Abstract
Entrainment has been studied in a variety of contexts including music perception, dance, verbal communication and motor coordination more generally. Here we seek to provide a unifying framework that incorporates the key aspects of entrainment as it has been studied in these varying domains. We propose that there are a number of types of entrainment that build upon pre-existing adaptations that allow organisms to perceive stimuli as rhythmic, to produce periodic stimuli, and to integrate the two using sensory feedback. We suggest that social entrainment is a special case of spatiotemporal coordination where the rhythmic signal originates from another individual. We use this framework to understand the function and evolutionary basis for coordinated rhythmic movement and to explore questions about the nature of entrainment in music and dance. The framework of entrainment presented here has a number of implications for the vocal learning hypothesis and other proposals for the evolution of coordinated rhythmic behavior across an array of species.
What a man danced, that was his tribe, his social custom, his religion. –Havelock Ellis We dedicate this paper to the memory of our mentor Margo Wilson.
Introduction
There is perhaps no stronger behavior to unite humans than coordinated rhythmic movement. This is possible because humans have the capacity to become entrained with one another or with an external stimulus. Entrainment can facilitate complex and interdependent coordination that can be seen in human activities including sport and play, verbal communication and emotional expression, and in the epitome of rhythmic entrainment: music and dance (McNeill, 1995). These kinds of activities are powerful, perhaps because they indicate a mutual perceptual and social experience originating from the sharing in time and space of embodied rhythm. Entrainment also provides a mechanism for physical mirroring, as in gestural mimicking in communication or in dance, and for metaphorical mirroring, as in empathy (Richardson, Dale & Kirkham, 2007; Calvo-Merino, Glaser, Grèzes, Passingham & Haggard, 2005; Gallese, Keysers & Rizzolatti, 2004). Such spatiotemporal mirroring is also manifest in the bond between parent and infant, as has been observed in coordinated gestures and vocalizations (Condon & Sander, 1974; Trevarthen, 1979; Thelen, 1981; Jaffe et al., 2001; Trehub, 2003; Feldman, 2007; Papoušek, 2007). It seems then that entrainment is rooted in physical, emotional and social aspects of the human experience, aspects that are quitessentially captured in music and dance.
We argue here that the tremendous and flexible human capacities to produce music and dance are rooted in the capacity for entrainment to rhythmic signals in the physical and social environment. The ability to entrain to an external auditory pulse or complex rhythm enables multiple individuals to time-lock their behavior by integrating information across different sensory modalities (e.g., Drake, Penel & Bigand, 2000; Keller, 2008; Repp & Keller, 2008; Merker et al. 2009). Sensorimotor synchronization of this kind can even occur when there is a high degree of rhythmic complexity and ambiguity in music (e.g., an isochronous beat can be inferred and expressed at various metrical levels and from syncopated rhythms (London, 2004; Repp, 2005; Toiviainen & Snyder, 2003; Patel, 2006). If we can better understand the origins and nature of the capacity for entrainment, it may provide important insights into the unparalleled human capacities to produce and appreciate rhythmic sounds and movements in highly coordinated ways, as we see in music and dance.
Here, we provide an ecological account of how a basic entrainment system might be structured, and how the capacity for rhythmic entrainment might have been built upon simple components involved in sensory detection and production. This general model can extend to various types of coordinated rhythmic movement, including non-pulse-based exchanges such as verbal and gestural communication, and pulse-based entrainment such as synchronization in music and dance. We also describe how complex forms of entrainment might result when individuals can transmit and share rhythmic information in a social context. By this account, a form of social entrainment can emerge from the simple capacity for responsiveness to rhythmic signals in the environment. We aim to 1) provide a common framework across domains for defining entrainment as coordinated rhythmic movement, 2) describe several categories of rhythmic entrainment and their perceptual and somatic foundations, while considering their potential functional origins, and 3) discuss the implications of this approach for discussions on the evolution of music and dance ability in humans. We believe the present framework is compatible with current views on the evolution of rhythmic entrainment, and lays a theoretical foundation that can facilitate a deeper understanding of the nature of entrainment and its role in social behavior and complex interactions.
1. Existing approaches to entrainment and behavioral synchrony
Entrainment and behavioral synchrony have been studied in a variety of disciplines, with experimental work and models informing our understanding of the processes underlying these capacities. Foremost, dynamic systems theory provides an empirical account of entrainment that illustrates the importance of the integration of information across multiple sensory modalities in various contexts. This approach accounts for human performance in synchronizing movements to complex musical rhythms in which a regular beat is perceived despite variations in executed and expressive timing, and in which a metrical structure is inferred from hierarchically-organized accent cues (Jones, 1976; Large & Jones, 1999; Large, 2000). The dynamic systems approach describes musical rhythmic entrainment as an active, self-sustained, periodic oscillation at multiple time scales, enabling the listener to use predictive timing to maintain a stable multi-periodicity pattern and synchronize movements at the tactus or other metrical levels (Large, 2000). This can facilitate the production of coordinated movements including gestures, vocalizations, and movements resulting in other sound production (Large, 2000).
Empirical work shows that the capacities to perceive and synchronize to a beat are possible across different sensory modalities, though music synchronization might capitalize on a network integrating auditory, motor, and vestibular systems (Large & Jones, 1999; Janata & Grafton, 2003; Patel, Iversen & Chen, 2005; Zatorre, Chen & Penhune, 2007; Phillips-Silver & Trainor, 2008; Trainor, Gao, Lei, Lehtovaara & Harris, 2009). For example, auditory-motor interactions are well documented in music, especially in beat-based rhythm processing (Patel, Iversen, Chen & Repp, 2005; Chen, Penhune & Zatorre, 2008; Zatorre, Chen & Penhune, 2007; Grahn & Rowe, 2009) as supported by evidence from both healthy adults and patient populations such as basal ganglia patients (i.e., Parkinson's disease) (Gran & Brett, 2007; Grahn & Brett, 2009).
In addition to auditory and motor systems, the vestibular system has been proposed as a potential contributor to entrainment in the context of beat perception in adults and infants (Todd, 1993; Todd & Cody, 2000; Phillips-Silver & Trainor, 2008; Trainor, Gao, Lei, Lehtovaara & Harris, 2009; Trainor, 2007; see also Todd & Lee, 2007), the latter of whom may rely especially on passive movement cues rather than motor planning such as that received as parents rock infants in their arms while singing a lullaby. More generally, the vestibular system is known to be sensitive to sound and vibration in various animal species, and may transmit movement sensation without overt movement (Todd, 2001; Todd & Cousins, 2007). Thus, vestibular information may participate along with auditory and motor information in beat-based and other forms of entrainment.
