Abstract
The purpose of this study was to examine whether the association between daily light-intensity physical activity (LPA) and total body fat mass changes during childhood. The study sample was 577 children participating in the longitudinal Iowa Bone Development Study. Body fat mass and physical activity (PA) were measured using dual energy X-ray absorptiometry (DXA) and accelerometers, respectively, at approximately 5, 8, and 11 years of age. Age- and gender-specific multivariable linear regression models were fit to predict fat mass by LPA, adjusted for actual age, birth weight, fat-free mass, height, moderate- to vigorous-intensity PA, and physical maturity (only for girls). Among boys, LPA was negatively associated with fat mass at age 11, but not age 5 or 8. Among girls, LPA was negatively associated with fat mass at ages 8 and 11, but not at age 5. LPA may have a beneficial effect against excess adiposity among older children.
The benefit of regular moderate- to vigorous-intensity physical activity (MVPA) for obesity prevention is well acknowledged by the public. The effect of MVPA on adiposity is mainly explained by energy expenditure associated with MVPA. Light-intensity PA (LPA) is defined as any activities with intensity between sedentary behavior and moderate-intensity PA. LPA involves lower energy expenditure than MVPA, but higher energy expenditure than inactivity. Therefore, it is conceivable that daily LPA may have a protective effect against excess fat mass. Given the fact that a substantial proportion of American children do not meet MVPA recommendations (22), LPA promotion may be an alternative strategy in preventing childhood obesity.
Exercise studies have traditionally focused on MVPA, which has often been measured by self-report PA recall questionnaires. Because LPA frequently occurs in daily life, it is more difficult to accurately quantify LPA than MVPA when using PA questionnaires (18). The development of accelerometry as an objective measure of PA provides new possibilities for objectively assessing the full range of PA intensities, from inactivity to vigorous activity, in free-living subjects over a number of days, and for studying the health effects of all PA intensity levels (15).
Recently, several studies (1,2,4,19,20,23) examined the association between accelerometer-measured daily LPA and adiposity in children. Two cross-sectional studies (1,20) demonstrated a negative association between accelerometer-measured time spent in LPA (Time LPA) and adiposity indicators, such as body mass index (BMI) percentile, fat mass, and percent body fat (BF%), among children and adolescents. However, others (2,4,19,23) have reported no association between Time LPA and BMI. At this time, research is limited regarding the association between LPA and adiposity during childhood; the association may differ by childhood age, because the pattern of PA changes during childhood.
Previously, we reported the beneficial effects of accelerometer-measured MVPA on adiposity and bone health in childhood using the longitudinal Iowa Bone Development Study data (8,9). We herein examined whether the association between daily LPA and body fat mass change during childhood using the data. We hypothesized that LPA may play an important role in determining fat accumulation in late childhood, but not in early childhood. Previous LPA studies have often used Time LPA as a LPA indicator. From an energy expenditure perspective, however, not only duration of LPA, but also its intensity is assumed to be a significant factor in establishing a relationship between adiposity and LPA. Fat mass is expected to better indicate an actual body fat level than BMI in children. Therefore, we examined the association between accelerometer-measured intensity-weighted LPA (IW-LPA) and dual energy X-ray absorptiometry (DXA)-derived total body fat mass during childhood.
Methods
Participants
The study sample was a cohort of children participating in the Iowa Bone Development Study, which is an ongoing longitudinal study of bone health during childhood and adolescence. The study participants are a subset of Iowa children recruited during 1998–2001 from a cohort of 890 families participating in the Iowa Fluoride Study. Detailed information about the study design is described elsewhere (5,8,10). Accelerometer and DXA measurements were conducted at approximately 5, 8, and 11 years of age (4.3–6.8 years of age range at the first examination, 7.6–10.8 years at the second examination, and 10.5–12.4 years at the third examination). Even if a cohort member did not participate in the age 5 examination, s/he was invited to participate in the age 8 and 11 examinations. If the time interval between the accelerometer measurement and DXA scanning was greater than 1.5 years for any age examination, the data for that examination were excluded. Four hundred thirty-six children completed both the accelerometer and DXA assessments at the age 5 examination from February 1998 to November 2000; 502 at the age 8 examination from September 2000 to December 2004; and 454 at the age 11 examination from October 2003 to September 2006. A total of 577 children (51% girls, 95% white) who completed at least one of those three examinations were included in the data analysis. The study was approved by the University of Iowa Institutional Review Board (Human Subjects). Written informed consent was provided by the parents of the children and assent was obtained from the children.
