearson S. Non-veridical size perception of expanding and contracting objects. Vision Research. 1999;39:2999–3009. doi: 10.1016/s0042-6989(99)00010-3. [DOI] [PubMed] [Google Scholar]
- Whitney D. The influence of visual motion on perceived position. Trends in Cognitive Sciences. 2002;6:211–216. doi: 10.1016/s1364-6613(02)01887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D. Motion distorts perceived position without awareness of motion. Current Biology. 2005;15:R324–R326. doi: 10.1016/j.cub.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D. Contributions of bottom–up and top–down motion processes to perceived position. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:1380–1397. doi: 10.1037/0096-1523.32.6.1380. [DOI] [PubMed] [Google Scholar]
- Whitney D, Bressler DW. Second order motion without awareness: Passive adaptation to second-order motion produces a motion aftereffect. Vision Research. 2007;47:567–579. doi: 10.1016/j.visres.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D, Cavanagh P. Motion adaptation shifts apparent position without the motion after-effect. Perception & Psychophysics. 2003;65:1011–1018. doi: 10.3758/bf03194830. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth A. On the after-effect of seen movement. British Journal of Psychology Monograph Supplements. 1911;1:1–117. [Google Scholar]
- Zaidi Q, Sachtler WL. Motion adaptation from surrounding stimuli. Perception. 1991;20:703–714. doi: 10.1068/p200703. [DOI] [PubMed] [Google Scholar]