Some of the abilities for cross-modal integration of timing and “beat” information in music begin to emerge early in infancy. For example, the transfer of information about body movement to the auditory encoding of a musical beat develops in the first months of life (Phillips-Silver & Trainor, 2005), and the ability to detect cross-modal asynchrony (versus synchrony) such as in dance may begin in infancy as well (Hannon, 2008). The developmental literature stresses the importance of interpersonal synchrony in many parent-infant interactions, including not only music but also non-musical vocal, gestural, and gaze exchanges (Condon & Sander, 1974; Trevarthen, 1979; Jaffe et al., 2001; Crown, Feldsteing, Jasnow, Beebe & Jaffe, 2002; Feldman, 2007). Thus, the general foundations for interpersonal synchrony are established early in life, through various types of social communication behaviors.
In adults, synchronization with acoustic signals has been studied extensively. Rhythmic musical behavior is based upon the ability to process and respond to a regular pulse (Arom, 1991; Fraise, 1982). Synchronization of motor output to sensory input requires the ability to adjust one's own motor output based on incoming rhythmic information. Repp (2005) reviewed the range of work examining feedback-based error-correction in sensorimotor synchronization (SMS) in coordinated tapping studies. He found that error correction involves at least two distinct processes: period correction and phase correction. Repp proposed that phase-related adjustments involve unconscious (dorsal) processes involved with controlling action, and period adjustments involve conscious (ventral) processes related to perception and planning. Similarly, Merker (2009) proposed that two different modes of error-correction are implemented by distinct neural systems. Bispham (2006) argued that the capacity for period correction is particularly well suited for musical entrainment (i.e., synchronizing to a perceived isochronous pulse) whereas phase correction can apply in various ways across domains when the updating of expectancies is needed for behavioral synchronization.
The musical pulse can range from the basic isochronous pulse (as in a metronome) to myriad forms of complex rhythmic and metrical structures. This introduces one of the distinguishing features of human rhythmic entrainment: it typically occurs in much more complex contexts than a mere isochronous pulse. The quintessential examples of human entrainment occur in musical contexts involving rhythms that are metrically organized (Patel et al., 2009). We can hear the work song of a group of individuals whose productivity relies on their coordination of effortful movement. We can imagine the swaying, the clapping hands and the wailing voices of a gospel choir singing praises in spiritual unity. Or, we can envision a couple in a dance of courtship, perhaps one body leading the other in musical movement. The rhythms may be simple or syncopated, the voices and bodies may be in phase or antiphase, but the multi-level pulse enables the subtle interplay of sound and gesture in entrainment.
A second distinguishing feature of human entrainment is the ability to entrain to a wide range of tempi (limits of tempo range from the shortest physically reproducible interval (around 100ms) to the longest interval that can be retained as a memory trace (around 2s), though the optimal tempo range is around 300–800ms for humans (Fraisse, 1982; see also London, 2004). A third distinguishing feature is its crossmodal nature, illustrated by the types of rhythmic movement that are aimed at synchronization but not sound production, such as in dance. These three features have been described as reflecting a special instance of rhythmic entrainment called musical beat perception and synchronization, and some have suggested that these abilities may be unique to animals equipped with vocal learning capacities (see discussion) (Patel, 2006; Patel et al., 2009; Schachner et al., 2009).
2. Towards a unified theory of entrainment
We propose the operational definition of entrainment that we believe captures the key components as defined and studied across domains: spatiotemporal coordination resulting from rhythmic responsiveness to a perceived rhythmic signal. In this section, we seek to extend our operationalization of entrainment by describing foundational components of the capacity for entrainment across domains, and providing a non-mathematical, qualitative model that is consistent with the existing work on entrainment. We believe this can provide a coherent framework for reasoning about entrainment across a variety of domains.
In our view, entrainment or coordinated rhythmic movement is based on the capacities for perception and production of rhythmic information, and the real-time transmission of this information between sensory and motor systems. Here, “coordinated rhythmic movement” refers to an organism's coordinated response to a signal, but does not specify the source or modality of the signal—coordinated rhythmic movement in response to both social and other (e.g., auditory) signals can be incorporated within this framework. Moving rhythmically in space and time with others is a complex computational task requiring well-tuned sensory systems, the capacity to produce rhythmic output, and the ability to adjust that rhythmic output based on sensory input.
Coordinated rhythmic movement, and thus entrainment, can occur in the context of signals that vary in their nature of periodicity or predictability, from musical rhythm to conversation and language processing, nonverbal communication, gesture, play and sharing of attentional gaze (e.g., Crown et al., 2002; Cummins, 2009; Kotz, Swartze & Schmidt-Kassow, 2009; Schmidt-Kassow & Kotz, 2008; Wilson & Wilson, 2005). For example, it has been proposed that conversational turn-taking is governed by the entrainment of mandibular oscillations during vocal production—interlocutors can predict upcoming pauses in a speech signal and begin conversational turns with precise timing (Wilson & Wilson, 2005). Elements common to all of these behaviors, as described in the following section, include rhythmic signal detection and response, and integration of these via entrainment.
Todd, Lee & O'Boyle (2002) proposed a sensorimotor theory of `beat induction' that consists of the three components of entrainment: rhythmic detection (by the auditory system), rhythmic action (by the musculoskeletal system), and the integration of input and output (by a parieto-cerebellar feedback system). We adopt a similar approach, describing how these three components can lead to several distinct types of rhythmic entrainment. However, while Todd, Lee and O'Boyle (2002) focused on the mathematical formalization of a single entrainment system, our approach aims to provide an account of entrainment encompassing both function at the level of a single entrainment system as well as the interaction that such systems can have with one another. This provides a more general approach to entrainment that can incorporate complex phenomena that are likely to involve feedback between or among rhythmic entrainment systems, such as in music, dance, and other forms of social entrainment.