Physical Activity Measurements
Physical activity was measured using Actigraph uniaxial accelerometers (model number 7164, Pensacola, FL). Accelerometer movement counts were collected in one-minute intervals (one-minute epochs). At the age 5 and 8 examinations, children were asked to wear the monitors during waking hours for four consecutive days, including one weekend day, during the fall season (September through November). At the age 11 examination, they were asked to wear the monitor during waking hours for five consecutive days, including both weekend days, during the fall season. The procedure for PA measurement is described in detail elsewhere (6,7). In the data reduction process, an interval of 20 or more consecutive minutes of zero accelerometer counts was considered as not wearing the monitor and invalid data (3). The two inclusion criteria for accelerometer data for data analysis were having valid data for more than eight hours per day and wearing the monitor for at least three days. The mean number of valid days of monitor-wearing was 4.0 days at age 5, 4.0 days at age 8, and 4.9 days at age 11.
Measure of Light-Intensity Physical Activity
Light intensity was defined by accelerometer counts per minute between inactivity and moderate to vigorous intensity. Moderate to vigorous intensity was defined as 3,000 or more accelerometer movement counts per minute (ct·min1; 16, 21). There is no consensus on an accelerometer count cut-point to define inactivity in children. We selected two extremes (low and high) among cut-points suggested in previous studies: 99 ct·min1 (13,21) and 1,099 ct·min1 (17). This approach allowed us to identify the influence of the cut-points in examining the association between LPA and adiposity (two light-intensity definitions: 100–2,999 ct·min1 or 1,100–2,999 ct·min1). Intensity-weighted LPA (IW-LPA) was determined as the daily sum of accelerometer counts derived during LPA. Intensity-weighted MVPA (IW-MVPA) was determined as the daily sum of accelerometer counts derived during MVPA. Total activity was defined as the daily sum of accelerometer counts during any intensities of PA (3 100 ct·min1).
Total Body Fat Mass Measurements
At the age 5 and 8 examinations, whole body scans using a Hologic QDR 2000 DXA (Hologic, Waltham, MA) were acquired using software version 7.20B in the fan-beam mode. At the age 11 examination, the Hologic QDR 4500 DXA (Delphi upgrade) with software version 12.3 in the fan-beam mode was used for scan acquisition. Quality control scans were performed daily using the Hologic phantom. To adjust for measurements differences between the two DXA machines, linear regression equations were used to translate 4500 DXA measures to 2000 DXA measures for age 11 examinations. The translational equation was developed specifically for the two scanners in a pilot study where 60 of the children (32 boys, 28 girls) aged 9.9 to 12.4 years (mean = 11.4 years, SD = 0.4 years) were scanned on each machine in random order during one clinic visit (8). The equation was [2000 DXA-estimated body fat (kg) = 1.238 × 4500 DXA-estimated body fat (kg)—1.694]. Total body fat mass (kg) was derived from the scan images. Fat-free mass (kg) was calculated as the sum of bone-free soft tissue and bone mineral content.
Covariate Measurements
At each DXA visit, research nurses trained in anthropometry measured the child’s height and weight. Sitting height was measured at the age 11 examination to calculate maturity offset (year from peak height velocity) using predictive equations established by Mirwald and colleagues (14). To estimate physical maturity status, the maturity offset variable was dichotomized as prepeak height velocity (premature) or postpeak height velocity (mature). Dietary assessment was conducted using a three-day food diary at the age 5 examination and the Block Kid’s Food Frequency Questionnaire (the Block Kid’s FFQ; 12) at the age 8 and 11 examinations. Approximately 40% of the age 5 participants completed a 3-day food diary, while 94% of the age 8 participants and 98% of the age 11 participants completed the Block Kid’s FFQ, respectively. Birth weight data were available in the original Fluoride Study database.
Statistical Analysis
Age group (5, 8, and 11 years)- and gender-specific analyses were performed using SAS version 9.2 (Cary, NC). Descriptive analyses were conducted. The distribution of times spent in different intensities of activity during waking hours was determined. The distribution of total fat mass by age was depicted in a scatter plot. Because the fat mass variable was not normally distributed, a Box-Cox power transformation of the fat mass variable (hereafter ‘transformed fat mass’) was performed (the lambda is noted in Tables 2 and 3). Age-, height-, and fat-free mass-adjusted partial correlation coefficients between the LPA variables and the transformed fat mass variable were estimated.
Table 2.
