Abstract
Programmed death-1 (PD-1), a member of the CD28 costimulatory receptor family, is expressed by germinal center-associated T cells in reactive lymphoid tissue. In a study of a wide range of lymphoproliferative disorders, neoplastic T cells in 23 cases of angioimmunoblastic lymphoma were immunoreactive for PD-1, but other subtypes of T cell and B cell non-Hodgkin lymphoma, as well as classic Hodgkin lymphoma, did not express PD-1. The pattern of PD-1 immunostaining of neoplastic cells in angioimmunoblastic lymphoma was similar to that reported for CD10, a recently described marker of neoplastic T cells in angioimmunoblastic lymphoma. Tumor-associated follicular dendritic cells in cases of angioimmunoblastic lymphoma were found to express PD-L1, the PD-1 ligand. In addition, PD-1-positive reactive T cells formed rosettes around neoplastic L&H cells in 14 cases of nodular lymphocyte predominant Hodgkin lymphoma studied. These findings, along with data from previous studies, suggest that angioimmunoblastic lymphoma is a neoplasm of germinal center-associated T cells and that there is an association of germinal center-associated T cells and neoplastic cells in nodular lymphocyte predominant Hodgkin lymphoma. PD-1 is a useful new marker for angioimmunoblastic lymphoma and lends further support to a model of T-cell lymphomagenesis in which specific subtypes of T cells may undergo neoplastic transformation and result in specific, distinct histologic, immunopheno-typic, and clinical subtypes of T-cell neoplasia.
Keywords: non-Hodgkin lymphoma, CD28 family, nodular lymphocyte predominant, Hodgkin lymphoma
Programmed death-1 (PD-1) is a member of the CD28 family of receptors that includes CD28, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), inducible costimulator (ICOS), and B- and T-lymphocyte attenuator (BTLA; reviewed by Riley and June4 and Sharpe and Freeman2). These receptors play a role in the cellular immune response. For example, CD28 serves as a costimulatory receptor that enhances T-cell activation, whereas CTLA-4 serves as an inhibitor of T-cell activation.1,2 PD-1 also has an inhibitory function on T cells and B cells, and is important in peripheral tolerance.1–3 There are at least 2 ligands for PD-1, PD-L1, and PD-L2, which are expressed on a range of cells.4
CD28 is constitutively expressed on most or all CD4+ T cells and approximately 50% of CD8+ T cells, whereas CTLA-4 is not expressed on resting T cells.1 PD-1 is also expressed on activated T cells, B cells, and myeloid cells.5 Iwai and coworkers5 studied the micro-anatomic distribution of PD-1 in human tonsil and found that PD-1 is expressed on most T cells and a small subset of B cells in the light zone of germinal centers, but not elsewhere in the tonsil. On that basis, it was postulated that PD-1 may play a role in the process of clonal selection of centrocytes, which occurs in this subanatomic site in germinal centers.5
Because of the limited and specific distribution of PD-1 expression in lymphoid tissue, we utilized a monoclonal antibody to PD-1 to examine its expression in a wide range of B-cell and T-cell lymphoproliferative disorders, to see if PD-1 expression is associated with any particular subset of B-cell and/or T-cell lymphoproliferative disorders.
MATERIALS AND METHODS
Case material was obtained from the Brigham & Women’s Hospital, Boston, MA, in accordance with institutional policies. All diagnoses were based on the histologic and immunophenotypic features described in the World Health Organization Lymphoma Classification system6 and in all cases diagnostic material was reviewed by a hematopathologist.
PD-1 antibody (EH12) was generated by immunization of mice with recombinant human PD-1 fusion protein.4 Spleen cells were fused with SP2/0 myeloma cells, cloned, and hybridoma supernatants screened by cell surface staining of PD-1 transfected 300.19, Jurkat, and CHO cells and for lack of reactivity with vector alone transfected cells. Clone EH12 (mouse IgG1) was chosen for further analysis based on its capacity to stain paraffin-embedded tissue. PD-L1 antibody 29E.2A3 was previously described.4 Antibodies for CD3 and CD20 (L26) were obtained from DakoCytomation (Carpinteria, CA); antibodies BU36 for CD21 and BU38 for CD23 were obtained from Binding Site (San Diego, CA); antibodies 56C6 for CD10 and P1F6 for bcl-6 were obtained from Novocastra (Vector Laboratories, Burlingame, CA).
