Summary
Early in development, the ocular lens establishes its distinctive architecture, and this is maintained throughout life as the lens continues to grow. This growth is tightly regulated through the proliferation of the lens epithelial cells and their subsequent differentiation into specialised elongated fiber cells. Although much work has been carried out to define these patterns of growth, very little has been reported on the detailed fate and kinetics of lens cells during embryogenesis. Using BrdU-incorporation, the present study has attempted to follow the fate of lens cells that have undergone at least one round of DNA synthesis during the early stages of lens morphogenesis. Results from this work have confirmed that the rate of lens cell proliferation and new fiber cell differentiation progressively slows as the lens differentiates and grows. In addition, these studies have shown that early in lens development, not all DNA synthesis is restricted to the lens epithelium, with some elongating fiber cells retaining the ability to undergo DNA synthesis. Adopting this system we have also been able to place the initiation of secondary fiber cell differentiation in the mouse lens by E12.5, concomitant with the loss of the lens vesicle lumen by the elongating primary fiber cells. Overall, this study has allowed us to revisit some of the mechanisms involved in early lens development, has provided us with insights into the fate of cells during this rapid phase of murine lens growth, and has provided a novel method to study the rate of new fiber cell differentiation over a defined period of lens development and growth.
Keywords: lens cell proliferation, fiber differentiation, lens growth, BrdU
Introduction
Cell proliferation is a fundamental process involved in the establishment and development of many embryonic tissues, including the ocular lens. Not only is this cellular process important for providing the precise number of cells that contribute to the establishment of the lens during embryogenesis, but it continues to provide precursor cells, albeit at a progressively reduced rate, that will subsequently undergo specialised differentiation to support the continued growth of the lens throughout life.
Lens morphogenesis is initiated upon contact of the evaginating optic vesicles with the overlying embryonic surface head ectoderm (see Robinson and Lovicu, 2004; Lovicu and McAvoy, 2005, for review). This close association initiates the thickening of this defined region of head ectoderm to form the lens placode. This subsequently invaginates to form the lens pit that then pinches off from the overlying ectoderm to form a hollow ball of cells known as the lens vesicle. At this developmental stage, defined differentiation events lead to the lens becoming polarized, with the posterior lens vesicle cells elongating and differentiating to form primary lens fiber cells, while the more anterior lens vesicle cells are retained as a differentiated epithelial monolayer.
During early lens morphogenesis, all cells have been shown to actively proliferate (Lovicu and McAvoy, 1999), but it is not until the lens vesicle cells begin to differentiate, that proliferation is primarily restricted to the anterior lens epithelium. During the rapid growth of the embryonic lens, many of these epithelial cells continue to proliferate; however, as the rate of lens growth begins to slow (most evident during postnatal development) proliferation is restricted to a region of the epithelium above the lens equator, generally known as the germinative zone (see Srinivasan and Harding, 1965). It is the turnover of cells in this region that has previously been reported to provide the precursor cells for the transitional zone, where lens epithelial cells exit the cell cycle and begin to elongate and differentiate into secondary fiber cells. With the reduction in lens cell proliferation with age, there is a corresponding reduction in the rate of secondary fiber cell differentiation (Hanna & O'Brien, 1961; Srinivasan and Harding, 1965; Treton and Courtois, 1981). Much of these findings have come about through earlier studies on the postnatal lens that have introduced precursors of DNA synthesis, such as H3-thymidine, to effectively tag cells entering S-phase. The fate of these cells was then followed after a prescribed period of time using autoradiography (Hanna & O'Brien, 1961; Hanna, 1965; Srinivasan and Harding, 1965; Rafferty and Rafferty, 1981; Zhou et al., 2006). These classical studies established some fundamentals of cell population kinetics in the postnatal lens including the following; (i) that labeled epithelial cells move from the germinative zone into the early fiber cell mass (Hanna & O'Brien, 1961; Hanna, 1965; Rafferty and Rafferty, 1981), (ii) that an equivalent number of labeled cells remain in the germinative zone after they divide (Rafferty and Rafferty, 1981); and (iii) that the rate of cell proliferation and subsequent migration into the fiber cell mass decreases with age (Hanna & O'Brien, 1961; Srinivasan and Harding, 1965; Treton and Courtois, 1981). Most of these studies examined the labeling patterns and changes in the postnatal lens of several species and reported similar trends. However, to date there is still no detailed study of the fate of proliferating cells in the differentiating embryonic lens, in particular no studies have addressed the question of what is the exact contribution of these cells to the growth of the lens.
Although new approaches for labeling proliferating cells are emerging (Wiley et al., 2010), in the present study we employed the more commonly adopted precursor of DNA synthesis, namely 5-bromo-2-deoxyuridine (BrdU), and used this to tag lens cells undergoing DNA synthesis throughout the different stages of lens morphogenesis and early postnatal growth, following their fate over a defined period of time. Findings from the present study, examining the key stages in lens morphogenesis, have not only provided support for some of the earlier pioneering studies described above, but offer new insights into the timing of key events during lens development, including the period of initiation of secondary fiber cell differentiation as well as the kinetics and fate of proliferating epithelial cells.
Methods
All experimental procedures involving animals in this study conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Ethics Committee of The University of Sydney.
Tissue Collection
To obtain embryonic tissues, FVB/N female mice (6-8 weeks old) were superovulated by injection with 5 IU of pregnant mare serum gonadotrophin (Lyppard, Sydney, Australia) followed by injection with 5 IU of human chorionic gonadotrophin (HCG; Lyppard) 47.5 hours later. After administration of HCG, superovulated females were individually placed overnight with males. The following morning, females were examined for the presence of a copulation plug (embryonic day 0.5). Pregnant mice at various stages following copulation were sacrificed and embryos/foetuses were delivered by Caesarean section. Embryonic mouse heads or postnatal eyes were removed, fixed overnight in 10% phosphate-buffered formalin, dehydrated, embedded in paraffin and processed for routine histology.
BrdU-immunolabeling
To examine the patterns of DNA replication (cells in S-phase of the cell cycle), incorporation of 5-bromo-2′-deoxyuridine (BrdU) was analysed using immunohistochemistry. Pregnant female mice at 10.5, 11.5, 12.5, 13.5, 14.5, 15.5 and 18.5 days post-copulation were injected intraperitoneally with 100 μg/g of BrdU diluted with sterile saline (0.9% NaCl). Two hours following injection, eyes from mothers, and embryonic mouse heads were collected, fixed and processed as previously described (Robinson et al., 1995). Alternatively, for BrdU-tracer experiments, animals were sacrificed and tissues collected either one day, two days, three days or one and two weeks (only for collection of postnatal eyes) following administration of BrdU.
