Abstract
Systemic Salmonella infection commonly induces prolonged splenomegaly in murine or human hosts. Although this increase in splenic cellularity is often assumed to be due to the recruitment and expansion of leukocytes, the actual cause of splenomegaly remains unclear. We monitored spleen cell populations during Salmonella infection and found that the most prominent increase is found in the erythroid compartment. At the peak of infection, the majority of spleen cells are immature CD71−Ter119+ reticulocytes, indicating that massive erythropoiesis occurs in response to Salmonella infection. Indeed, this increase in RBC precursors corresponded with marked elevation of serum erythropoietin (EPO). Furthermore, the increase in RBC precursors and EPO production required innate immune signaling mediated by Myd88/TRIF. Neutralization of EPO substantially reduced the immature RBC population in the spleen and allowed a modest increase in host control of infection. These data indicate that early innate immunity to Salmonella initiates marked splenic erythropoiesis and may hinder bacterial clearance.
Salmonella infections are typically transmitted by contaminated food or water and can cause a spectrum of clinical manifestations, ranging from uncomplicated local gastro-enteritis to typhoid (1, 2). The outcome of Salmonella infection depends on the particular bacterial serovar and both the genetic susceptibility and immune competence of the infected host (1, 2). The increasing incidence of Salmonella antibiotic resistance and the limited potential for the development of new antibiotics (3–5) necessitate the generation of new Salmonella vaccines. However, for this goal to be realized, a greater understanding of bacteria–host interactions and immunity to infection is required.
Salmonella spp. are facultative intracellular bacteria that are able to survive macrophage phagocytosis and replicate within a modified endosome, called the Salmonella-containing vacuole (6, 7). Salmonella-containing vacuole formation is regulated by multiple Salmonella virulence genes, which allow adaptation of the macrophage intracellular environment for optimal bacterial growth (7). Infected phagocytes therefore require an activation signal provided by IFN-γ to initiate bactericidal processes that overcome phagosome modifications and eliminate the infecting bacteria (8). During the early stages of Salmonella infection, IFN-γ is provided by cells of the innate immune system, such as NK cells, neutrophils, and macrophages, thus allowing the host to restrain initial bacterial growth while a Salmonella-specific adaptive immune response develops (9, 10). Although Salmonella-specific CD4 T cells are activated rapidly postinfection, it takes several weeks to develop Salmonella-specific Th1 cells that are capable of producing large amounts of IFN-γ and controlling intracellular bacterial replication (11, 12). In the absence of Th1 cells or IFN-γ, the infected host is unable to regulate bacterial growth and eventually succumbs to infection (13, 14).
During systemic infection, Salmonella spp. replicate extensively within phagocyte populations of the GALTs, spleen, liver, and bone marrow (15, 16). The early innate immune response to infection invokes chemokine-dependent recruitment of neutrophils and monocytes to the infected site, where they can be activated to produce inducible NO synthase and kill bacteria (16, 17). However, recruitment of phagocytes can also be counterproductive, because these newly recruited phagocytes provide a rich source of new cells for additional infection. Indeed, prior depletion of phagocytes actually increases host resistance to Salmonella infection (18). In addition to recruitment of phagocytes, Salmonella infection also induces marked activation and expansion of CD4 and CD8 T cells (19–21), the majority of which appear to be Salmonella-specific and eventually control bacterial replication (22). Thus, a hallmark of Salmonella infection is increased cellularity of the spleen, owing in part to recruitment and expansion of phagocyte and lymphocyte populations responding to infection. Although the splenomegaly accompanying Salmonella infection is sometimes attributed to this increase in splenic leukocytes, the contribution of other cell populations is not always carefully examined.
The expansion and differentiation of erythroid cells usually occurs in the bone marrow and is tightly regulated by the production of the hormone erythropoietin (EPO). Erythroid progenitor cells respond to EPO and progress through a series of well-characterized differentiation stages before extruding nuclei and forming mature circulating erythrocytes (23, 24). This process of erythroid differentiation is normally regulated so that sufficient erythrocytes are produced in the bone marrow. However, under conditions of stress, erythroid differentiation can become dys-regulated, and a large increase in erythrocytes can be initiated in the spleen (25), termed “extramedullary erythropoiesis”.