Pearson Correlation Coefficients Between IW-LPA and IW-MVPA and Fat Mass by Gender and Age Group
| Boys |
Girls |
|||||
|---|---|---|---|---|---|---|
| Age 5 (n = 203) |
Age 8 (n = 242) |
Age 11 (n = 216) |
Age 5 (n = 232) |
Age 8 (n = 257) |
Age 11 (n = 233) |
|
| r (95% CI) | r (95% CI) | |||||
| IW-MVPA | ||||||
| IW-LPA100 | 0.22 (0.08, 0.35) |
0.22 (0.10, 0.34) |
0.29 (0.17, 0.41) |
0.30 (0.18, 0.42) |
0.20 (0.08, 0.32) |
0.33 (0.21, 0.44) |
| IW-LPA1100 | 0.34 (0.21, 0.45) |
0.34 (0.23, 0.45) |
0.37 (0.25, 0.48) |
0.36 (0.25, 0.47) |
0.30 (0.19, 0.42) |
0.42 (0.31, 0.52) |
| Fat mass† | ||||||
| IW-LPA100 | −0.04 (−0.18, 0.09) |
−0.14 (−0.27, −0.02) |
−0.27 (−0.39, −0.14) |
−0.11 (−0.24, 0.02) |
−0.17 (−0.29, −0.05) |
−0.20 (−0.32, −0.07) |
| IW-LPA1100 | −0.07 (−0.21, 0.07) |
−0.20 (−0.31, −0.07) |
−0.32 (−0.43, −0.19) |
−0.12 (−0.24, 0.01) |
−0.21 (−0.32, −0.09) |
−0.25 (−0.36, −0.12) |
Age-, height-, and fat-free mass-adjusted Pearson partial correlation coefficients between IW-LPA and transformed fat mass are presented. In the Box-Cox transformation of fat mass, the lambda (l) was −1.0, −0.5, and 0 for boys, −0.5, 0, and 0 for girls at ages 5, 8, and 11, respectively. If l = 0, then y(l) = log y; if l 1 0, then y(l) = y −1/ l.
CI = confidence interval, IW-LPA100 = the daily sum of accelerometer counts during light-intensity physical activity defined as 100 ct·min1£ accelerometer counts £ 2,999 ct·min1, IW-LPA1100 = the daily sum of accelerometer counts during light-intensity physical activity defined as 1,100 ct·min1£ accelerometer counts £ 2,999 ct·min1, IW-MVPA = the daily sum of accelerometer counts during moderate- to vigorous-intensity physical activity defined as accelerometer counts 3 3,000 ct·min1.
Table 3.
Parameter Estimates for IW-LPA From Multivariable Linear Regression Models to Predict Total Body Fat Mass
| Age 5 |
Age 8 |
Age 11 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure variable |
β | SE | P | β | SE | P | β | SE | P | |
| Boys | IW-LPA100 (×103 ct/d) | 0.38 | 0.96 | 0.69 | −2.27 | 2.31 | 0.33 | −13.91 | 4.85 | <0.01 |
| IW-LPA1100 (×103 ct/d) | 0.38 | 1.12 | 0.74 | −2.96 | 3.08 | 0.34 | −20.79 | 6.13 | <0.01 | |
| Girls | IW-LPA100 (×103 ct/d) | −0.25 | 1.09 | 0.82 | −5.41 | 2.60 | <0.05 | −8.93 | 4.88 | 0.07 |
| IW-LPA1100 (×103 ct/d) | 0.03 | 1.32 | 0.98 | −8.14 | 3.34 | <0.05 | −16.12 | 6.61 | <0.05 | |
β = regression parameter estimate, IW-LPA100 = the daily sum of accelerometer counts during light-intensity physical activity defined as 100 ct·min1≤ accelerometer counts ≤ 2,999 ct·min1, IW-LPA1100 = the daily sum of accelerometer counts during light-intensity physical activity defined as 1,100 ct·min1≤ accelerometer counts ≤ 2,999 ct·min1, IW-MVPA = the daily sum of accelerometer counts during moderate- to vigorous-intensity physical activity defined as accelerometer counts ≥ 3,000 ct·min1, SE = standard error of the estimate.
In the Box-Cox transformation of fat mass, the lambda was −1.0, −0.5, and 0 for boys, −0.5, 0, and 0 for girls at ages 5, 8, and 11, respectively. If λ = 0, then y(λ) = log y; if λ 1 0, then y(λ) = y −1/ λ.