To detect BrdU incorporation, 6 μm thick sections of tissues were hydrated, quenched with 3% H2O2 in 10% methanol to block endogenous peroxidase activity and incubated with 0.02% pepsin (20 minutes at 37°C). Following pepsin digestion, sections were treated with 2N HCl to denature the nucleic acids to facilitate epitope recognition with the primary antibody. Sections were rinsed in phosphate buffered saline supplemented with 0.1% BSA (PBS/BSA) and incubated for 30 minutes in 3% normal goat serum to reduce non-specific staining. Sections were then incubated overnight at 4°C with a monoclonal anti-BrdU antibody (Bioclone, Australia) diluted 1:100 with PBS. Binding of the primary antibody was visualised using a goat-anti-mouse IgG conjugated to horseradish peroxidase (HRP; diluted 1:50 with PBS; Silenus, Australia) for 2 hours at room temperature. Sections were rinsed with PBS/BSA, followed by a rinse in deionised water, before applying the 3,3′-diaminobenzidine tetrahydrochloride (DAB; 0.5 mg/ml, diluted in 0.05M Tris, 0.01% H2O2) substrate. The DAB was rinsed off and cell nuclei were counterstained with Harris haematoxylin. Sections were visualised using bright-field microscopy (Leica-DMLB, Germany) and photography was carried out using a digital camera (Leica DC-280, Germany).
Analysis of Cell Counts
For each developmental stage, we examined five or six BrdU-labeled mid-sagittal tissue sections from four different animals. Microscopic images from each of these sections were scored, with manual counts for BrdU-labeled cell nuclei recorded for both central and peripheral lens epithelium (for Figure 7B) and fiber cells (for Figure 6). Analysis of the percentage of BrdU-labeled fiber cell nuclei was conducted for all time periods. For the analysis of percentage of BrdU-labeled cells in the central and peripheral epithelium, only the 2 hour time period was assessed. The central and peripheral epithelial regions were demarcated by the most anterior margin of the developing optic cup (for later stages we used the developing iris). Total cell counts were based on total cell nuclei. The average percentage of labeled cell nuclei for each lens region was determined. Error bars for histograms were incorporated using standard errors for each grouping.
Figure 7.
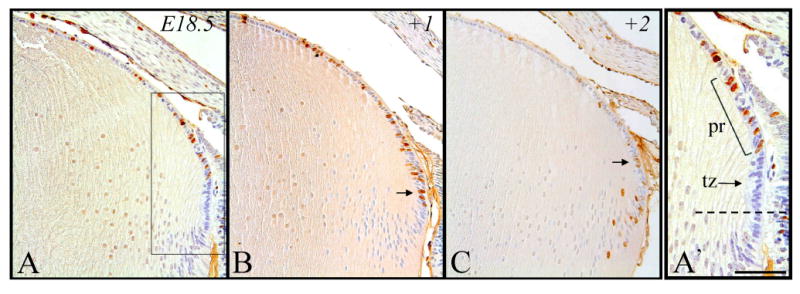
Representative micrographs of BrdU-labeled cells in murine embryonic sections of developing eyes, counterstained with haematoxylin. Embryonic tissue incorporated BrdU at E18.5 and cells were subsequently labeled after 2 hrs (A), 24 hrs (B) and 48 hrs (C). A′. Higher magnification of boxed region shown in A. Above the lens equator (broken line), a cluster of BrdU-labelled cells are present in the peripheral region (pr), followed posteriorly by another region of cells (transitional zone; tz) that have exited the cell cycle (not labeled for BrdU) as they elongate and differentiate into secondary fiber cells. B. Labeled cells in transitional zone. C. Labeled cells in peripheral region. Scale bar, A-C, 200μm; A′, 100μm.
Figure 6.
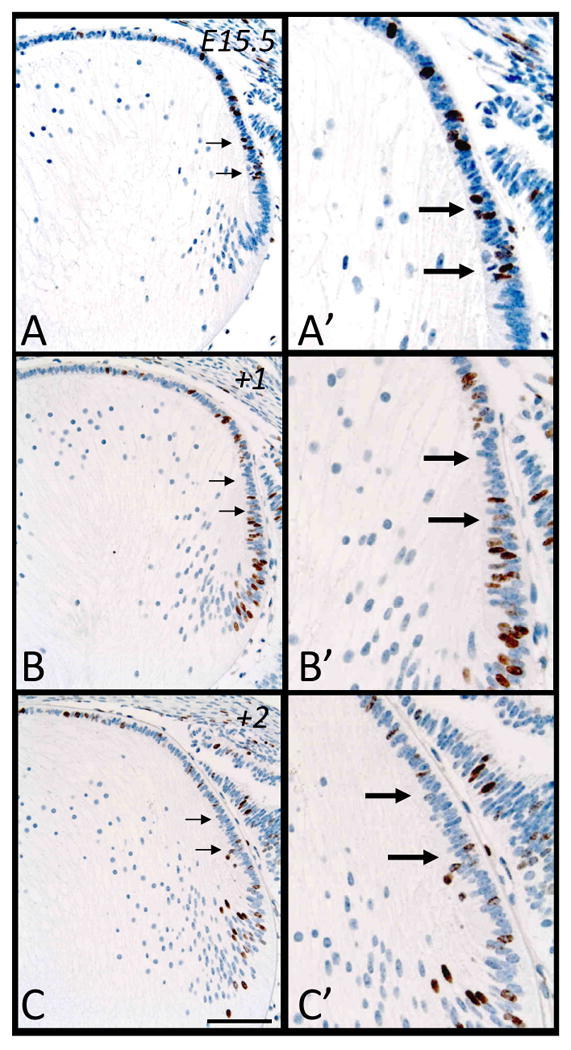
Representative micrographs of BrdU-labeled cells in murine embryonic sections of developing eyes, counterstained with haematoxylin. Embryonic tissue tagged with BrdU at E15.5 was labeled after 2 hrs (A, A′), 24 hrs (B, B′) and 48hrs (C, C′). Arrows indicate changes in patterns of labeling in the peripheral region of the epithelium, with progressively fewer labeled cells. Scale bar, A-C, 200μm; A′-C′,100μm.
Results
For each embryonic developmental age, tissues were examined for the incorporation of BrdU after being exposed to this compound for 2 hours, 24 hours (+1 day) or 48 hours (+2 days). In the case of postnatal eyes, tissues were also exposed for extended periods ranging from 7 days (+7) to 15 days (+15). It should be noted that precursors of DNA synthesis such as BrdU and H3-thymidine, upon administration, are only available to cells for up to two hours (Cenedella, 1987; Cenedella, 1989a; Cameron and McKay, 2001), hence no ‘new’ incorporation is expected to take place beyond this time. Once cells take up BrdU, for example, they are permanently tagged, making it ideal to trace their fates, as carried out in this study.