Immunostaining for PD-1, CD3, CD10, CD21, CD23, and bcl-6 was performed on formalin-fixed paraffin-embedded tissue sections following microwave antigen retrieval in 10 mM citrate buffer, pH 6.0, using a standard indirect avidin-biotin horseradish peroxidase method and diaminobenzidine color development, as previously described.7,8 Immunostaining for PD-L1 and CD20 was performed as above, except that no antigen retrieval was employed. Cases were regarded as immunoreactive for PD-1 if at least 20% of neoplastic cells exhibited positive staining. PD-1 and PD-L1 immunostaining was compared with that of mouse IgG isotype control antibody diluted to identical protein concentration for all cases studied, to confirm staining specificity. Two color immunostaining was performed using NovaRED (Vector Laboratories, Burlingame, CA) as an additional reagent for color development, in addition to diaminobenzidine.
RESULTS
PD-1 Immunostaining in Reactive Lymphoid Tissue
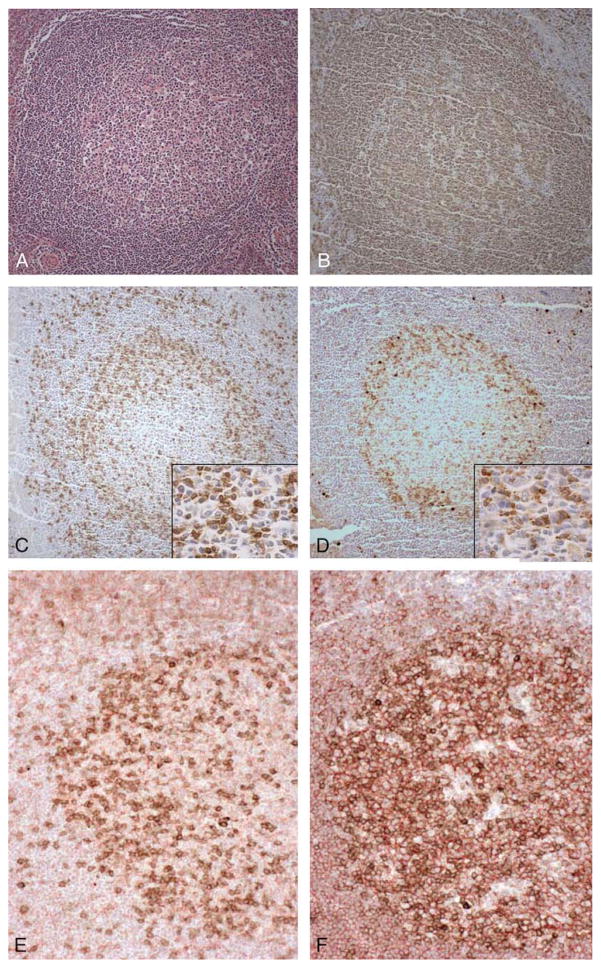
Monoclonal antibody EH12 for PD-1 was used to stain formalin-fixed, paraffin-embedded specimens of reactive lymphoid tissue, thymus, and a range of cases of B-cell and T-cell lymphoproliferative disorders. In specimens of tonsil exhibiting reactive changes, including follicular hyperplasia, a subset of predominantly small lymphocytes in the germinal centers exhibited cell surface and cytoplasmic staining for PD-1, with few PD-1-positive cells seen in the interfollicular T-cell zones (Fig. 1D). The PD-1 staining pattern within germinal centers was virtually identical to that seen with an antibody to CD3 (Fig. 1C), a pan-T-cell marker, whereas an antibody to CD20, a pan B-cell marker, stained the vast majority of germinal center B cells (Fig. 1B). Similar results were seen in histologic sections of reactive lymph node and spleen (data not shown). No PD-1 staining was observed in adult or fetal thymus (data not shown). Two color immunostaining was performed to definitively identify PD-1-positive germinal center cells. PD-1-positive cells in germinal centers were double-stained with antibody for T-cell marker CD3 (Fig. 1E), but not with antibody for B-cell marker CD20, which stained adjacent B cells (Fig. 1F).
FIGURE 1.
PD-1 immunostaining in tonsil. Tonsil with follicular hyperplasia and germinal center formation (A). Germinal centers contain numerous CD20-positive B cells (B), and CD3-positive T cells (C), which are also present in the interfollicular T-cell zone. PD-1 immunostaining highlights the vast majority of germinal center-associated T cells (D), but few T cells in the interfollicular T-cell zone. (A, hematoxylin and eosin (H&E), × 100; B-D, immunostaining with hematoxylin counterstain, × 100, with insets at × 400). Double immunostaining for PD-1, B-cell, and T-cell markers in tonsil with follicular hyperplasia and germinal center formation (E, F), stained for PD-1 (brown) and CD3 (red), showing dual stained cells (E). Tonsil was also stained for PD-1 (brown) and CD20 (red) showing separately stained PD-1-positive and CD20-positive cells (F). (A, H&E, × 100; B-D, immunostaining with hematoxylin counterstain, × 100; C and D insets, immunostaining with hematoxylin counterstain, × 200; E and F, immunostaining with hematoxylin counterstain, × 200).