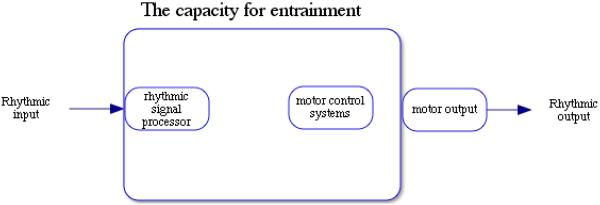
We propose that the capacity for the simplest form of entrainment emerges when three critical building blocks are in place, all of which can be favored by natural selection. These building blocks are 1) the ability to detect rhythmic signals in the environment, 2) the ability to produce rhythmic signals (including rhythmic signals that are byproducts of other functions, such as locomotion or feeding behavior), and 3) the ability to integrate sensory information and motor production which enables adjustment of motor output based on rhythmic input (see Figure 1). Once organisms evolve each of these abilities, the capacity for entrainment can emerge. In the example of the duet courtship dance, the dancers may hear the pulse of a song (detection), move their feet (production), and change tempo depending on the match between their movement and the music (integration between sensory and motor systems). In addition to entrainment with the music (external pulse), social entrainment between partners may also emerge, where partners detect rhythmic signals via auditory, tactile/vestibular or visual cues coming from their partner and adjust their own movements accordingly. If these processes occur accurately, the partners produce a dance that is congruent with the rhythms of the music and one another's movement.
Figure 1.
The ability to connect rhythmic information processing with motor control systems provides the necessary cognitive foundation to allow individuals to produce rhythmic output that is entrained with an external rhythm.
2.1. Building block 1: The ability to detect rhythmic signals
The world is filled with information that is perceived as rhythmic in nature, from familiar acoustic patterns such as lapping waves on the shore or approaching footsteps, to ecological rhythms such as changes in light level and nutrient availability that occur because of tides, weather changes, day/night cycles, and even predator-prey dynamics (Lokta, 1925; Volterra 1926). Organisms synchronize their own biological rhythms to these and other cyclical processes (Foster & Kreitzman 2005), presumably because the capacity to `tune in' to such ecological rhythms and respond systematically provides evolutionary advantages to those organisms capable of doing so. In the ethnomusicological entrainment literature, the ability of organisms to respond to ecological and environmental rhythms has been described as asymmetrical entrainment (Clayton, Sager, & Will, 2004) because in these cases individuals respond in such a way as to entrain to rhythmic information in the environment, while the environmental rhythms do not change. Organisms would not necessarily be selected to detect and process all kinds of rhythms; rather, these abilities should be specific to the adaptive demands of the given recurrent environmental context.
In addition to the ability to detect to ecological rhythms such as those suggested above, there are many additional adaptive domains in which the ability to detect rhythmic information could have provided an evolutionary advantage, including predation/hunting, predator avoidance, and detection of conspecifics. Again, in each of these domains, the sensory information available is likely to be dependent on the particular ecological circumstances that a species inhabits. For example, a predatory bird species with a highly developed visual system for detecting prey that have certain patterns of rhythmic movement might be more attuned to visual rhythmic input. Alternatively, a land-dwelling predator in a dense habitat that uses auditory information (e.g., the sound of footsteps) might be more attuned to auditory rhythmic input.
2.2. Building block 2: The production of rhythmic information
In this framework, the ability to produce rhythmic output is a prerequisite for entrainment. Rhythm production can be a byproduct of any number of adaptations, but can also constitute a design feature of an adaptive system. For example, physical locomotion, respiration and feeding behavior can endogenously generate rhythmic information (Potts, Rybak & Paton, 2005), and the production of this rhythmic information will have differential impacts on survival and reproduction depending on the ecological context. Any organism capable of locomotion generates auditory, visual, and vestibular rhythmic cues as a result of movement in space. This is the case when a bacterium rotates its flagella, when a bird flaps its wings, or when a land animal moves its limbs. Indeed, rhythmic cues of other organisms' movement are often highly relevant to the survival and reproduction of many animals, including both predators (who need to seek out moving prey) and prey (who need to detect and avoid moving predators). There is likely strong selection pressure favoring the capacity to detect and process rhythmic information that is a byproduct of other organisms' locomotion, respiration and feeding.
There are also situations in which it may have benefited an organism to produce rhythmic information that could be detected by others. For example, many organisms produce rhythmic information to attract individuals of the opposite sex (e.g., crickets, fireflies, frogs, and katydids) (Greenfield, 1994b). Rhythmic production for the purposes of enabling individuals to find one another may have also been selected for in situations in which social aggregation provides a benefit (Allee, 1949). In general, the ability to produce rhythmic information is likely to have been selected in situations in which social proximity provided a benefit, whether for the purposes of mating, evading predators, or other mutually beneficial behaviors.
2.3. Building block 3: the integration of sensory and motor production systems
The capacity for entrainment necessitates connectivity between systems designed for the detection of rhythmic information and those capable of producing rhythmic information. This enables an organism to produce rhythmic information in response to rhythmic sensory input. Such a process can occur at the neuronal level (Sumbre, Muto, Baier & Poo, 2008) and the capacity of individuals to perform both period and phase correction has been well established (e.g., Repp, 2005), providing the final building block for entrainment: the capacity to systematically alter rhythmic production based on the perception of rhythmic signals from the environment.
3. Extending entrainment to social domains: Complexity emerges from feedback
We have defined entrainment as spatiotemporal coordination resulting from rhythmic responsiveness, and we have described how the capacity for entrainment is likely to be built upon the abilities to connect the detection and production of rhythmic information. In this section we expand upon these ideas, extending the general framework of entrainment into the social domain. We propose that social entrainment is a special case of entrainment in which the rhythmic signal originates from another individual. In social entrainment, mechanisms capable of sensing rhythmic sensory stimuli are activated by cues from the social environment in ways that generate coordinated behavior and can potentially lead to complex feedback loops between rhythmic information production and detection.
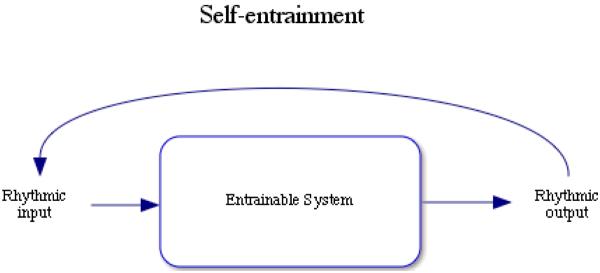
3.1. Self-Entrainment
When organisms have the ability to produce rhythmic output in response to rhythmic sensory input, the capacity for self-entrainment can arise (Figure 2). Self-entrainment can be defined as the rhythmic responsiveness to self-generated rhythmic signals. This kind of process might be at work in certain aspects of solitary vocal production and motor behavior. Self-entrainment in rhythmic music production has been observed in musicians (Clayton, Sager & Will, 2004), and this might involve similar feedback as do respiration and locomotion, capacities that could provide a biomechanical basis for nuances of timing in music production and perception (Friberg & Sundberg, 1999; Styns, van Noorden, Moelants & Leman, 2007; Todd, Cousins & Lee, 2007).