Multivariable linear regression model: transformed total body fat mass (g) = exposure variable + age (year) + birth weight (g) + fat-free mass (g) + height (cm) + IW-MVPA (×103 ct/d). Physical maturity was only included in the model for girls at age 11.
For boys, n = 203 at age 5, n = 242 at age 8, and n = 216 at age 11. For girls, n = 232 at age 5, n = 257 at age 8, and n = 233 at age 11.
The LPA associated with fat mass was tested using the two IW-LPA variables derived based on two different light-intensity definitions (100–2,999 ct·min1 and 1,100–2,999 ct·min1; these IW-LPA variables were labeled as IW-LPA100 and IW-LPA1100, respectively). Multivariable linear regression models were fit to predict transformed fat mass based on IW-LPA. The models also included actual age, birth weight, height, fat-free mass, and IW-MVPA as covariates. Physical maturity status was additionally included as a covariate in models for girls at age 11; none of the boys were classified as postpeak height velocity at age 11. Another set of multivariable linear regression models were fit, additionally including energy intake as a covariate to investigate its potential confounding effect. Herein, we present the results from final multivariable linear regression models excluding energy intake for the following three reasons: energy intake did not change the direction or magnitude of the association between LPA and transformed fat mass; energy intake measured by the Block Kid FFQ was found to have a low validity (12); and there was a low completion rate at age 5.
After model fitting, model diagnostics were performed to test whether the data met model assumptions. If an observation significantly changed the estimated model coefficients (influence, residuals exceeding +3 or −3), the observation was excluded. Linearity, normality of residuals, and heteroscedasticity were tested. For the collinearity assumption, if a tolerance value was lower than 0.1, the variable was considered as a linear combination of other predictor variables. Based on model diagnostics, one boy at age 5, three boys at age 8, and two boys and three girls at age 11 were excluded from the final models. The significance level wasset at 0.05 (two-sided).
Results
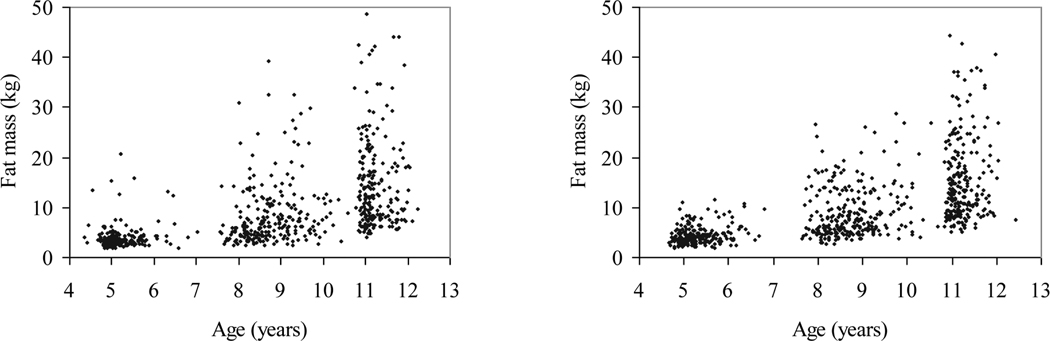
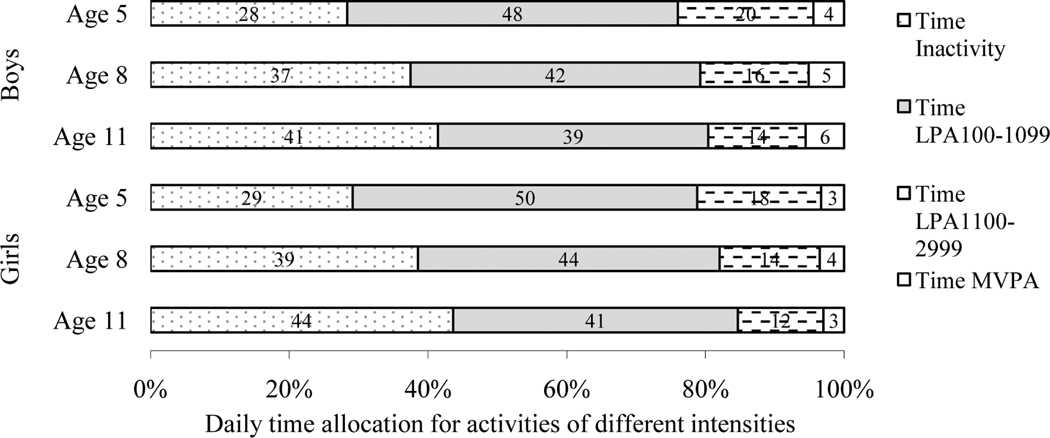
Table 1 presents the characteristics of participants according to gender and age group. The mean fat mass increased 9.2 kg in boys and 9.5 kg in girls over six years. Birth weight was 3.6 ± 0.6 kg for boys and 3.4 ± 0.5 kg for girls (not shown in Table 1). The range of the fat mass distribution increased wider with age (Figure 1). As illustrated in Figure 2, inactive time tended to increase and Time LPA tended to decrease with age. The proportion of time spent in MVPA (Time MVPA) during waking hours was minimal at each age. Bivariate analyses showed that the correlation coefficients of IW-LPA with IW-MVPA were low to moderate (IW-LPA100: r = 0.22–0.29 for boys and r = 0.20–0.33 for girls; IW-LPA1100: r = 0.34–0.37 for boys and r = 0.36–0.42 for girls, data not shown). As presented in Table 2, age-, height-, and fat-free mass-adjusted Pearson correlation coefficients between transformed fat mass and IW-LPA were more strongly negative with increasing age; significant at ages 8 and 11 (p < .05) for both boys and girls, but not significant at age 5 for either boys or girls.