PD-1 Immunostaining in Paraffin-embedded Tissue Sections of B-cell and T-cell Lymphoproliferative Disorders
We studied a range of B-cell and T-cell lymphoproliferative disorders for PD-1 expression; the results are summarized in Table 1. Forty-two cases of B-cell lymphoproliferative disorders were examined for PD-1 expression, including representative cases of precursor B lymphoblastic leukemia/lymphoblastic lymphoma, and a range of lymphoproliferative disorders of mature B cells, including a number of B-cell non-Hodgkin lymphomas of follicular origin, including 6 cases of follicular lymphoma and 7 cases of Burkitt lymphoma. None of the B-cell lymphoproliferative disorders showed staining for PD-1. In some cases, non-neoplastic reactive lymphoid tissue was present, and showed a PD-1 staining pattern as seen in tonsil and other reactive lymphoid tissue noted above (data not shown).
TABLE 1.
PD-1 Immunostaining in Lymphoproliferative Disorders
| PD-1 Immunostaining | |
|---|---|
| B-cell LPDs | 0/42* |
| B-LL/LL | 0/3 |
| CLL | 0/4 |
| MCL | 0/4 |
| FL | 0/6 |
| MZL | 0/3 |
| HCL | 0/3 |
| DLBCL | 0/6 |
| BL | 0/7 |
| LPL | 0/3 |
| MM | 0/3 |
| Hodgkin lymphoma | 0/25 |
| Classical | 0/11 |
| Nodular lymphocyte-predominant | 0/14† |
| T cell LPDs | 23/60 |
| T-LL/LL | 0/5 |
| T-PLL | 0/3 |
| AIL | 23/23 |
| PTCL, unspecified | 0/14 |
| ALCL | 0/12 |
| ATLL | 0/3 |
Number of immunoreactive cases/total number of cases.
PD-1-positive T cells form rosettes around neoplastic L&H cells in 14/14 cases.
B-LL/LL indicates precursor B-cell lymphoblastic lymphoma/lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; HCL, hairy cell leukemia; DLBCL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; LPL, lympho-plasmacytic lymphoma; MM, multiple myeloma; T-LL/L, precursor T-lympho-blastic leukemia/lymphoblastic lymphoma; T-PLL, T-cell prolymphocytic leukemia; AIL, angioimmunoblastic lymphoma; PTCL, peripheral T-cell lymphoma, unspecified; ALCL, anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma.
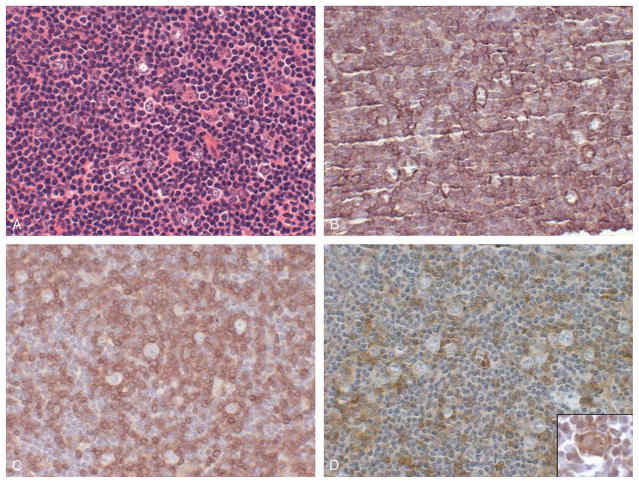
Similarly, in 25 cases of Hodgkin lymphoma, including 11 cases of classic Hodgkin lymphoma and 14 case of lymphocyte-predominant Hodgkin lymphoma, the neoplastic cells did not exhibit staining for PD-1. Interestingly, in all 14 cases of lymphocyte-predominant Hodgkin lymphoma, the T cells surrounding neoplastic CD20-positive lymphocytic and/or histiocytic (L&H) cells were immunoreactive for PD-1, similar to the staining pattern reported for CD57+ T cells in lymphocyte-predominant Hodgkin lymphoma (Fig. 2; Ref. 6). These PD-1-positive cells were a subset of the total CD3+ T-cell population present (Fig. 2).
FIGURE 2.