Figure 2.
Self-entrainment occurs when an individual capable of entrainment uses self-generated rhythmic output as a signal for rhythmic input systems.
3.2. Social Entrainment
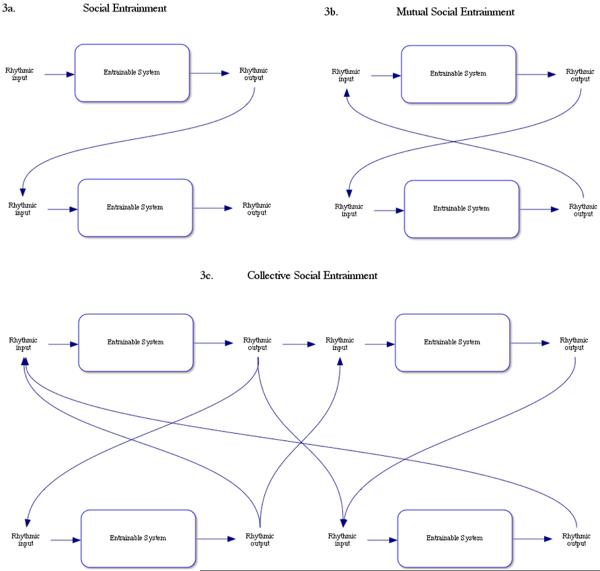
Social entrainment is a special category of entrainment characterized by responsiveness to rhythmic information generated by others. This can occur whenever rhythmic output from one organism becomes input for another organism's rhythmic signal processing system. Cases of social entrainment (also called interpersonal entrainment) have been observed in many species (as reviewed in Clayton, Sager & Will, 2004). Social entrainment, in addition to self-entrainment, clearly plays a large role in music or dance ensembles, and empirical data are consistent with the idea that inter-agent synchrony can drive musical timing and expression (Keller, 2008; Keller & Repp, 2008). In addition to simple social entrainment (Figure 3a), we delineate two additional subtypes of social entrainment: mutual social entrainment and collective social entrainment.
Figure 3.
a) Social entrainment occurs when an individual capable of entrainment uses rhythmic output from another social entity as the incoming signal for rhythmic processing systems. b) Mutual social entrainment results between two individuals capable of entrainment when each uses output from the partner as input to their rhythmic processor. c) Collective social entrainment occurs when a group of individuals who are capable of entrainment use rhythmic output from one another as input for their rhythm processing systems.
In mutual social entrainment (Figure 3b), rhythmic responsiveness during bidirectional information processing between two individuals results in a `loop' where the output of each individual's rhythmic production provides input for the other's rhythmic processing system. This kind of process may be at work in duetting behavior (Merker, 2000) or conversational turn taking (Wilson & Wilson, 2005). We also speculate that mutual social entrainment will enhance partner-based social dance, where partners `tune in' to features of one another's rhythmic movement and adjust their own motor behavior to bring their movement into synchrony with their partner's. This may create a more physically coordinated dance, a more enjoyable experience for the dancers, and a more cohesive visual experience for observers. There may be an important evolutionary advantage to mutual social entrainment as it could enable the coordination of movement during fitness-relevant activities such as shelter construction, resource extraction and inter-tribal warfare.
Collective social entrainment (Figure 3c) is similar to mutual entrainment, but rather than being characterized by a `loop' between two rhythmically responsive individuals, it is characterized by a network of input/output connections among individuals in a group. We speculate that collective social entrainment may play a similar role in groups to that which mutual entrainment serves in dyads. For example, collective social entrainment may underlie certain forms of collaborative music production and dance, including both structured (e.g., formal performances) and unstructured forms (e.g., `jamming', celebratory dance, the work song and the gospel choir). Collective social entrainment may facilitate groupwise interactions more generally, such as those underlying group conversation, or the coordination of any work that requires individuals to be sensitive to one another's movement and effort. Thus, processes such as these could play an important role in promoting higher-level functions in a variety of domains including, but not limited to, music and dance.
Systems of social entrainment may be facilitated by the ability to detect intentional signals produced by an agent. In many species, entraining behaviors in any number of modalities will often incorporate the deliberate acts of conspecifics. In the case of human social entrainment, the recognition of a rhythmic signal as the ostensive act of a potential coordination partner might play a role in the activation of the system. Intentional acts often have particular attributes that allow for their detection. In the case of entrainment in human music production, many sources of information can be used to infer intentionality such as eye gaze, vocal signals, particular body postures, and explicit instruction (e.g., Seddon, 2005).
Moreover, a shared social context is likely necessary for entrainment processes to operate properly and efficiently, given its importance in a variety of coordinated activities in humans such as learning language and music (e.g., Tomasello & Carpenter, 2007). For example, Kirschner and Tomasello (2009) found that children as young as 2.5 years were able to show some synchronizion in drumming to an isochronous beat, but only when that drumming occurred with a live partner in a social context (as opposed to a machine, or a recording). While intentionality might play an important role in social entrainment in humans and other species, we do not consider it a necessary feature. Rather, our view is that social entrainment may be facilitated by intentionality, but can also occur among systems that lack the cognitive complexity to process intentional components of signals. Nonetheless, the capacity to processes intentional signals may enable more complex and novel collective (i.e., entrainment-based) behaviors than are possible without this ability. For example, shared intentionality is likely to be necessary for activities such as collective dance, music production and other open-ended creative activities. Moreover, shared intentionality is likely important for other activities that require coordinated movement in novel or uncertain situations including hunting, warfare, and collective work (e.g., shelter construction) in new environments.