Table 1.
Descriptive Characteristics of Study Participants by Gender and Age Group
| Boys |
Girls |
|||||
|---|---|---|---|---|---|---|
| Age 5 (n = 204) | Age 8 (n = 245) | Age 11 (n = 218) | Age 5 (n = 232) | Age 8 (n = 257) | Age 11 (n = 236) | |
| Mean (95% CI) | Mean (95% CI) | |||||
| Age (years) | 5.2 (5.2, 5.3) | 8.8 (8.7, 8.9) | 11.2 (11.2, 11.3) | 5.3 (5.2, 5.4) | 8.8 (8.7, 8.8) | 11.2 (11.2, 11.3) |
| Height (m) | 1.12 (1.11, 1.13) | 1.35 (1.34, 1.36) | 1.49 (1.48, 1.50) | 1.11 (1.10, 1.12) | 1.33 (1.32, 1.34) | 1.49 (1.48, 1.50) |
| Body weight (kg) | 20.5 (20.0, 21.1) | 33.8 (32.6, 35.1) | 45.3 (43.5, 47.0) | 20.0 (19.5, 20.5) | 31.9 (30.8, 32.9) | 44.5 (42.9, 46.0) |
| Fat mass (kg) | 3.9 (3.6, 4.3) | 8.4 (7.6, 9.1) | 13.1 (11.8, 14.3) | 4.5 (4.2, 4.8) | 9.2 (8.5, 9.9) | 14.0 (12.9, 15.1) |
| Monitor-wearing time (hr/d) | 12.1 (12.0, 12.2) | 12.5 (12.4, 12.6) | 12.4 (12.2, 12.5) | 12.1 (12.0, 12.2) | 12.4 (12.3, 12.5) | 12.3 (12.2, 12.4) |
| IW-LPA100 (×103 ct/d) | 416 (406, 425) | 351 (342, 360) | 315 (307, 324) | 397 (388, 406) | 333 (324, 341) | 291 (283, 298) |
| IW-LPA1100 (×103 ct/d) | 253 (245, 261) | 214 (206, 221) | 190 (184, 197) | 228 (221, 235) | 190 (184, 197) | 160 (154, 166) |
| IW-MVPA (×103 ct/d) | 150 (137, 164) | 181 (167, 196) | 216 (189, 224) | 114 (104, 125) | 129 (116, 141) | 109 (99, 120) |
CI = confidence interval, IW-LPA100 = the daily sum of accelerometer counts during light-intensity physical activity defined as 100 ct·min1£ accelerometer counts £ 2,999 ct·min1, IW-LPA1100 = the daily sum of accelerometer counts during light-intensity physical activity defined as 1,100 ct·min1£ accelerometer counts £ 2,999 ct·min1, IW-MVPA = the daily sum of accelerometer counts during moderate- to vigorous-intensity physical activity defined as accelerometer counts 3 3,000 ct·min1.
Figure 1.
Scatter plots of fat mass over age in boys and girls (a) Boys (n = 204 at age 5, n = 245 at age 8, and n = 218 at age 11; b) Girls (n = 232 at age 5, n = 257 at age 8, and n = 236 at age 11)
Figure 2.