PD-1 immunostaining in nodular lymphocyte predominant Hodgkin lymphoma. Lymph node involved by nodular lymphocyte-predominant Hodgkin lymphoma, showing a nodular proliferation of L&H (popcorn) cells surrounded by small lymphocytes (A). The neoplastic L&H cells show immunostaining for the B-cell marker CD20 (B), whereas the surrounding small lymphocytes show immunostaining for T-cell marker CD3 (C). A subset of the CD3-positive T cells form rosettes around the neoplastic L&H cells and are PD-1 positive (D). L&H cells are immunoreactive for PD-1 ligand PD-L1 (D, inset). (A, H&E, × 400; B-D, immunostaining with hematoxylin counterstain, × 400; D inset, immunostaining with hematoxylin counterstain, × 400).
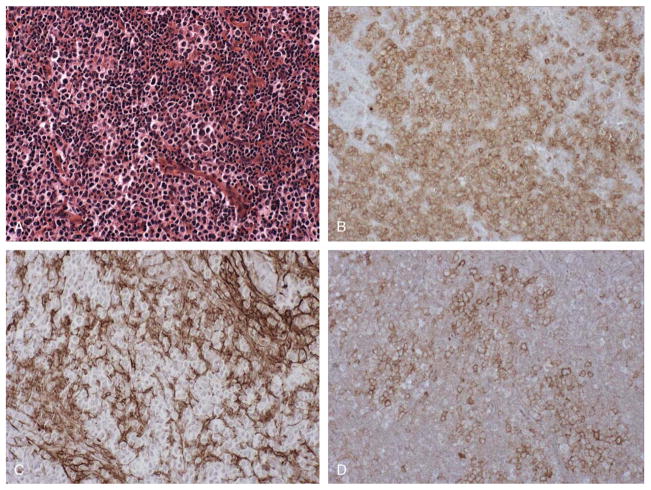
A range of T-cell lymphoproliferative disorders was studied for expression of PD-1; the results are summarized in Table 1. Cases of precursor T-cell lymphoblastic leukemia/lymphoblastic lymphoma, a neoplasm of immature T cells, were negative for PD-1, as were neoplasms of peripheral, post-thymic T cells, including cases of T-cell prolymphocytic leukemia, peripheral T-cell lymphoma, unspecified, anaplastic large cell lymphoma, and adult T-cell leukemia/lymphoma. In contrast, all 23 cases of angioimmunoblastic lymphoma, primarily diagnosed in lymph node biopsies from patients ranging in age from 36 to 86 years, with mean age 65 years, contained foci of PD-1-positive cells that were also immunoreactive for pan T-cell markers such as CD3. PD-1-positive cells represented 20% to 50% of CD3-positive T cells in lesional tissue (Table 2). PD-1-positive cells were consistently found at foci of expanded CD21+ follicular dendritic cells (FDCs) networks, a characteristic feature of angioimmunoblastic lymphoma. Figure 3 shows a representative case of angioimmunoblastic lymphoma with multiple foci of PD-1-positive cells found at foci of expanded FDC networks. Two-color immunostaining was performed to definitively identify PD-1-positive cells in cases of angioimmunoblastic lymphoma. In cases of angioimmunoblastic lymphoma studied, the PD-1-positive cells were double stained with antibody for T-cell marker CD3, but not with antibody for B-cell marker CD20, which stained adjacent B cells (data not shown).
TABLE 2.
PD-1, CD10, and bcl-6 Immunostaining in Angioimmunoblastic Lymphoma
| Case No. | PD-1/CD3* | CD10/CD3* | Bcl-6/CD3* |
|---|---|---|---|
| 1 | 20 | 10 | <5 |
| 2 | 50 | 25 | <5 |
| 3 | 50 | 20 | 10 |
| 4 | 20 | 10 | <5 |
| 5 | 50 | <5 | <5 |
| 6 | 50 | 5 | 5 |
| 7 | 20 | 20 | <5 |
| 8 | 50 | 50 | 5 |
| 9 | 20 | 20 | 5 |
| 10 | 50 | 50 | <5 |
| 11 | 30 | <5 | <5 |
| 12 | 20 | 5 | <5 |
| 13 | 50 | <5 | 5 |
| 14 | 50 | 25 | 25 |
| 15 | 20 | 10 | <5 |
| 16 | 20 | 10 | <5 |
| 17 | 50 | 25 | <5 |
| 18 | 50 | 50 | <5 |
| 19 | 25 | 25 | 10 |
| 20 | 25 | <5 | ND |
| 21 | 50 | <5 | 50 |
| 22 | 50 | 30 | 5 |
| 23 | 20 | <5 | ND |
Percentage staining of CD3-positive T cells.
FIGURE 3.