Embryonic Day 10.5
During murine lens morphogenesis, at E10.5, the presumptive lens ectoderm has invaginated to form the lens pit. Following a two hour exposure to BrdU, most of these cells demonstrate DNA synthesis (more cells labeled for BrdU than not; Figure 1A), consistent with the fact that many of these cells are actively proliferating (presence of mitotic figures; see McAvoy, 1978). A day later (+1, Figure 1B), these BrdU-tagged cells, that now comprise the majority of the new lens vesicle, are present in the anterior lens vesicle and throughout the posterior lens vesicle cell population which has started to elongate into the primary fibers (Figure 1B, arrows). These mostly amitotic primary fiber cells retain the strongest label for BrdU a further day in development (+2, equivalent to E12.5 of embryogenesis) as they continue to elongate, obliterating the lumen of the lens vesicle as they make contact with the overlying epithelium (Figure 1C). In contrast, the epithelial cells are more weakly reactive for BrdU (Figure 1C, arrowheads) as they continue to dilute this BrdU label through subsequent mitotic divisions (see Figure 4G).
Figure 1.
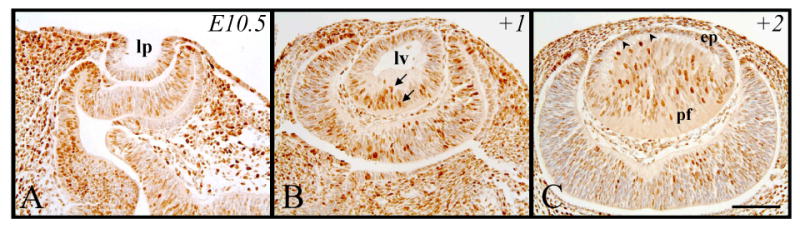
Representative micrographs of BrdU-labeled cells in murine embryonic sections of developing eyes, counterstained with haematoxylin. Embryonic tissue incorporated BrdU at E10.5 and cells were labeled after 2 hrs (A), 24 hrs (B, +1) and 48 hrs (C, +2). B. Arrows indicate labeled primary fiber cells. C. Arrowheads indicate labeled epithelial cells. Abbreviations: ep, lens epithelium; lp, lens placode; lv, lens vesicle; pf, primary fibers. Scale bar, 200μm.
Figure 4.
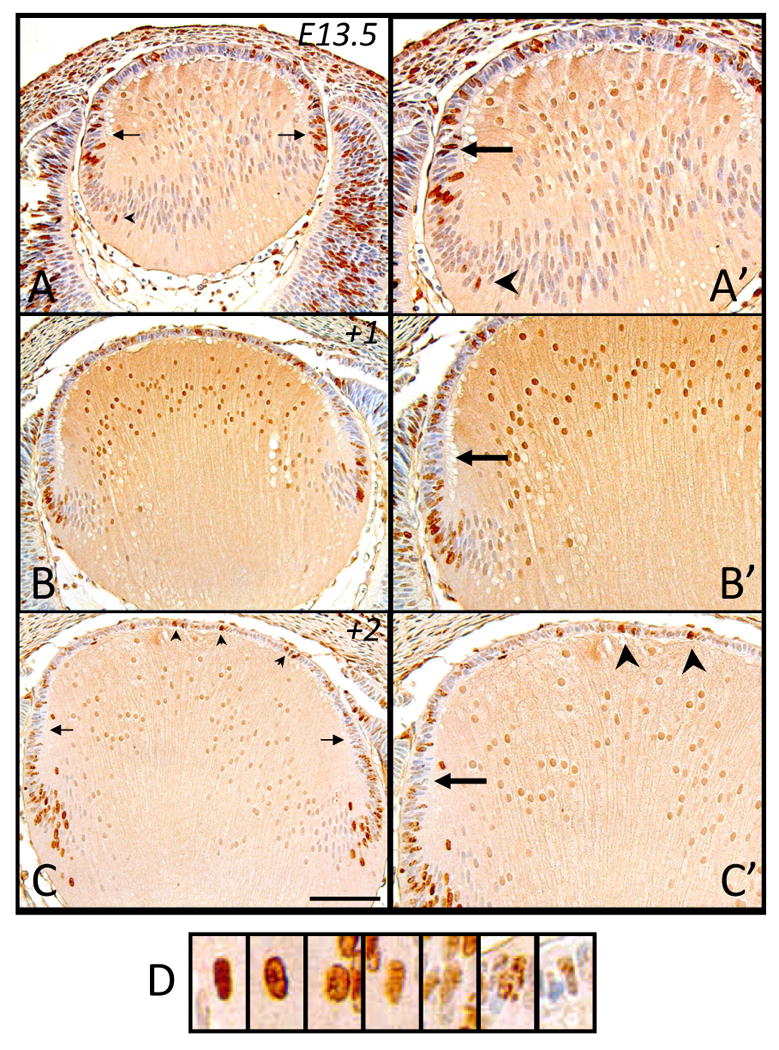
Representative micrographs of BrdU-labeled cells in murine embryonic sections of developing eyes, counterstained with haematoxylin. Embryonic tissue tagged with BrdU at E13.5 was labeled after 2 hrs (A, A′), 24hrs (B, B′) and 48hrs (C, C′). A, A′. Arrows indicate stronger labeled cells in peripheral region. B′, C, C′. Arrows indicate peripheral region devoid of strongly labeled cells while arrowheads show strongly labeled epithelial cells. D. Representative higher magnification images of lens cell nuclei from C, showing differential labeling of BrdU incorporation. The label weakens from left to right, becoming ‘diluted’, as would be the case through consecutive mitotic divisions. Note that high background staining in B, B′ has resulted in artifactual labeling of the central lens fiber cell nuclei. Scale bar, A-C, 200μm; A′-C′,130μm.
Embryonic Day 11.5
At E11.5, the posterior cells of the early lens vesicle are just beginning to elongate into primary fiber cells (Figure 2A). After a two hour exposure to BrdU, most of the BrdU-incorporation is observed in the anterior lens vesicle cells (Figure 2A); however, many posterior lens vesicle cells elongating into primary fibers still retain the ability to undergo DNA synthesis (Figure 2A, arrowheads). This is supported by examining BrdU-tagged cells one day later (+1), at E12.5, where some of the more mature primary fibers (closer to the lens core) are labeled for BrdU (Figure 2B′, arrowhead). At this stage, we note that the strongest BrdU-labeled cells are found in the transitional zone of the lens, indicating that these cells most likely underwent a final round of cell division a day earlier, prior to becoming secondary fiber cells (Figure 2B, 2B′, arrows). There are also many BrdU-labeled cells throughout the epithelium (Figure 2B) indicating that these anterior cells are increasing in number as well as beginning to provide the precursors for secondary fiber cells. A day later (+2, equivalent to E13.5 of embryogenesis), as the lens continues to grow, more labeled cells (originating from the equatorial region of the lens; see Figure 2B) now comprise the lens fiber mass (Figure 2C), indicating the progression of secondary fiber differentiation. Many of the epithelial cells continue to label for BrdU. It is interesting to note that the peripheral region of the lens epithelium that would normally contain the highest mitotic index by this developmental age (see McAvoy 1978; compare with equivalent stage of E13.5, Figure 4A), does not contain many cells that have strong BrdU-labeling (Figure 2C, arrowheads).