The capacities for mutual social entrainment and collective social entrainment echo ideas captured in Bluedorn's (2002) description of entrainment as “the process in which the rhythms displayed by two or more phenomena become synchronized, with one of the rhythms often being more powerful or dominant and capturing the rhythm of the other. This does not mean, however, that the rhythmic patterns will coincide or overlap exactly; instead, it means the patterns will maintain a consistent relationship with each other” (p. 148). Large & Jones (1999) and Large (2000) discussed similar ideas in their theory of oscillator resonance and coupling, adopting a mathematical approach. These quantitative approaches are compatible with the qualitative model of social entrainment presented here to analyze the processes by which bodies can become coordinated through social entrainment.
4. Social entrainment across species: the example of synchronous chorusing
The primary goal of this paper is to provide a framework for entrainment that can be applied across different domains, including entrainment in a variety of species and through multiple processes. This includes complex coordinated activities involving rhythmic information such as music and dance, which may have their origins in simpler types of social entrainment. Synchronous chorusing is an excellent example of social entrainment in other species, and as such, may be a sort of precursor to more complex types of social entrainment, such as those underlying music and dance in humans. The existence of social entrainment across species, as evidenced by synchronous chorusing, also illustrates the generality of this framework.
Collective social entrainment can be observed in displays of synchronous chorusing in a variety of species including crickets and frogs (Greenfield, 1994a, 1994b; Backwell, Jennions, Passmore & Christy, 1998). In these displays, actions of stridulation (such as legor wing-rubbing, or chirping) produce acoustic signals timed to occur with precise inter-individual simultaneity (Greenfield, 1994a; Merker, 2000). Such behavior is distinct from endogenously generated rhythmic movements, such those that occur in respiratory and locomotor rhythms (Potts, Rybak & Paton, 2005). These endogenous rhythms are inherently periodic but do not rely on an external signal for their rhythmicity (Merker, 2000). In collective social entrainment characteristic of synchronous chorusing, external acoustic signals serve to enable the precise synchronization of two or more individuals, and this is achieved through the use of predictive timing (Fraise, 1982; Merker, 2000). Entrainment can also occur in response to rhythmic visual signals, as in the synchronized `chorusing' observable in firefly bioluminescense (Buck & Buck, 1978). This capacity for collective social entrainment in synchronous chousing can serve as a mechanism of signal amplification, and enable more effective signaling in the context of sexual advertisement (Greenfield, 1994a; Merker, 2000).
Synchronous chorusing demonstrates the critical role of entrainment in the production of coordinated sound and movement, and we speculate that similar entrainment processes are likely to be at work in the human ability to produce music and dance. However, we also recognize that the products of social entrainment in humans are arguably much more complex (e.g., drum circles and tangos) than those that result from synchronous chorusing. This might be due to a higher degree of flexibility and integration of sound and motor production in humans compared with other species, or perhaps (as suggested earlier) it is the capacity for shared intentionality which leads to more open-ended and creative products of social entrainment.
5. Discussion
The evolutionary origins of music and dance abilities have been a source of recent discussion. The framework presented here might help to frame such discussions and integrate information from multiple domains. The systematic account of entrainment that we provide suggests that the evolutionary pathway leading to complex forms of entrainment, such as those underlying music and dance, may begin with much simpler abilities.
5.1. Does selection for vocal learning enable musical ability?
Recent hypotheses about the neural substrates underlying beat perception and synchronization in music implicate mechanisms that may have evolved to facilitate vocal learning, such as tightly coupled auditory input and motor output systems (e.g., via the basal ganglia) (Doupe, Perkel, Rheiner & Stern, 2005; Fitch, 2006; Patel 2006; Patel et al., 2009; Schachner, Brady, Pepperberg & Hauser, 2009; Grahn & Rowe, 2009). The `vocal learning hypothesis' (Patel 2006; Patel et al., 2009) specifically proposes that beat perception and synchronization emerged as byproducts of mechanisms that enabled individuals to mimic the vocalizations of others. This is consistent with a number of components of the framework for entrainment we describe here—in particular, the coupling of auditory input and motor output that is a critical part of vocal learning maps onto the third building block of entrainment delineated in our framework. However, our model of entrainment is more general and does not necessarily require that the modality of incoming information be auditory, for example.
While both music and conversational synchronization behaviors are considered forms of entrainment in our framework, an important distinction is worth noting, which is in the role of a perceived isochronous pulse (Merker et al., 2009) in music/dance, but not in speech and verbal communication. While speech and conversational rhythms manifest patterns of stress or syllable accents, empirical data are consistent with the idea that the timing patterns in speech and conversation are not driven by the same kind of regular isochrony that is characteristic of music (Patel, 2006). This regularity of rhythm has been observed across culture in music and dance (Arom, 1991). It has also been established in behavioral and neuroimaging studies of both rhythm perception and production (e.g., Repp, 2005; Calvo-Merino et al., 2005; Zanto, Snyder & Large, 2006). It has been argued that the critical role that auditory-motor feedback plays in beat perception and synchronization in music rests on the pulse-based nature of measured music (Patel et al., 2005; Brown, Martinez & Parsons, 2006; Zatorre, Chen & Penhune, 2007; Grahn & Brett, 2007; Chen, Penhune & Zatorre, 2008). Thus, isochrony (or perceived isochrony) provides a special condition for entrainment in music and dance.
Nevertheless, there appear to be aspects of vocal communication that are rhythmic in nature. For example, the oscillator model of conversational turn taking by Wilson and Wilson (2005) describes mandibular oscillations that drive consonant vowel production entrainment between conversationalists. This model of conversational speech can account for the rapid time course of spontaneous conversation—the rate of syllabic production in ordinary speech is often greater than 200ms per syllable. Timely reaction to the end of a conversational turn, and the subsequent speech production in response, requires a mechanism that can accurately predict a break in the sequence. This suggests that there may be aspects of verbal communication and vocal production in humans that have inherently rhythmic components that may not be strictly isochronous, but do have important rhythmic features. Such rhythmic features seem to be inherent to vocal learning.
5.2. Have music and dance been selected by evolution?
Evolutionary theories of coordinated rhythmic movement in general, and pulse-based musical synchronization in particular, consider their potential roles in sexual selection (Miller, 2000), social bonding, and group cohesion (Huron, 2006; Fitch 2006; Merker et al., 2009), as well as coalition signaling and territorial advertisement (Hagen & Bryant, 2003; Hagen & Hammerstein, 2009). While questions of specific adaptive design loom large (Hauser & McDermott, 2003; Mithen, 2005; Fitch 2006; Merker et al., 2009; Patel, Iversen, Bregman & Schulz, 2009), natural selection somehow shaped a complex system for not only detecting and producing rhythmic information, but also for integrating these types of information into an entrainment processor.