Daily time allocation for physical activity intensities according to gender and age group. Note: Time LPA100–1099 = the amount of daily time spent in light-intensity physical activity defined as 100 ct·min1≤ accelerometer counts ≤ 1,099 ct·min1, Time LPA1100–2999 = the amount of daily time spent in light-intensity physical activity defined as 1,100 ct·min1≤ accelerometer counts £ 2,999 ct·min1, Time MVPA = the amount of daily time spent in moderate- to vigorous-intensity physical activity defined as accelerometer counts ≥3,000 ct·min1, Time Inactivity = the amount of daily inactive time defined as accelerometer counts < 100 ct·min1. For boys, n = 204 at age 5, n = 245 at age 8, and n = 218 at age 11. For girls, n = 232 at age 5, n = 257 at age 8, and n = 236 at age 11.
Table 3 shows the fitted multivariable linear regression models predicting transformed fat mass by IW-LPA, adjusted for actual age, birth weight, height, fat-free mass, IW-MVPA, and physical maturity at age 11 (only for girls). Among boys, both IW-LPA100 and IW-LPA1100 were negatively associated with transformed fat mass at age 11 (p < .01), but not at ages 5 or 8. Among girls, IW-LPA1100 was negatively associated with transformed fat mass at age 11 (p < .05). The negative association between IW-LPA1100 and transformed fat mass at age 11 was suggestive (p < .10). Both IW-LPA100 and IW-LPA1100 were negatively associated with transformed fat mass at age 8 (p < .05), but not at age 5.
Discussion
Main Findings and Consistency
The purpose of this study was to examine whether the association between daily LPA and total body fat mass changes during childhood. We found that IW-LPA was negatively associated with fat mass in children at age 11, but not at age 5. The negative association between IW-LPA and fat mass at age 8 was observed only among girls. A few studies examined the association between accelerometer-measured Time LPA and adiposity in youth. Two cross-sectional studies (1,20) demonstrated a significant negative association between Time LPA and adiposity. Treuth et al. (20) examined a cross-sectional relationship between several adiposity indicators (BMI, fat mass, and BF%) and Actiwatch accelerometer-measured Time LPA in 229 elementary-, middle school-, and high school-age boys and girls. They found that Time LPA was negatively associated with adiposity indicators at all three school levels among girls, but not among boys. Butte et al. (1) examined the cross-sectional relationship between DXA-measured BF% and Actiwatch accelerometer-measured Time LPA among 897 children 4–19 years of age. Time LPA was negatively associated with BF%, adjusted for gender and age. On the other hand, four other cross-sectional studies (2,4,19,23) have shown no association between Time LPA and BMI. For example, in a study of 790 boys and girls in grades 3 and 7, Thompson et al. (19) found no difference in Time LPA among healthy-weight, overweight, and obese groups.
It may be inappropriate to directly compare results across studies, because of the use of different types of accelerometer and different cut-points. In the current study, however, the use of two distinct LPA accelerometer cut-points did not influence the association between LPA and fat mass. Potential confounding factors may partially explain the inconsistency across studies. Previous studies did not take into account MVPA and birth weight, which were found to be significant confounding factors in the current study. Controlling for possible confounding factors is critical for the internal validity of study results. For example, in the current study, the age-, height-, and fat-free mass-adjusted correlation coefficient between IW-HLPA and fat mass at age 8 among boys was significant; however, multivariable linear regression analysis revealed that IW-HLPA was not associated with fat mass, after further adjustment for birth weight and IW-MVPA. In future research to examine the role of LPA in determining adiposity, such factors, particularly MVPA, should be taken into account as potential confounding factors. In addition, the difference of monitor-wearing time could lead to measurement error in estimating LPA. However, when we additionally examined the association between average IW-LPA (IW-LPA divided by monitor-wearing time) and fat mass, the results again demonstrated a negative association only among older children.
Measurement Issues of Physical Activity
It is a concern that despite the need to consider LPA, there is no consensus on an accelerometer count cut-point to define LPA in children. Suggesting an appropriate cut-point for defining LPA is beyond the scope of this study. However, this study demonstrates that the analysis results using two distinct cut-points in the association between LPA and fat mass were fairly consistent.
Previous studies have often used Time LPA as the indicator of LPA. In exploratory analyses, we found that fat mass was more strongly negatively associated with the two IW-LPA variables (p < .05 for boys and p < .10 for girls) than with two Time LPA variables (p > .10 for boys and p > .70 for girls) at age 11. These findings suggest that not only LPA duration, but also its intensity should be considered when investigating the association between LPA and adiposity. However, researchers should be cautious because the use of IW-LPA may amplify measurement error, which is derived from the difference of relative intensities between individuals when absolute intensity is given.