Figure 2.
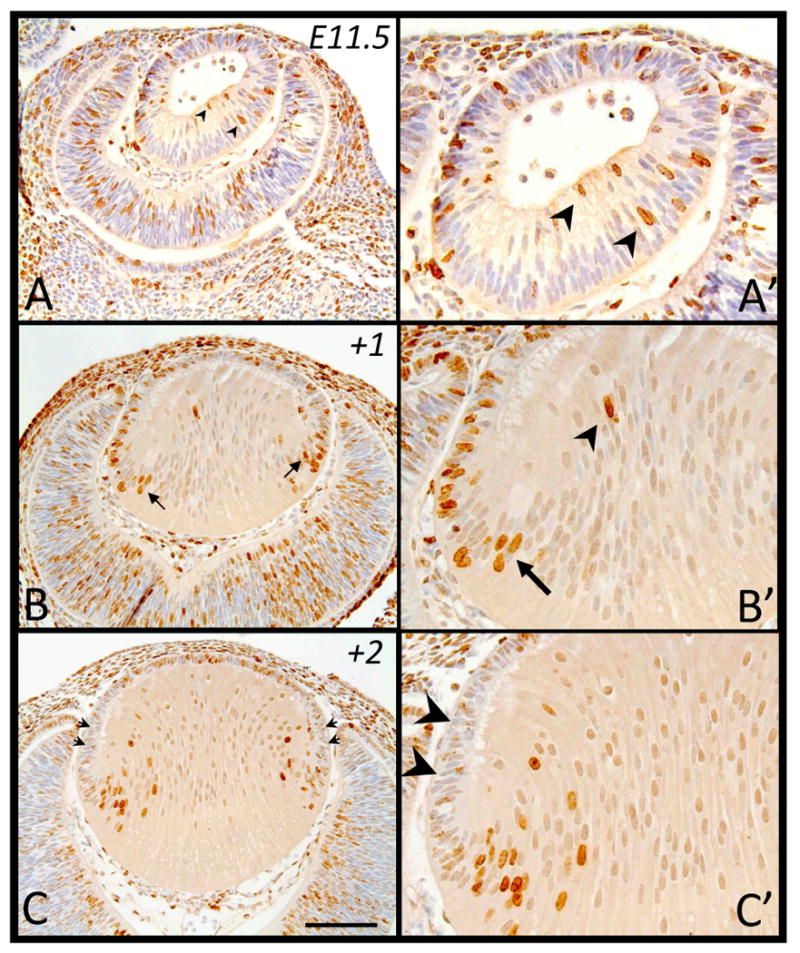
Representative micrographs of BrdU-labeled cells in murine embryonic sections of developing eyes, counterstained with haematoxylin. Embryonic tissue tagged with BrdU at E11.5 was labeled after 2 hrs (A, A′), 24hrs (B, B′) and 48hrs (C, C′). A, A′. Arrowheads indicate labeled primary fiber cells. B, B′. Arrows indicate labeled primary fiber cells while arrowhead shows a labeled mature fiber cell. C, C′. Arrowheads indicate the peripheral epithelium devoid of strongly labeled cells. Scale bar, A-C, 200μm; A′-C′,100μm.
Embryonic Day 12.5
At E12.5, the primary fiber cells have made contact with the lens epithelium (Figure 3A). When we examine for DNA synthesis following the two hour exposure to BrdU, the epithelial cells of the lens retain most of the BrdU-labeled cells (Figure 3A), with prominently labeled cells at the lens equator (Figure 3A, 3A′, arrow), adjacent to the anterior margins of the differentiating optic cup. Once again, similar to our observation at E11.5, in some lenses, some posterior lens cells, now prominent primary fiber cells, also show a strong BrdU label (Figure 3A, 3A′, arrowhead). These sporadic labeled cells (sometimes appearing in pairs, not shown) may have arisen from epithelial cell divisions during the period of BrdU exposure; however, we can not exclude the possibility that they are young fiber cells that have retained the ability to synthesise DNA. A day later (+1), at E13.5, BrdU-tagged cells have shifted from the lens equator and are becoming incorporated into the fiber cell mass as they differentiate (Figure 3B, 3B′). Anterior epithelial cells are also labeled, with most displaying a weaker label to that seen in the fiber cells. Further in development, two days after initial BrdU incorporation (+2), the trend noted earlier is more apparent, with weakly labeled peripheral epithelial cells (3C, 3C′, arrows) and an influx of strongly labeled cells observed in the lens fiber mass (Figure 3C).
Figure 3.
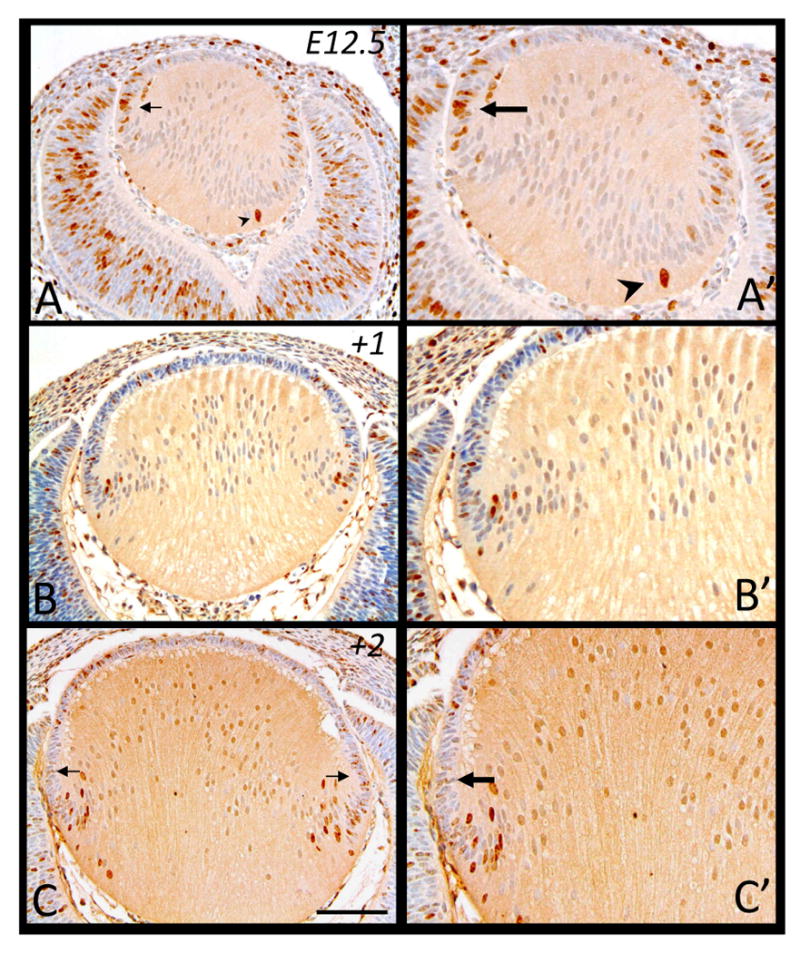
Representative micrographs of BrdU-labeled cells in murine embryonic sections of developing eyes, counterstained with haematoxylin. Embryonic tissue incorporated BrdU at E12.5 and cells were labeled after 2 hrs (A, A′), 24 hrs (B, B′) and 48 hrs (C, C′). A, A′. Arrow indicates labeled equatorial epithelial cell with arrowhead identifying a labeled primary fiber cell. C. Arrows indicate weaker labeled cells in the peripheral region. Scale bar, A-C, 200μm; A′-C′,130μm.