PD-1 immunostaining in angioimmunoblastic lymphoma. Lymph node involved by angioimmunoblastic lymphoma, showing numerous intermediate size lymphoid cells with round to irregular nuclei and clear cytoplasm, along with a proliferation of small blood vessels (A). Immunostaining reveals the presence of numerous CD3-positive T cells (B), and a CD23-positive FDC meshwork is associated with the T-cell infiltrate (C). A significant subset of CD3-positive cells express PD-1 (D). Scattered CD20-positive B cells were also present (not shown). (A, H&E, × 200; B-D, immunostaining with hematoxylin counterstain, × 200).
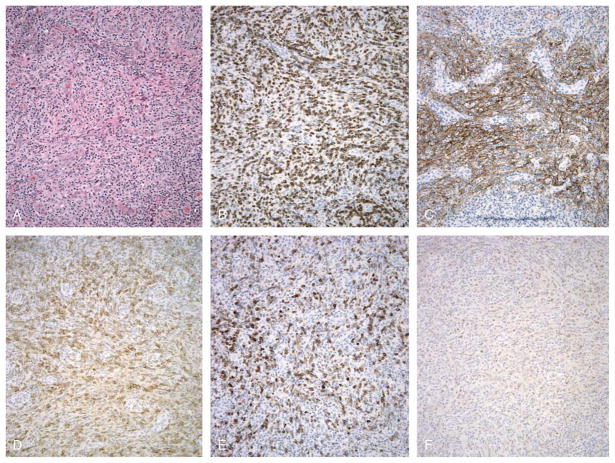
We compared PD-1 immunostaining in cases of angioimmunoblastic lymphoma to CD10 and bcl-6 immunostaining, because both markers have been reported to mark neoplastic cells in cases of angioimmunoblastic lymphoma; the results are summarized in Table 2. In general, anti-CD10 antibody 56C6 stained neoplastic cells in a pattern similar to that observed for PD-1; representative results are shown in Figure 4. CD10-positive cells typically represented 5% to 50% of CD3-positive cells, and represented a smaller subset of CD3-positive cells than PD-1-positive cells. In 6 of 23 cases of angioimmunoblastic lymphoma, the CD10-positive cells represented less than 5% of CD3-positive cells present, and in 6 of 23 cases of angioimmunoblastic lymphoma, the percentage of CD3-positive cells that were positive for PD-1 and CD10 was equal (Table 2). In general, anti-bcl-6 antibody P1F6 stained a small subset of cells at foci of PD-1-positive, CD10-positive cells, in cases of angioimmunoblastic lymphoma; representative results are shown in Figure 4. In 12 of 21 cases, bcl-6-positive cells represented less than 5% of CD3-positive cells present; in 1 case of angioimmunoblastic lymphoma, the percentage of CD3-positive cells that were positive for PD-1 and bcl-6 was equal, and in 6 cases, the percentage of CD3-positive that were positive for bcl-6 was equal to or greater than the percentage of CD10-positive cells (Table 2).
FIGURE 4.
Comparison of PD-1, CD10, and bcl-6 immunostaining in angioimmunoblastic lymphoma. Lymph node involved by angioimmunoblastic lymphoma, showing numerous intermediate size lymphoid cells with round to irregular nuclei and clear cytoplasm, along with a proliferation of FDCs and small blood vessels (A). Immunostaining reveals the presence of numerous CD3-positive T cells (B), CD21-positive FDCs (C), PD-1-positive cells, which account for a significant subset of CD3-positive cells (D), CD10-positive cells, which account for a smaller subset of CD3-positive cells (E), and bcl-6-positive cells, which account for a minor percentage of cells present (F). (A, H&E, × 200; B-F, immunostaining with hematoxylin counterstain, × 200).
Immunostaining for PD-L1
We performed immunostaining for PD-1 ligand PD-L1 using monoclonal antibody 29E.2A3, on 19 of 23 cases of angioimmunoblastic lymphoma. This antibody was previously demonstrated to stain germinal centers in reactive lymphoid tissue in a pattern consistent with FDC staining.4 In cases of angioimmunoblastic lymphoma, clusters of neoplastic CD3-positive T cells typically are found in association with expanded networks of FDCs, which are immunoreactive for CD21 (Fig. 3). We also found that CD23 stained FDCs in cases of angioimmunoblastic lymphoma in a pattern similar to that found with CD21 immunostaining, with more pronounced staining of FDCs for CD23 than CD21 in 7 cases, and less pronounced staining of FDCs for CD23 than CD21 in 1 case (data not shown). In the cases of angioimmunoblastic lymphoma studied, CD21- and CD23-positive FDCs were immunoreactive with PD-L1 antibody 29E.2A3, with staining noted adjacent to foci of PD-1-immunoreactive T cells in the pattern observed for FDC staining (Fig. 5). PD-L1 antibody also stained small vessels in reactive lymphoid tissue and lesional tissue studied. In cases of lymphocyte-predominant Hodgkin lymphoma studied, PD-L1 antibody stained CD20-positive L&H cells, and FDCs and scattered small lymphocytes; representative findings are shown in the inset to Figure 2D.