In contrast with previous studies, the current study suggests the potential difference in the association between LPA and fat mass by age, which may be partially explained by the change of PA patterns during childhood (e.g., greater time spent in inactivity or more fat mass variability in older children than younger children). However, potential measure error should be considered in interpreting the results. First, because the relative intensity of a given absolute accelerometer counts per minute value is higher in younger children than in older children, the application of the same cut-point for PA intensity categorization to different age groups may have caused misclassification of the exposure. Second, PA patterns of young children may include more various types of activities in addition to ambulatory activities than those of older children. Therefore, when PA was measured by waist-worn accelerometers which are known to best capture ambulatory activities of walking and running, larger measurement error of PA at age 5 may have occurred than for any other age group. Lastly, as illustrated in Figure 1, given a low level of fat mass and its narrow distribution at age 5 in this study sample, the observational data may be inappropriate to demonstrate an association between LPA and excess body fat. However, considering that IW-MVPA was negatively associated with fat mass at age 5 (data not shown), it would seem that adiposity has, at the most, a weaker association with LPA than with MVPA among younger children.
Public Health Implications
First, the study supports the current PA recommendations focusing on MVPA. LPA seems to have some beneficial effect on adiposity among older children, but little to no effect among younger children, whereas the beneficial effect of MVPA on adiposity is consistent throughout childhood. Therefore, promoting MVPA would be an appropriate recommendation for obtaining a health benefit of PA. Second, this study suggests that the effect of LPA on adiposity may become apparent with age throughout childhood. The promotion of LPA, such as slow walking and playing active electronic games (11), may be an alternative intervention strategy for obesity prevention in older children. It would be a realistic and practical strategy for inactive children to gradually increase PA, by moving from inactivity to LPA and to MVPA. Promoting LPA may be particularly meaningful for girls who are likely to engage in less MVPA than boys.
Limitations and Strengths
The study sample was not randomly selected; selection bias may have occurred. Therefore, an association between LPA and adiposity observed in this sample may not represent that in the general child population and caution should be taken in generalizing the results. In the participant cohort, 95% were white, which is a lower risk population for childhood obesity than the Hispanic or African American population. Genetic predisposition to body composition was not accounted for. This observational study cannot eliminate error by residual and unmeasured confounding factors. However, the use of objective and accurate measures for both PA and adiposity helped reduce measurement error and increase the confidence of internal validity. Considering potential confounders such as MVPA and birth weight also helped protect study results against threats to internal validity. In conclusion, this study suggests there is a possibility that LPA has a preventive effect against excess adiposity in older children. More research is required to further examine the possible beneficial effect of LPA on obesity prevention throughout childhood.
Acknowledgments
This study was supported by the National Institute of Dental and Craniofacial Research R01-DE12101 and R01-DE09551, and the General Clinical Research Centers Program from the National Center for Research Resources, M01-RR00059.
Contributor Information
Soyang Kwon, Dept. of Health Studies, University of Chicago, Chicago, IL..
Kathleen F. Janz, Dept. of Health and Human Physiology and Dept. of Epidemiology, University of Iowa, Iowa City, IA.
Trudy L. Burns, Dept. of Epidemiology and Dept. of Pediatrics, University of Iowa, Iowa City, IA.
Steven M. Levy, Dept. of Preventive and Community Dentistry, and Dept. of Epidemiology, University of Iowa, Iowa City, IA.