Embryonic Day 13.5
With increasing growth of the lens at E13.5, after a two hour exposure to BrdU, we observe that immunoreactivity for BrdU is primarily localized to the lens epithelium (Figure 4A). Like E12.5; however, the label in the epithelium is most pronounced in the peripheral region (Figure 4A, 4A′, arrows), with the occasional fiber cell still incorporating BrdU (Figure 4A, 4A′, arrowhead). A day after these cells are tagged, although many are retained in the central epithelium, we note that they just begin to infiltrate the lens fiber mass as they differentiate (Figure 4B). The increased height and girth of the lens (based on tissue sections) over this day is most likely due to the increased size of the primary fiber cells, as the rate of secondary fiber differentiation, based on the numbers of BrdU-tagged cells contributing to the fiber mass (see Figure 9 for cell counts), does not appear to be markedly different to that observed at E12.5 (compare Figures 3B and 4B). Similarly, although more BrdU-labeled cells are recruited to the lens cortex after another day of development (+2, Figure 4C; equivalent to E15.5), the rapid growth of the lens over this period can not solely be attributed to an increase in cell number but must, as for the earlier period, be largely due to the increased size of primary fiber cells. Consistent with earlier ages, the peripheral region of the epithelium is mostly devoid of strongly labeled BrdU-tagged cells (Figure 4C, 4C′, arrows), with prominently labeled cells still evident through the anterior central lens epithelium (Figure 4C, 4C′, arrowheads).
Figure 9.
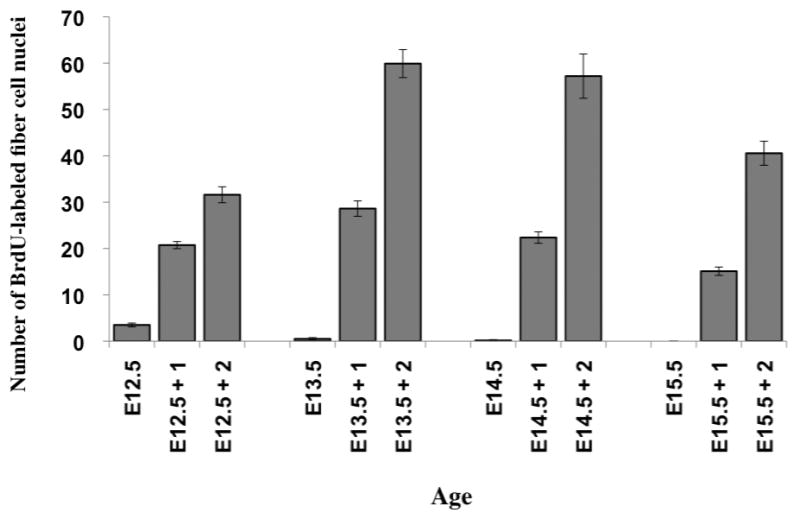
Number of BrdU-labeled fiber cell nuclei, following a 2 hour, 24 hour (+1) or 48 hour (+2) period from when the lens epithelial cell nuclei were labeled at each respective gestational age.
Embryonic Day 14.5
At E14.5, following a two hour exposure to BrdU we observe a consistent pattern of labeling to that seen in earlier developmental stages, however, this is the earliest stage whereby DNA synthesis is exclusively localized to the lens epithelium (Figure 5A). Unlike earlier stages, we do not see any BrdU-incorporation in any of the mature primary or early secondary fiber cells. Although BrdU-labeled cells in the lens epithelium are still clustered in the peripheral epithelium, at this stage, there is a similar number of BrdU-labeled cells distributed throughout the more anterior central epithelium (Figure 5A, Figure 10B). A day later (+1), most of these labeled cells are still evident in these same regions, with numbers increasing in the central epithelium as well as a few posterior labeled cells entering the transitional zone, concomitant with their elongation into secondary fibers (Figure 5B). At E16.5, two days (+2) after the cells first incorporated BrdU, cells have clearly become incorporated into the fiber cell mass, extending beyond the transitional zone (Figure 5C). This event appears to be somewhat comparable to that observed at earlier stages indicating that the rate of lens growth, based on the numbers of new secondary fiber cells differentiating, is similar between embryonic days 13.5 to 15.5 (Figure 4A-4C), and E14.5 to E16.5 (Figure 5A-C, see Figure 9 for cell counts). Again this indicates that an increase in fiber cell size is a major contributing factor to the increase in lens size between these latter stages.
Figure 5.
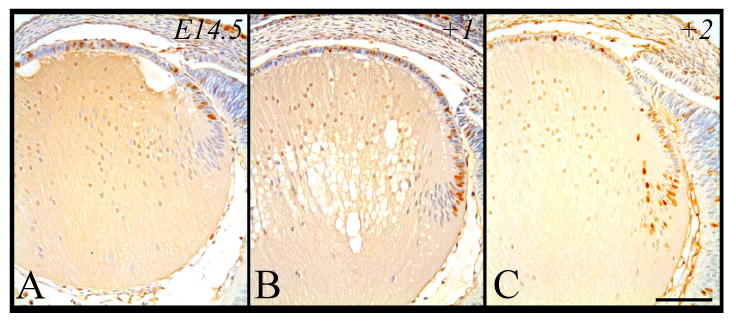
Representative micrographs of BrdU-labeled cells in murine embryonic sections of developing eyes, counterstained with haematoxylin. Embryonic tissue incorporated BrdU at E14.5 and cells were labeled after 2 hrs (A), 24 hrs (B) and 48 hrs (C). Scale bar, 200μm.
Figure 10.