Discussions of function necessarily raise questions of biological adaptation, but it is not our intention to address in detail the adaptive function of social entrainment in the present paper. Rather, the framework proposed here describes how the capacity for social entrainment (which we believe to be the foundation for music and dance ability) could have emerged from the simpler ability to entrain to rhythmic information in the physical environment. We believe it is likely that selection acted on this capacity to produce more nuanced rhythmic social responsiveness, but future work should address the questions of how exactly social entrainment mechanisms evolved and what kinds of selection pressures might have shaped the human capacity for music and dance. Nevertheless, social entrainment processes appear to play an important role in many aspects of social behavior, as evidenced by recent research showing how synchrony promotes cooperation (Wiltermuth & Heath, 2009), and affiliation (Hove & Risen, 2008). The relation between entrainment and social behavior is clearly a rich area for future exploration.
We offer the additional speculation that a potential function of social entrainment may be the facilitation of higher-level organization that requires real-time information sharing between or among individuals that are producing and/or processing rhythmic information. In complex systems with highly interdependent components, the ability to quickly and effectively transmit information can promote efficiency at a higher level, and even enable otherwise impossible higher-level functionality. In a variety of species coordinated activities may play a role in promoting higher-level function. As discussed earlier, synchronous chorusing is thought to enable more effective sexual advertising (Merker et al., 2009). Another domain in which coordination may facilitate higher-level function is that of niche construction (i.e., building dwellings, cultivating land), where the coordinated activity of multiple individuals may allow the construction of structures impossible to create without coordinated movement. Collective foraging, collective predation, collective predator evasion, and collective migration may be additional situations in which the ability of individuals to effectively coordinate their movement through social entrainment may lead to increased survival and/or reproduction. We are not suggesting that social entrainment is necessary for these processes, but that social entrainment might make them more effective and efficient. For example, social insects exhibit highly coordinated behaviors in a variety of domains, using complex chemical communication to organize themselves spatially and temporally (Wilson & Wilson, 2007). Whether they accomplish this through entrainment as we have defined it remains to be seen. The present approach focuses on rhythmic information as the basis for entrainment, and it is possible that there are periodic components of signals in these insect colonies that provide a basis for rhythmic entrainment. It may also be the case that social insects accomplish highly coordinated activities using underlying mechanisms that are different from entrainment as we describe it here.
The historical importance of the work song (Edwards & Haas, 2000; McNeill, 1999), suggests a potential role for music in organizing coordinated and effortful activities. If the power to unite bodies in coordinated rhythmic movement is enhanced in pulse-based music and dance, this may have important implications for the ability of humans to engage in complex and highly interdependent large-scale activities that require behavioral coordination.
Our view that entrainment facilitates large-scale coordination is consistent with the view of Hagen and Hammerstein (2009). These authors propose an analogy between human groups engaging in coordinated music/dance and complex and highly ordered biological systems. They suggest that music and dance might facilitate large-scale coordination that requires the transmission of information, just as signaling systems amongst cells in a multicellular organism allows the whole organism to act as a strategic individual. Hagen and collaborators emphasize the potential function of music and dance for the signaling of coalition quality (Hagen & Bryant, 2003) and territorial advertisement more generally (Hagen & Hammerstein, 2009). This is consistent with the basic framework we present here, although we extend this reasoning to a number of other domains. We suggest that social entrainment (including music and dance) may play a more general role in facilitating larger-scale functional behavior that requires information transmission between individuals.
Our proposed framework can provide a tool for exploring questions about the social function and evolutionary history of entrainment in music and dance. We characterized several classes of entrainment, and discussed the ways that the environment and the body provide a substrate that could have scaffolded the emergence of entrainment ability. The framework presented here suggests that the capacity for entrainment may be based on very simple and evolutionarily ancient abilities, yet it may have allowed for the emergence of some of the most complex types of behavioral coordination including music and dance.
References
- Allee WC, Emerson AE, Park O, Park T, Schmidt KP. Principles of animal ecology. W. B. Saunders; Philadelphia: 1949. [Google Scholar]
- Arom S. African Polyphony and Polyrhythm: Musical Structure and Methodology. Cambridge University Press; Cambridge, UK: 1991. [Google Scholar]
- Backwell P, Jennions M, Passmore N, Christy J. Synchronized courtship in fiddler crabs. Nature. 1998;391:31–32. 1998. [Google Scholar]
- Bispham J. Rhythm in music: What is it? Who has it? And why? Music Perception. 2006;24(2):125–134. [Google Scholar]
- Bluedorn AC. The human organization of time: Temporal realities and experience. Stanford University Press; Palo Alto, CA: 2002. p. 148. [Google Scholar]
- Brown S, Martinez MJ, Parsons LM. The neural basis of human dance. Cerebral Cortex. 2006;16:1157–1167. doi: 10.1093/cercor/bhj057. [DOI] [PubMed] [Google Scholar]
- Buck J, Buck E. Toward a functional interpretation of synchronous flashing in fireflies. American Naturalist. 1978;112:471–492. [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. Action Observation and Acquired Motor Skills: An fMRI Study with Expert Dancers. Cerebral Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Chen JL, Penhune VB, Zatorre RJ. Moving on time: Brain network for auditory synchronization is modulated by rhythmic complexity and musical training. Cerebral Cortex. 2008;18:2844–2854. doi: 10.1162/jocn.2008.20018. [DOI] [PubMed] [Google Scholar]