FIGURE 5.
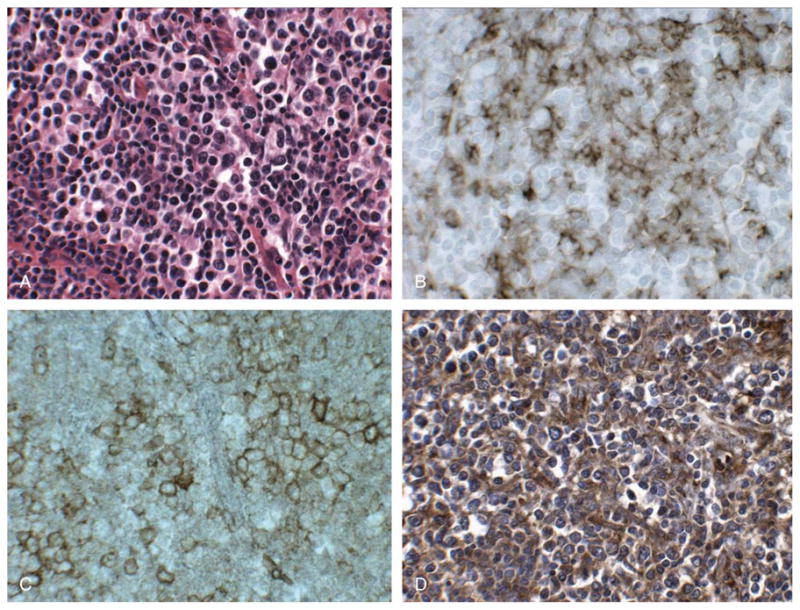
PD-L1 immunostaining in angioimmunoblastic lymphoma. Lymph node involved by angioimmunoblastic lymphoma (A) with CD21-positive FDCs present in expanded networks (B) where foci of PD-1-positive cells are present (C). Immunostaining reveals the presence of numerous PD-L1-positive cells (D) closely associated with PD-1-positive cells. The pattern of PD-L1 staining is similar to that seen with CD21, a marker of FDCs (B). (A, H&E, × 400; B-D, immunostaining with hematoxylin counterstain, × 400).
DISCUSSION
Here we report that PD-1, which is a marker of germinal center-associated T cells, is expressed by neoplastic cells in angioimmunoblastic T-cell lymphoma, and is expressed by T cells associated with neoplastic B cells in nodular lymphocyte-predominant Hodgkin lymphoma. Recently, Chtanova and coworkers9 described a distinctive transcriptional profile in T-follicular helper cells which home to the B-cell areas of secondary lymphoid tissue. A number of genes were found to be expressed specifically in T-follicular helper cells, including PD-1, which was not expressed at significant levels in other T-cell subsets.9 Our finding of PD-1 expression by immunostaining in germinal center-associated T cells provides evidence that PD-1 is not only transcriptionally up-regulated in follicular-associated T cells, but in addition, PD-1 protein is produced in these cells, and is not found in T cells of other subanatomic zones. The absence of PD-1 by immunostaining in other T-cell zones, and in a range of T-cell lymphomas, including those that appear to be derived from Th1 and Th2 committed T cells,10,11 is further support of the finding that PD-1 expression is limited to germinal center-associated T cells. Although germinal center-associated T-helper cells are required for antibody production, neoplastic transformation of these cells may contribute to the immunodeficiency and immune dysfunction commonly seen in angioimmunoblastic lymphoma. Expression of PD-1 should allow the T cell to be receptive to down-regulatory signals via the PD-1/PD-1 ligand pathway and limit the expansion and cytokine production of T cells in the B cell zone.
PD-1 expression by neoplastic T cells in angioimmunoblastic lymphoma suggests that this neoplasm is derived from follicular or germinal center-associated T cells. There are other findings that support this conclusion. We have previously shown that FDCs, and fibroblastic reticulum cells, characteristically proliferate and are associated with the neoplastic cells in cases of angioimmunoblastic lymphoma.12 FDCs are closely associated with B-cell germinal centers in secondary lymphoid tissue and are associated with the neoplastic cells in virtually all cases of follicular lymphoma, a neoplasm of germinal center B cells.6 FDC proliferation is not seen in T-cell lymphoproliferative disorders, except angioimmunoblastic lymphoma, which suggests that the cell of origin of angioimmunoblastic lymphoma is closely associated with FDCs, as we would expect would be the case for germinal center-associated T cells. We have shown that FDCs in cases of angioimmunoblastic lymphoma express PD-L1, the ligand for PD-1, and that PD-L1-positive FDCs are closely associated with PD-1-positive neoplastic T cells.