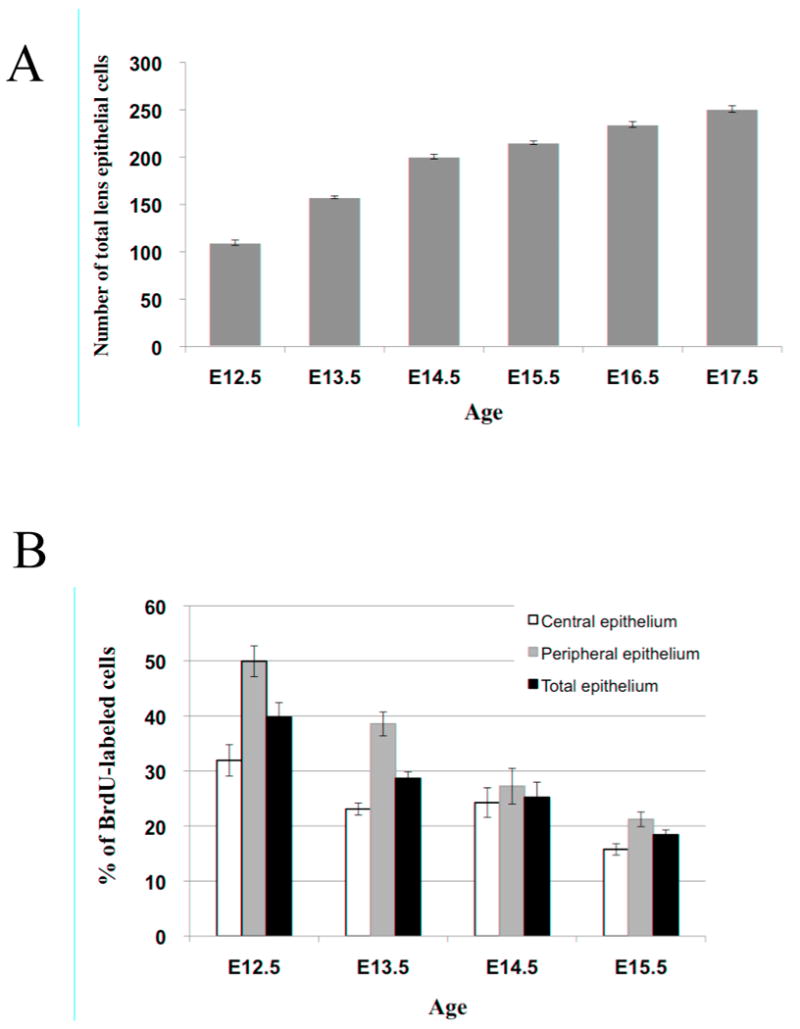
(A). Average number of lens cells comprising the lens epithelium of embryos from gestational ages ranging from E12.5 to E17.5, demonstrating a progressive increase in the total number of lens epithelial cells with age. (B). Percentage of labeled cells after a 2 hour period in either the central lens epithelium, peripheral lens epithelium or total lens epithelium at each gestational age examined, demonstrating a progressive reduction in the rate of lens epithelial cell proliferation with increasing embryonic age.
Embryonic Day 15.5
The changes we observe in the fate of cells labeled with BrdU at E15.5 through to E17.5 (Figure 6A-C) are very similar to those observed between E14.5 to E16.5 (Figure 5A-C). BrdU-tagged cells are prominent in the peripheral region of the epithelium at E15.5 (Figure 6A, 6A′, arrows) and over a 48 hour period, these cells progressively vacate this region (Figure 6B-C, 6B′-C′, arrows), becoming displaced primarily towards the transitional zone where they elongate into fibers (Figure 6B-C). The number of labeled cells in the anterior central epithelium also increases. The fact that there are fewer BrdU-labeled cells in the fiber cell mass after 48 hours (Figure 6C), compared to those of E14.5 after the same period (see Figure 5C), indicates that lens growth dependent on new secondary fiber cell differentiation is progressively slowing (see Figure 9 for cell counts).
Embryonic Day 18.5
At embryonic day 18.5, after a two hour exposure to BrdU, labeled cells are found throughout the lens epithelium, both in the peripheral and central regions (Figure 7A). After 24 hours (+1), cells in the peripheral region appear to have increased in number and have progressed more posteriorly, with the occasional cell observed in the transitional zone (Figure 7B, arrow). The fact that many of these cells after 24 hours still retain a relatively dark label (not diluted out) indicates that the rate of cell proliferation has also slowed. After 48 hours (+2, close to birth age), the most posterior BrdU-tagged cells have become incorporated into the fiber cell mass; however, some are still present in the peripheral region of the epithelium (Figure 7C, arrow). Cells throughout the central epithelium are still labeled strongly for BrdU but their numbers are reduced (Figure 7C).
Young adults (P40-50)
The postnatal eyes examined for this stage were primarily collected from the pregnant mice sacrificed for embryo collection; injected with BrdU and exposed for comparable periods as described above. As reported previously, much of the cell proliferation (DNA synthesis) in adult mice is confined to the peripheral region of the epithelium (at postnatal stages this is generally referred to as incorporating the germinative zone) and this was clearly evident after a two hour exposure to BrdU (Figure 8A, arrowhead). Unlike earlier stages examined, labeled cells in this peripheral region were also markedly decreased in number (approximately 1 to 2 labeled cells), with very few (an occasional labeled epithelial cell was observed near the line of iris contact) to no cells labeled throughout the remainder of the epithelium (Figure 8A). The following day (+1), these BrdU-tagged cells approximately doubled in number but were still primarily situated peripherally (Figure 8B). Following a further 24 hrs (+2), cell numbers had again approximately doubled (Figure 8C, arrowheads). After another day (+3), there was no appreciable difference in the number of BrdU-tagged cells and there was no apparent displacement from the peripheral region (data not shown, see Figure 8C). It was not until after 7 days (+7) from when the cells were originally tagged (Figure 8D), and even more obvious after 15 days (+15, Figure 8E), that some of the cells were displaced posteriorly into the fiber cell mass (see Figure 8D, E, arrowheads). While some labeled cells were retained in the peripheral epithelium after 7 days (sometimes observed as pairs; Figure 8D, arrowhead with asterisk), cells had become displaced into the lens cortex, reaching the bow zone (most posterior lens fiber nuclei) and further into the cortex after 15 days (Figure 8E, 8E′, arrowheads and asterisk). Consistent with the reduced rate of postnatal lens growth, it was not too surprising that the rate of cell proliferation and subsequent differentiation into secondary fiber cells was clearly reduced compared to that shown in the early embryo.
Figure 8.