- Clayton M, Sager R, Will U. In time with the music: The concept of entrainment and its significance for ethnomusicology. ESEM Counterpoint. 2004;1:1–45. [Google Scholar]
- Condon WS, Sander LW. Synchrony demonstrated between movements of the neonate and adult speech. Child Development. 1974;45(2):456–462. [PubMed] [Google Scholar]
- Crown CL, Feldstein S, Jasnow MD, Beebe B, Jaffe J. The cross-modal coordination of interpersonal timing: Six week-olds infants' gaze with adults' vocal behavior. Journal of Psycholinguistic Research. 2002;31:1–23. doi: 10.1023/a:1014301303616. [DOI] [PubMed] [Google Scholar]
- Cummins F. Rhythm as entrainment: The case of synchronous speech. Journal of Phonetics. 2009;37:16–28. [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends in Neurosciences. 2005;28:353–363. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Drake C, Penel A, Bigand E. Tapping in time with mechanically and expressively performed music. Music Perception. 2000;18:1–23. [Google Scholar]
- Edwards G, Haas K. Flamenco. Thames & Hudson, Ltd.; London: 2000. [Google Scholar]
- Ellis H. In: What is dance? Readings in theory and criticism. Copeland R, Cohen M, editors. Oxford University Press; Oxford: 1983. p. 479. [Google Scholar]
- Feldman R. Parent-Infant Synchrony: Biological Foundations and Developmental Outcomes. Current Directions in Psychological Science. 2007;16(6):340–345. [Google Scholar]
- Fitch T. The biology and evolution of music: A comparative perspective. Cognition. 2006;100(1):173–215. doi: 10.1016/j.cognition.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Foster RG, Kreitzman L. Rhythms of Life: The Biological Clocks that Control the Daily Lives of Every Living Thing. Yale University Press; Oct 10, 2005. [Google Scholar]
- Fraisse P. Rhythm and tempo. In: Deutsch D, editor. The Psychology of Music. Academic Press; London: 1982. pp. 149–180. [Google Scholar]
- Friberg A, Sundberg J. Does music performance allude to locomotion? A model of final ritardandi derived from measurements of stopping runners. Journal of the Acoustical Society of America. 1999;105(3):1469–1484. [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Grahn J, Brett M. Rhythm and beat perception in motor areas of the brain. Journal of Cognitive Neuroscience. 2007;19(5):893–906. doi: 10.1162/jocn.2007.19.5.893. [DOI] [PubMed] [Google Scholar]
- Grahn J, Brett M. Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex. 2009;45:54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Rowe JB. Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. Journal of Neuroscience. 2009;29(23):7540–7548. doi: 10.1523/JNEUROSCI.2018-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield MD. Cooperation and conflict in the evolution of signal interactions. Annual Review of Ecological Systems. 1994a;25:97–126. [Google Scholar]
- Greenfield MD. Synchronous and alternating choruses in insects and anurans: Common mechanisms and diverse functions. American Zoologist. 1994b;34(6):605–615. [Google Scholar]
- Hagen EH, Bryant GA. Music and dance as a coalition signaling system. Human Nature. 2003;14(1):21–51. doi: 10.1007/s12110-003-1015-z. [DOI] [PubMed] [Google Scholar]
- Hagen EH, Hammerstein P. Did Neanderthals and other early humans sing? Seeking the biological roots of music in the territorial advertisements of primates, lions, hyenas, and wolves. Musicae Scientiae. 2009 in press. [Google Scholar]
- Hannon E. Infants know bad dancing when they see it. Abstract. International Conference on Infant Studies; Vancouver, Canada. 2008. [Google Scholar]
- Hauser MD, McDermott J. The evolution of the music faculty: A comparative perspective. Nature Neuroscience. 2003;6(7):663–668. doi: 10.1038/nn1080. [DOI] [PubMed] [Google Scholar]
- Hove MJ, Risen JL. It's all in the timing: Interpersonal synchrony increases affiliation. In: Adachi M, et al., editors. 10th Intl. Conf. on Music Perception and Cognition; Sapporo, Japan. Adelaide: Causal Productions; 2008. [Google Scholar]
- Huron D. Is Music and Evolutionary Adaptation? In: Wallin NL, Merker B, Brown S, editors. The Origins of Music. MIT Press; Cambridge, MA: 2006. pp. 57–75. [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown CL, Jasnow MD, Rochat P, Stern DN. Rhythms of dialogue in infancy: Coordinated timing in development; Monographs of the Society for Research in Child Development; 2001. pp. i–149. [PubMed] [Google Scholar]
- Janata P, Grafton ST. Swinging in the brain: shared neural substrates for behaviors related to sequencing and music; Nature Neuroscience; 2003. pp. 682–687. [DOI] [PubMed] [Google Scholar]
- Jones MR. Time, our lost dimension: Toward a new theory of perception, attention, and memory; Psychological Review; 1976. pp. 323–355. [PubMed] [Google Scholar]
- Keller PE. Joint action in music performance. In: Morganti F, Carassa A, Riva G, editors. Enacting intersubjectivity: A cognitive and social perspective to the study of interactions. IOS Press; Amsterdam: 2008. pp. 205–221. [Google Scholar]
- Keller PE, Repp BH. Multilevel coordination stability: Integrated goal representations in simultaneous intra-personal and inter-agent coordination. Acta Psychologica. 2008;128(2):378–386. doi: 10.1016/j.actpsy.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner S, Tomasello M. Joint drumming: Social context facilitates synchronization in preschool children. Journal of Experimental Child Psychology. 2009;102:299–314. doi: 10.1016/j.jecp.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Swartze M, Schmidt-Kassow M. Non-motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. Cortex. 2009;45:982–990. doi: 10.1016/j.cortex.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Large E. On synchronizing movements to music. Human Movement Science. 2000;19:527–566. [Google Scholar]
- Large E, Jones M. The dynamics of attending: How people track time-varying events. Psychological Review. 1999;106:121–156. [Google Scholar]
- Lotka AJ. Elements of physical biology. Williams & Wilkins company; 1925. [Google Scholar]
- McFarland DH. Respiratory markers of conversational interaction. Journal of Speech, Language, & Hearing Research. 2001;44:128–143. doi: 10.1044/1092-4388(2001/012). [DOI] [PubMed] [Google Scholar]
- McNeill WH. Keeping Together in time: Dance and drill in human history. Harvard University Press; Cambridge, MA: 1995. [Google Scholar]
- Merker B. Synchronous chorusing and human origins. In: Wallin NL, Merker B, Brown S, editors. The origins of music. MIT Press; Cambridge, MA: 2000. pp. 315–327. [Google Scholar]