Ree and coworkers have reported that bcl-6 protein, a transcription factor involved in B-cell proliferation and differentiation characteristically expressed by germinal center B cells and the neoplastic cells in follicular lymphoma,6 is expressed by a subset of neoplastic CD3-positive T cells in angioimmunoblastic lymphoma.13 We also found that bcl-6 stained a subset of cells in cases of angioimmunoblastic lymphoma at foci of PD-1-positive cells. Although a smaller number of bcl-6-positive cells than PD-1-positive cells was typically seen in cases of angioimmunoblastic lymphoma, the pattern and distribution of bcl-6-positive cells was similar to that seen for PD-1 positive cells. Interestingly, bcl-6 was also identified as a marker of T-follicular helper cells in the transcription profiling study of Chtanova and coworkers.9
Recently, neoplastic cells in the majority of cases of angioimmunoblastic lymphoma have been reported to express CD10, a marker of germinal center B cells and B-cell precursors.14 Attygalle and coworkers14 identified CD10 as an immunophenotypic marker of neoplastic T cells in angioimmunoblastic lymphoma in 87% of cases, and found that CD10 is not expressed in other peripheral T-cell lymphomas. We found that anti-CD10 antibody 56C6-stained neoplastic cells in cases of angioimmunoblastic lymphoma in a pattern similar to that observed for PD-1, although CD10-positive cells typically represented a smaller subset of CD3-positive cells than PD-1-positive cells. In 6 of 23 cases of angioimmunoblastic lymphoma, the CD10-positive cells represented less than 5% of CD3-positive cells present, and in 6 of 23 cases of angioimmu-noblastic lymphoma, the percentage of CD3-positive cells that were positive for PD-1 and CD10 was equal. Similarly, Attygalle and coworkers14 found that CD10-positive and CD3-positive cells ranged from 0% to 30% and represented greater than 5% of cells present in 50% of cases of angioimmunoblastic lymphoma. They postulated that CD10 expression in angioimmunoblastic lymphoma cells may be a result of a disturbance of apoptosis in these cells,14 similar to that seen in bcl-2-overexpressing follicular lymphoma cells. CD10, an endopeptidase, is expressed by a wide range of cell types, including a subset of thymic T cells.15
The expression of a number of regulatory and functional proteins by germinal center B cells as well as germinal center-associated T cells was observed in transcriptional profiling experiments.9 Similarly, we have observed that angioimmunoblastic lymphoma, which we suggest may be a neoplasm of germinal center-associated T cells, expresses a number of protein markers of neoplastic, germinal center B cells. Previously, we reported that a transcription factor, T-bet, a T-box transcription factor required for Th1 T-cell development, which is expressed in a subset of T-cell lymphomas, is also expressed in B cells and a subset of B-cell lymphoma.16 Similarly, B-cell-associated transcription factor OCT-1 and coactivator BOB.1, expressed as well in B-cell lymphomas, were found to be expressed in a wide range of T-cell neoplasms and thought to possibly play a role in neoplastic transformation of T cells.17 These findings suggest that a number of proteins are needed for both B-cell and T-cell development and function, and that this shared expression persists in lymphoid neoplasia.
Nodular lymphocyte-predominant Hodgkin lymphoma is a B-cell neoplasm that appears to be derived from germinal center B cells with mutated, nonfunctional immunoglobulin genes.18,19 Similar to angioimmunoblas-tic lymphoma, neoplastic cells are associated with a meshwork of FDCs. PD-1 expression is seen in T cells closely associated with neoplastic CD20-positive cells in nodular lymphocyte-predominant Hodgkin lymphoma, in a pattern similar to that reported for CD57-positive T cells.6 CD57 has been identified as another marker of germinal center-associated T cells, along with CXCR5.20 We also found that neoplastic CD20-positive B cells in cases of nodular lymphocyte-predominant Hodgkin lymphoma studied express PD-L1 ligand. Taken together, these findings support the conclusion that the neoplastic cells in nodular lymphocyte-predominant Hodgkin lym-phoma have a close association with germinal center-associated T cells.