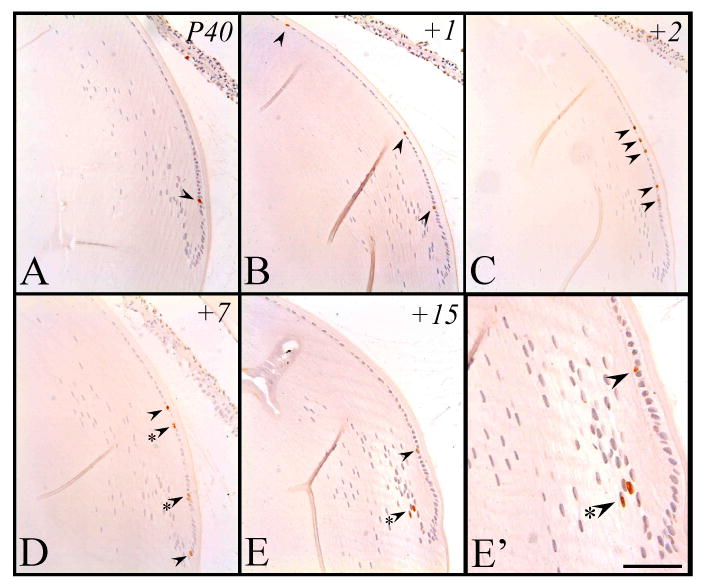
Representative micrographs of BrdU-labeled cells in postnatal murine eye sections, counterstained with haematoxylin. Tissue incorporated BrdU at approximately P40 and cells were subsequently labeled after 2 hrs (A), 24 hrs (B), 48 hrs (C), 7 days (D) and 15 days (E, E′). Arrowheads point to labeled cells. Labeled cell pairs are also highlighted with an asterisk. Scale bar, A-E, 100μm; E′, 50μm.
Discussion
In the present study, by permanently tagging lens cells in vivo using BrdU incorporation, we have been able to follow their fate during the very rapid growth associated with lens morphogenesis and differentiation. Given the fact that the rates of cell proliferation are generally high in early stages of embryogenesis, it was not too surprising that most cells involved in early lens formation, namely in the lens pit, were labeled for BrdU after a two hour exposure. Following the fate of these early labeled cells over 48 hours was not very informative, other than to confirm that both forms of lens cells, epithelial and fiber, that are present at E12.5, are derived from the ‘stem’ cell population of the lens pit. By E11.5, the murine lens begins to establish its polarity as the posterior lens vesicle cells start to elongate to become the primary lens fibers. Interestingly, some of these elongating cells still retain the ability to synthesise DNA. This phenomenon was not restricted to E11.5, as early differentiating fiber cells were also BrdU-labeled at E12.5 and E13.5, albeit at reduced numbers (see Figure 4A). This is consistent with an earlier report of an occasional BrdU-labeled cell detected below the lens equator in the E13.5 murine lens (Martinez et al., 2009). Whether this is a property of some late differentiating primary fiber cells or early secondary fiber cells could not be resolved; however, these cells are unlikely to be secondary fibers, as it is improbable that within a 2 hour window, a lens epithelial cell tagged with BrdU during S-phase of the cell cycle, would have time to undergo mitosis, then exit from the cell cycle and migrate or become displaced as a new secondary fiber cell in the lens cortex. By E14.5, after a two hour exposure to BrdU, there was no longer any sign that fiber cells retained the ability to synthesise DNA. For these few differentiating fiber cells in the early embryonic lens that appear to retain the ability to synthesize DNA, although we can also not unequivocally discount the fact that the incorporation of BrdU may have resulted from these cells repairing small amounts of DNA (see Cameron and McKay, 2001), we consider this event unlikely as the immunolabeling of these cells was relatively strong, a feature more consistent with cells replicating their complete genome.
An important result from this labeling study has been resolution of the issue of when secondary fiber cell differentiation begins. Our analysis shows that this process begins by E12.5 and is concomitant with the obliteration of the lens vesicle lumen by the elongating primary fiber cell mass (see Figures 2B, 3A and 3B). We define the initiation of secondary fiber cell differentiation when we detect the presence of the earliest BrdU-labeled fiber cells that have originated from the lens epithelium. At E12.5, after a 2 hour exposure to BrdU, we report strong labeling throughout the lens epithelium, with an occasional BrdU-labeled cell deeper in the primary fiber cell mass (Figure 3A; as discussed earlier). If we examine an equivalent E12.5 stage of development, this time when BrdU was incorporated 24 hours prior to labeling (Figure 2B), we note the marked increase in numbers of BrdU-labeled fiber cells that have originated from the lens epithelium (originally labeled at E11.5). This difference in number of labeled fiber cells (comparing 3A and 2B) indicates that secondary fiber differentiation has commenced by E12.5. It could be also argued that this is an underestimation, and that this process begins even earlier. At E11.5, in the lens vesicle stage shown in Figure 1B (24 hours after BrdU-incorporation), all the primary fibers have begun to elongate towards the anterior lens cells. One day later in development, at E12.5 (now labeled 48 hours after BrdU incorporation; Figure 1C), we note that the fiber cells have at least doubled in number. This is mostly attributed to the prior addition of new fiber cells through the process of secondary fiber cell differentiation; however we cannot exclude the possibility that some labeled cells in the posterior of the lens vesicle at E11.5 (that are at early stages of primary fiber elongation) are still able to divide and generate some of the additional cell numbers evident at E12.5. Therefore, whilst it could be argued that some secondary fiber differentiation may occur earlier, this process is clearly established by E12.5.
It is also from E12.5, in early development, that lens growth and size dramatically increases, not only due to the fact that the number of lens epithelial cells increases (see Figure 10A) and that new secondary fibers are progressively being added to the fiber cell mass, but primarily because the first established primary fibers and the newly added secondary fibers are increasing in length. This was apparent throughout lens morphogenesis as the dramatic increase in height and girth of the lens, between E12.5 to E18.5, could not be attributed solely to increased numbers of differentiating secondary fibers (BrdU-tagged cells) contributing to the lens fiber mass. This is supported by the fact that the differentiating cells added to the fiber cell mass at E13.5 (Figure 4B) are comparable (or less) in number to the secondary fiber cells added to the larger lens at E14.5 (Figure 5B) and E15.5 (Figure 6B) after 24 hours (see Figure 9 for cell counts). With the increasing length of fiber cells contributing to the growth of the lens, we also see a large number of BrdU-labeled lens epithelial cells, contributing to the increasing area of the epithelial monolayer covering the anterior surface of the fiber cell mass. These epithelial cells appear to be continually proliferating as evident by the weaker (‘diluted’) BrdU label of numerous cell nuclei, in contrast to the strongly labeled nuclei of the tagged cells that have most likely exited the cell cycle and elongated to contribute to the lens cortex. Many of these latter cells are derived directly from the peripheral epithelium, as this region is typically devoid of strong BrdU-labeled cells two days after its administration (see Figures 4C, 5C & 6C).