Erythropoiesis can also be substantially altered during infection with microbial pathogens. For example, infection with Friend spleen focus-forming virus initiates erythroid hyperplasia in the absence of EPO (26), and studies published decades earlier clearly demonstrate that injection of bacterial endotoxin can reduce bone marrow, but increase splenic erythropoiesis (27–29). A more recent study reported that Salmonella infection can cause erythroid depletion of the bone marrow and a corresponding increase in splenic erythropoiesis (30). However, the initiation of splenic erythropoiesis during Salmonella infection has received relatively little attention.
In this study, we examined the process of splenomegaly during murine Salmonella infection. Our data show that a massive increase in immature erythroid reticulocytes accounts for the greatest single change in splenic cell populations following Salmonella infection. Expansion of this erythroid population was initiated by a marked increase in EPO production, which required recognition of bacteria by innate immune receptor signaling. These data document a major effect of Salmonella infection on erythroid cell development that alone accounts for much of the splenomegaly in response to infection.
Materials and Methods
Mouse and bacterial strains
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) and The Jackson Laboratory (Bar Harbor, ME), and they were used at 6–12 wk old. CD90.1 congenic, RAG-deficient SM1 TCR transgenic mice express a monoclonal TCR specific for Salmonella flagellin and have been described in detail previously (31, 32). Myd88/TRIF-deficient mice were bred in our animal facility from breeding stock provided by Dr. M. Jenkins (University of Minnesota, Minneapolis, MN). CD47-deficient mice were purchased from The Jackson Laboratory. All mice were given care in accordance with University of Minnesota Research Animal Resource guidelines. Salmonella BRD509 strain (33) was provided by Dr. D. Xu (University of Glasgow, Glasgow, U.K.).
Salmonella infection, spleen weights, and bacterial counts
BRD509 (AroA−D−) were grown overnight in Luria-Bertani broth without shaking, and bacterial numbers were estimated using a spectrophotometer (optical density at 600 nm). Stocks were prepared in PBS and mice were infected intravenously with 5 × 105 bacteria in the lateral tail vein. Bacterial doses were confirmed in every experiment by plating serial dilutions of the stock culture onto MacConkey agar plates and examining colony counts the following day. Spleens from infected and uninfected mice were surgically removed and weighed prior to further processing. To determine bacterial growth in vivo, spleens from infected mice were homogenized in PBS, serial dilutions were plated onto MacConkey agar, and colonies were counted the following day.
Flow cytometric analysis of spleen and blood
Spleens were harvested from infected and uninfected mice, and a single cell suspension was prepared in Eagle’s Ham’s amino acids supplemented with 2% FBS. Peripheral blood was obtained by retro-orbital bleeding of anesthetized mice using heparinized capillary tubes. Total cells were counted using a hemocytometer and 5 × 106 cells incubated on ice for 30 min in Fc receptor block containing FITC-, PE-, PE-Cy5-, or allophycocyanin-conjugated Abs specific for CD11b, NK1.1, Gr-1, CD4, CD8, B220, Ter119, and CD71 (eBioscience, San Diego, CA; BD Biosciences, San Jose, CA). After surface staining, cells were fixed using paraformaldehyde and examined by flow cytometry using a FACS Canto (BD Biosciences). All flow data were analyzed using FlowJo software (Tree Star, Ashland, OR).
TCR transgenic adoptive transfers
Spleen and lymph node cells were harvested from SM1 TCR transgenic mice. A single cell suspension was generated and stained using Abs to CD4 and Vβ2 to determine the percentage of TCR transgenic cells. The volume was adjusted accordingly, and 1 × 105-1 × 106 SM1 cells were injected intravenously into recipient mice. Three days postinfection, spleens were harvested and a single-cell suspension was generated in Eagle’s Ham’s amino acids medium containing 2% FBS. Samples were incubated on ice for 30 min in Fc block containing FITC-, PE-, PE-Cy5-, or allophycocyanin-conjugated Abs specific for CD4, CD11a, and CD90.1 (eBioscience; BD Biosciences). After staining, cells were fixed using paraformaldehyde and examined by flow cytometry using a FACS Canto. All flow data were analyzed using FlowJo software (Tree Star).