References
- 1.Butte NF, Puyau MR, Adolph AL, Vohra FA, Zakeri I. Physical activity in nonoverweight and overweight Hispanic children and adolescents. Med. Sci. Sports Exerc. 2007;39:1257–1266. doi: 10.1249/mss.0b013e3180621fb6. [DOI] [PubMed] [Google Scholar]
- 2.Byrd-Williams C, Kelly LA, Davis JN, Spruijt-Metz D, Goran MI. Influence of gender, BMI and Hispanic ethnicity on physical activity in children. Int. J. Pediatr. Obes. 2007;2:159–166. doi: 10.1080/17477160701369167. [DOI] [PubMed] [Google Scholar]
- 3.Cliff DP, Reilly JJ, Okely AD. Methodological considerations in using accelerometers to assess habitual physical activity in children aged 0–5 years. J. Sci. Med. Sport. 2009;12:557–567. doi: 10.1016/j.jsams.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Hughes AR, Henderson A, Ortiz-Rodriguez V, Artinou ML, Reilly JJ. Habitual physical activity and sedentary behaviour in a clinical sample of obese children. Int J Obes (Lond) 2006;30:1494–1500. doi: 10.1038/sj.ijo.0803334. [DOI] [PubMed] [Google Scholar]
- 5.Janz KF, Burns TL, Levy SM, et al. Tracking of activity and sedentary behaviors in childhood: the Iowa Bone Development Study. Am. J. Prev. Med. 2005;29:171–178. doi: 10.1016/j.amepre.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Janz KF, Burns TL, Torner JC, et al. Physical activity and bone measures in young children: the Iowa bone development study. Pediatrics. 2001;107:1387–1393. doi: 10.1542/peds.107.6.1387. [DOI] [PubMed] [Google Scholar]
- 7.Janz KF, Gilmore JM, Burns TL, et al. Physical activity augments bone mineral accrual in young children: The Iowa Bone Development study. J. Pediatr. 2006;148:793–799. doi: 10.1016/j.jpeds.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 8.Janz KF, Kwon S, Letuchy EM, et al. Sustained effect of early physical activity on body fat mass in older children. Am. J. Prev. Med. 2009;37:35–40. doi: 10.1016/j.amepre.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janz KF, Letuchy EM, Eichenberger Gilmore JM, et al. Early physical activity provides sustained bone health benefits later in childhood. Med. Sci. Sports Exerc. 2010;42:1072–1078. doi: 10.1249/MSS.0b013e3181c619b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janz KF, Levy SM, Burns TL, Torner JC, Willing MC, Warren JJ. Fatness, physical activity, and television viewing in children during the adiposity rebound period: the Iowa Bone Development Study. Prev. Med. 2002;35:563–571. doi: 10.1006/pmed.2002.1113. [DOI] [PubMed] [Google Scholar]
- 11.Maddison R, Mhurchu CN, Jull A, Jiang Y, Prapavessis H, Rodgers A. Energy expended playing video console games: an opportunity to increase children’s physical activity? Pediatr. Exerc. Sci. 2007;19:334–343. doi: 10.1123/pes.19.3.334. [DOI] [PubMed] [Google Scholar]
- 12.Marshall TA, Eichenberger Gilmore JM, Broffitt B, Stumbo PJ, Levy SM. Relative validity of the Iowa Fluoride Study targeted nutrient semi-quantitative questionnaire and the block kids’ food questionnaire for estimating beverage, calcium, and vitamin D intakes by children. J. Am. Diet. Assoc. 2008;108:465–472. doi: 10.1016/j.jada.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002;34:689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Pate RR, O’Neill JR, Lobelo F. The evolving definition of “sedentary”. Exerc. Sport Sci. Rev. 2008;36:173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- 16.Puyau MR, Adolph AL, Vohra FA, Butte NF. Validation and calibration of physical activity monitors in children. Obes. Res. 2002;10:150–157. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- 17.Reilly JJ, Coyle J, Kelly L, Burke G, Grant S, Paton JY. An objective method for measurement of sedentary behavior in 3- to 4-year olds. Obes. Res. 2003;11:1155–1158. doi: 10.1038/oby.2003.158. [DOI] [PubMed] [Google Scholar]
- 18.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. discussion 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson AM, Campagna PD, Durant M, Murphy RJ, Rehman LA, Wadsworth LA. Are overweight students in Grades 3, 7, and 11 less physically active than their healthy weight counterparts? Int. J. Pediatr. Obes. 2009;4:28–35. doi: 10.1080/17477160802170050. [DOI] [PubMed] [Google Scholar]
- 20.Treuth MS, Hou N, Young DR, Maynard LM. Accelerometry-measured activity or sedentary time and overweight in rural boys and girls. Obes. Res. 2005;13:1606–1614. doi: 10.1038/oby.2005.197. [DOI] [PubMed] [Google Scholar]
- 21.Treuth MS, Schmitz K, Catellier DJ, et al. Defining accelerometer thresholds for activity intensities in adolescent girls. Med. Sci. Sports Exerc. 2004;36:1259–1266. [PMC free article] [PubMed] [Google Scholar]
- 22.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 23.Wrotniak BH, Epstein LH, Dorn JM, Jones KE, Kondilis VA. The relationship between motor proficiency and physical activity in children. Pediatrics. 2006;118:e1758–e1765. doi: 10.1542/peds.2006-0742. [DOI] [PubMed] [Google Scholar]