Overall, the peripheral epithelium also shows a higher proportion of BrdU-labeled cells than the central region (Figure 10B; see also Figures 3A and 4A). However, this is only clearly the case for the early stages of lens morphogenesis (E12.5-E13.5). At later stages, particularly at E14.5, there is little or no distinction between these regions in terms of BrdU incorportation (Figure 10B). It is not clear if this reflects other events occurring in the epithelium at this time but it does preclude the identification of this peripheral region of the epithelium as the ‘germinative zone’, at least during all stages of embryonic development.
Although it has been reported previously (Hanna and O'Brien, 1961; von Sallman et al., 1962; Treton and Courtois, 1981; Cenedella, 1989b; Bozanic and Saraga-Babic, 2004, Rajagopal et al., 2008), and confirmed in the present study, that the rate of lens growth declines with its development, in this study we have also observed the earliest signs of this delayed growth. When comparing the number of BrdU-labeled cells after a two hour exposure in all the different embryonic ages examined, we clearly see that in general the percentage of lens cells incorporating BrdU (synthesising DNA) progressively decreases in both the central and more peripheral regions of the lens epithelium (Figure 10B). On a similar note, if we compare the number of BrdU-tagged cells from each of the different ages after 24 or 48 hours, we note that with increasing age, progressively fewer cells are recruited to the fiber cell mass over this period of examination (Figure 9). This is evident throughout lens morphogenesis (e.g. compare Figure 4C and 6C) and even more pronounced at postnatal stages where it takes up to a week to observe a labeled cell entering the lens cortex (see Figure 8D).
Overall, we see that when we adopt this established labeling technique to permanently tag lens cells, and examine this throughout the process of lens morphogenesis, we can readily trace the fate of these cells during this rapid phase of tissue growth. Now that we have observed specific trends in the kinetics of lens cells during stages of normal lens morphogenesis in the mouse, we can apply these new strategies and information to better characterize patterns of lens growth in different animal models, including other mouse models. Basic information on the contributions of proliferation and fiber elongation to lens growth, both embryonic and postnatal, is central to investigating mechanisms that ensure the lens acquires dimensions appropriate for each developmental stage.
Research Highlights.
Using BrdU-incorporation to follow the fate of lens cells during murine lens morphogenesis and growth we:
have confirmed that the rate of lens cell proliferation and fiber cell differentiation progressively slows with age.
have shown that not all DNA synthesis is restricted to the lens epithelium, with some primary fiber cells retaining the ability.
identified the initiation of secondary fiber cell differentiation
provide a novel method to study the rate of new secondary fiber cell differentiation over a defined period of lens development and growth.
Acknowledgments
The authors would like to acknowledge the support of the National Institutes of Health (R01 EY0-3177), National Health and Medical Research Council (NHMRC), the Sydney Foundation for Medical Research, and the International Student Placement Centre (ISPC), Sydney, Australia for organizing and providing support for a student internship (GK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bozanic D, Saraga-Babic M. Cell proliferation during the early stages of human development. Anat Embryol. 2004;208:381–388. doi: 10.1007/s00429-004-0410-5. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the Dentate Gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cenedella RJ. Direct chemical measurement of DNA synthesis and net rates of differentiation of rat lens epithelial cells in vivo: Applied to the selenium cataract. Exp Eye Res. 1987;44:677–690. doi: 10.1016/s0014-4835(87)80138-0. [DOI] [PubMed] [Google Scholar]
- Cenedella RJ. Cell cycle specific effects of selenium on the lens epithelium studied in vivo by the direct chemical approach. Curr Eye Res. 1989a;8:429–433. doi: 10.3109/02713688908996391. [DOI] [PubMed] [Google Scholar]
- Cenedella RJ. Aging and rates of lens-cell differentiation in vivo, measured by a chemical approach. Invest Ophthalmol Vis Sci. 1989b;30:575–579. [PubMed] [Google Scholar]
- Hanna C. Changes in DNA, RNA, and protein synthesis in the developing lens. Invest Ophthalmol. 1965;4:480–491. [PubMed] [Google Scholar]
- Hanna C, O'Brien JE. Cell production and migration in the epithelial layer of the lens. Archs Ophthal. 1961;66:103–107. doi: 10.1001/archopht.1961.00960010105023. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Spatial and temporal expression of p57KIP2 during murine lens development. Mech Dev. 1999;86:165–169. doi: 10.1016/s0925-4773(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Martinez G, Wijesinghe M, Turner K, Abud HE, Taketo MM, Noda T, Robinson ML, de Iongh RU. Conditional mutations of β-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest Opthalmol Vis Sci. 2009;50:4794–4806. doi: 10.1167/iovs.09-3567. [DOI] [PubMed] [Google Scholar]
- McAvoy JW. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morph. 1978;45:271–281. [PubMed] [Google Scholar]
- Rafferty NS, Rafferty KA. Cell population kinetics of the mouse lens epithelium. J Cell Physiol. 1981;107:309–315. doi: 10.1002/jcp.1041070302. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Dattilo LK, Kaartinen V, Deng CX, Umans L, Zwijsen A, Roberts A, Bottinger EP, Beebe DC. Functions of the type1 BMP receptor Acvr1 (Alk2) in lens development: cell proliferation, terminal differentiation, and survival. Invest Opthalmol Vis Sci. 2008;49:4953–4960. doi: 10.1167/iovs.08-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Lovicu FJ. The lens: historical and comparative perspectives. In: Lovicu FJ, Robinson ML, editors. Development of the ocular lens. Cambridge University Press; New York: 2004. pp. 3–26. [Google Scholar]
- Srinivasan BD, Harding CV. Cellular proliferation in the lens. Invest Ophthalmol. 1965;4:452–470. [PubMed] [Google Scholar]
- Treton JA, Courtois Y. Evolution of the distribution, proiliferation and ultraviolet repair capacity of rat lens epithelial cells as a function of maturation and aging. Mech Aging Dev. 1981;15:251–267. doi: 10.1016/0047-6374(81)90134-2. [DOI] [PubMed] [Google Scholar]
- von Sallman L, Grimes P, McElvain N. Aspects of mitotic activity in relation to cell proliferation in the lens epithelium. Exp Eye Res. 1962;1:449–456. doi: 10.1016/s0014-4835(62)80037-2. [DOI] [PubMed] [Google Scholar]
- Wiley LA, Shui YB, Beebe DC. Visualizing lens epithelial cell proliferation in whole lenses. Molecular Vision. 2010;16:1253–1259. [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Leiberman J, Xu J, Lavker RM. A hierarchy of proliferative cells exists in mouse lens epithelium: implications on lens maintenance. Invest Ophthalmol. 2006;47:2997–3003. doi: 10.1167/iovs.06-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]