PD-1 is a new marker of angioimmunoblastic lymphoma and suggests a unique cell of origin for this neoplasm. Unlike CD10 and bcl-6, PD-1 is expressed by few B cells, so it may be a more specific and useful diagnostic marker in angioimmunoblastic lymphoma. It also seems to stain a greater percentage of CD3-positive neoplastic cells in angioimmunoblastic lymphoma than either CD10 or bcl-6. In addition, PD-1 expression provides new evidence that angioimmunoblastic lymphoma is a neoplasm derived from germinal center-associated T cells. Previously, we reported that Th1- and Th2-differentiated T cells may give rise to unique subsets of T-cell lymphoproliferative disorders.10,11 PD-1 expression in angioimmunoblastic lymphoma lends further support to this model of T-cell oncogenesis, in which specific subtypes of T cells may undergo neoplastic transformation and result in specific distinct histologic, immunophenotypic, and clinical subtypes of T-cell neoplasia. Chtanova and coworkers9 identified a number of genes that are specifically up-regulated in expression in germinal center-associated T cells, in addition to PD-1. It will be interesting to determine whether the expression of these other genes can be studied by immunostaining in angioimmunoblastic lymphoma and other lymphoid neoplasms. Furthermore, it may be possible that one or more of these new markers of angioimmunoblastic lymphoma, such as PD-1, may provide the basis for an immunotherapeutic approach to the treatment of angioimmunoblastic lymphoma, similar to the use of anti-CD20 and anti-CD52 immunotherapy in B-cell neoplasia.
Acknowledgments
Supported in part by grants NIH AI39671 and AI56299.
The authors thank Anton Chestukhin and Bao-Gong Zhu for assistance in generating monoclonal antibodies.
References
- 1.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 3.Probst HC, McCoy K, Okazaki T, et al. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 4.Brown J, Dorfman DM, Ma F-R, et al. Blockade of PD-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 5.Iwai Y, Okaszaki T, Nishimura H, et al. Microanatomical localization of PD-1 in human tonsils. Immunol Lett. 2002;83:215–220. doi: 10.1016/s0165-2478(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. pp. 189–235. [Google Scholar]
- 7.Jones D, Fletcher CDM, Pulford K, et al. The T-cell activation markers CD30 and OX40/CD134 are expressed in nonoverlapping subsets of peripheral T-cell lymphoma. Blood. 1999;93:3487–3493. [PubMed] [Google Scholar]
- 8.Dorfman DM, Greisman HA, Shahsafaei A. Loss of expression of the WNT/Beta-catenin signaling pathway transcription factors lymphoid enhancer protein-1 (LEF-1) and T cell factor-1 (TCF-1) in a subset of peripheral T cell lymphomas. Am J Pathol. 2003:162. doi: 10.1016/s0002-9440(10)64287-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 10.Jones D, O’Hara C, Kraus MD, et al. Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood. 2000;96:685–690. [PubMed] [Google Scholar]
- 11.Dorfman DM, van den Elzen P, Weng AP, et al. Differential expression of T-bet, a T box transcription factor required for Th1 T cell development, in peripheral T cell lymphomas. Am J Clin Pathol. 2003;120:866–873. doi: 10.1309/MLUF-X0HR-5B96-GVAX. [DOI] [PubMed] [Google Scholar]
- 12.Jones D, Jorgensen JL, Shahsafaei A, et al. Characteristic proliferations of reticular and dendritic cells in angioimmunoblastic lymphoma. Am J Surg Pathol. 1998;22:956–964. doi: 10.1097/00000478-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ree HJ, Kadin ME, Kikuchi M, et al. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblas-tic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum Pathol. 1999;30:403–411. doi: 10.1016/s0046-8177(99)90115-6. [DOI] [PubMed] [Google Scholar]
- 14.Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627–633. doi: 10.1182/blood.v99.2.627. [DOI] [PubMed] [Google Scholar]
- 15.Arber DA, Weiss LM. CD10: a review. Appl Immunohistdochem. 1997;5:125–140. [Google Scholar]
- 16.Dorfman DM, Hwang ES, Shahsafaei A, et al. T-bet, a T-cell-associated transcription factor, is expressed in a subset of B-cell lymphoproliferative disorders. Am J Clin Pathol. 2004;122:292–297. doi: 10.1309/AQQ2-DVM7-5DVY-0PWP. [DOI] [PubMed] [Google Scholar]
- 17.Marafioti T, Ascani S, Pulford K, et al. Expression of B-lymphocyte-associated transcription factors in human T-cell neoplasms. Am J Pathol. 2003;162:861–871. doi: 10.1016/S0002-9440(10)63882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braeuninger A, Kuppers R, Strickler JG, et al. Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proc Natl Acad Sci USA. 1997;94:9337–9342. doi: 10.1073/pnas.94.17.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marafioti T, Hummel M, Anagnostopoulos I, et al. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med. 1997;337:453–458. doi: 10.1056/NEJM199708143370703. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Rott LS, Clark-Lewis I, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]