- Merker BH, Madison GS, Eckerdal P. On the role and origin of isochrony in human rhythmic entrainment. Cortex. 2009;45:4–17. doi: 10.1016/j.cortex.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Miller G. Evolution of human music through sexual selection. In: Wallin NL, Merker B, Brown S, editors. The origins of music. MIT Press; Cambridge, MA: 2000. pp. 315–327. [Google Scholar]
- Mithen S. The Singing Neanderthals: The Origins of Music, Language, Mind and Body. Weidenfeld & Nicolson; London: 2005. [Google Scholar]
- Papoušek M. Communication in early infancy: An arena of intersubjective learning. Infant Behavior and Development. 2007;30:258–266. doi: 10.1016/j.infbeh.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Patel AD. Musical rhythm, linguistic rhythm, and human evolution. Music Perception. 2006;24:99–104. [Google Scholar]
- Patel AD, Iversen JR, Bregman MR, Schulz I, Schulz C. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Current Biology. 2009;19(10):827–830. doi: 10.1016/j.cub.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Patel AD, Iversen JR, Chen Y, Repp BH. The influence of metricality and modality on synchronization with a beat. Experimental Brain Research. 2005;163:226–238. doi: 10.1007/s00221-004-2159-8. [DOI] [PubMed] [Google Scholar]
- Phillips-Silver J, Trainor LJ. Feeling the beat: Movement influences infants' rhythm perception. Science. 2005;308:1430. doi: 10.1126/science.1110922. [DOI] [PubMed] [Google Scholar]
- Phillips-Silver J, Trainor LJ. Vestibular influence on auditory metrical interpretation. Brain and Cognition. 2008;67:94–102. doi: 10.1016/j.bandc.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Potts JT, Rybak IA, Paton JFR. Respiratory rhythm entrainment by somatic afferent stimulation. Journal of Neuroscience. 2005;25(8):1965–1978. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repp BH. Sensorimotor synchronization: A review of the tapping literature. Psychonomic Bulletin & Review. 2005;12(6):969–992. doi: 10.3758/bf03206433. [DOI] [PubMed] [Google Scholar]
- Repp BH, Keller P. Sensorimotor synchronization with adaptively timed sequences. Human Movement Science. 2008;27:423–456. doi: 10.1016/j.humov.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Richardson DC, Dale R, Kirkham NZ. The art of conversation is coordination. Psychological Science. 2007;18(5):407–413. doi: 10.1111/j.1467-9280.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Seddon FA. Modes of communication during jazz improvisation. British Journal of Music Education. 2005;22(1):47–61. [Google Scholar]
- Schachner A, Brady TF, Pepperberg IM, Hauser MD. Spontaneous motor entrainment to music in multiple vocal mimicking species. Current Biology. 2009;19(10):831–836. doi: 10.1016/j.cub.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kassow M, Kotz SA. Entrainment of syntactic processing? ERP responses to predictable time intervals during syntactic reanalysis. Brain Research. 2008;1226:144–155. doi: 10.1016/j.brainres.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Styns F, van Noorden L, Moelants D, Leman M. Walking on music. Human Movement Science. 2007;26:769–785. doi: 10.1016/j.humov.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Sumbre G, Muto A, Baier H, Poo M. Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature. 2008;456:102–107. doi: 10.1038/nature07351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Rhythmical behavior in infancy: an ethological perspective. Developmental Psychology. 1981;17:237–257. [Google Scholar]
- Todd NP, McAngus Vestibular feedback in music performance. Music Perception. 1993;10(3):379–382. [Google Scholar]
- Todd NP, McAngus Evidence for a behavioural significance of saccular acoustic sensitivity in humans. Journal of the Acoustical Society of America. 2001;110(1):380–480. doi: 10.1121/1.1373662. [DOI] [PubMed] [Google Scholar]
- Todd NP, McAngus, Cody F. Vestibular responses to loud dance music: A physiological basis for the “rock and roll threshold”? Journal of the Acoustical Society of America. 2000;107(1):496–500. doi: 10.1121/1.428317. [DOI] [PubMed] [Google Scholar]
- Todd NPM, Lee CS. Reply to “Embodied Rhythm” by Bruno Repp and “Do preferred beat rate and entrainment to the beat have a common origin in movement?” by Laurel Trainor. Empirical Musicology Review. 2007;2(3):110–112. [Google Scholar]
- Todd NPM, Cousins R, Lee CS. The contribution of anthropomorphic factors to individual differences in the perception of rhythm. Empirical Musicology Review. 2007;2(1):1–13. [Google Scholar]
- Todd NPM, Lee CS, O'Boyle DJ. A sensorimotor theory of temporal tracking and beat induction. Psychological Research. 2002;66:26–39. doi: 10.1007/s004260100071. [DOI] [PubMed] [Google Scholar]
- Toiviainen P, Snyder J. Tapping to Bach: Resonance-based modeling of pulse. Music Perception. 2003;21(1):43–80. [Google Scholar]
- Tomasello M, Carpenter M. Shared intentionality. Developmental Science. 2007;10:121–125. doi: 10.1111/j.1467-7687.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Trehub S. The developmental origins of musicality. Nature Neuroscience. 2003;6(7):669–673. doi: 10.1038/nn1084. [DOI] [PubMed] [Google Scholar]
- Trainor LJ. Do preferred beat rate and entrainment to the beat have a common origin in movement? Empirical Musicology Review. 2007;2(1):17–20. [Google Scholar]
- Trainor LJ, Gao X, Lei J, Lehtovaara K, Harris LR. The primal role of the vestibular system in determining musical rhythm. Cortex. 2009;45:35–43. doi: 10.1016/j.cortex.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Trevarthen C. Communication and cooperation in early infancy : A description of primary intersubjectivity. In: Bullowa M, editor. Before speech: The beginnings of human communication. Cambridge University Press; London: 1979. [Google Scholar]
- Volterra V. Variazioni e fluttuazioni del numero d'individui in specie animali conviventi. Mem. Acad. Lincei. 1926;2(6):31–113. [Google Scholar]
- Wilson M, Wilson TP. An oscillator model of the timing of turn-taking. Psychonomic Bulletin and Review. 2005;12(6):957–968. doi: 10.3758/bf03206432. [DOI] [PubMed] [Google Scholar]
- Wilson DS, Wilson EO. Rethinking the theoretical foundation of sociobiology. The Quarterly Review of Biology. 2007;82(4):327–348. doi: 10.1086/522809. [DOI] [PubMed] [Google Scholar]
- Wiltermuth SS, Heath C. Synchrony and Cooperation. Psychological Science. 2009;20(1):1–5. doi: 10.1111/j.1467-9280.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Snyder JS, Large EW. Neural correlates of rhythmic expectancy. Advances in Cognitive Psychology. 2006;2(2–3):221–231. [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: Auditory-motor interactions in music perception and production. Nature Reviews Neuroscience. 2007;8:547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]