EPO measurement
Blood was collected by retro-orbital bleeding of anesthetized, infected, and uninfected mice and incubated at room temperature for 2 h. Each sample was centrifuged in a bench-top centrifuge to pellet the RBCs, and serum was collected and stored at −20°C. EPO concentrations in serum samples were measured using the Quantikine Mouse/Rat Epo Immunoasssay (R&D Systems, Minneapolis, MN) following the recommended protocol.
In vivo blockade of EPO
Salmonella-infected mice were injected i.v. with 250 μg monoclonal rat anti-mouse EPO or rat IgG2a isotype control (R&D Systems) in PBS, 2 and 4 d postinfection. Blood was collected by retro-orbital bleeding of anesthetized mice on days 4 and 7 postinfection, and serum was collected.
Statistical analysis
Statistical differences between groups of normally distributed data were examined using InStat (GraphPad Software, La Jolla, CA). For bacterial burdens, Log10 CFUs are normally distributed and were also compared using InStat. Data in each group were compared using an unpaired t test and were considered significantly different at p < 0.05.
Results
Salmonella infection causes a marked increase in erythroid cells in the spleen
We examined the development of splenomegaly in response to Salmonella infection by weighing spleens harvested from infected mice. One week postinfection of C57BL/6 mice, the average spleen weight increased markedly and continued to increase over subsequent weeks (Fig. 1A). This period of increasing spleen weight corresponded closely to the time when live bacteria could be cultured from the spleen (Fig. 1B). After bacterial clearance occurred (4 wk postinfection), the average spleen weight steadily declined at each subsequent time point (Fig. 1A).
FIGURE 1.
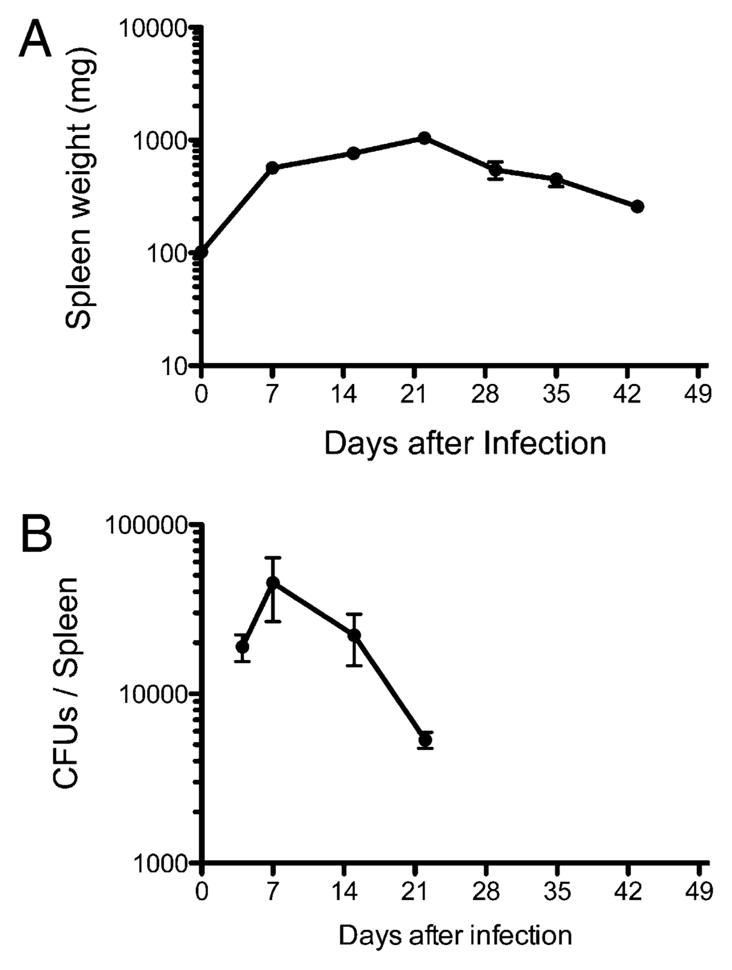
Infection with Salmonella causes marked splenomegaly. C57BL/6 mice were infected intravenously with 5 ×105 attenuated Salmonella, BRD509. Groups of infected mice were sacrificed every week, and spleen weight and bacterial loads were determined. A, Graph shows the mean spleen weight ± SD of Salmonella-infected mice from three to four mice per time points and is representative of two separate experiments. B, Graph shows mean bacterial CFUs ± SD from three to four mice per group and is representative of two separate experiments.
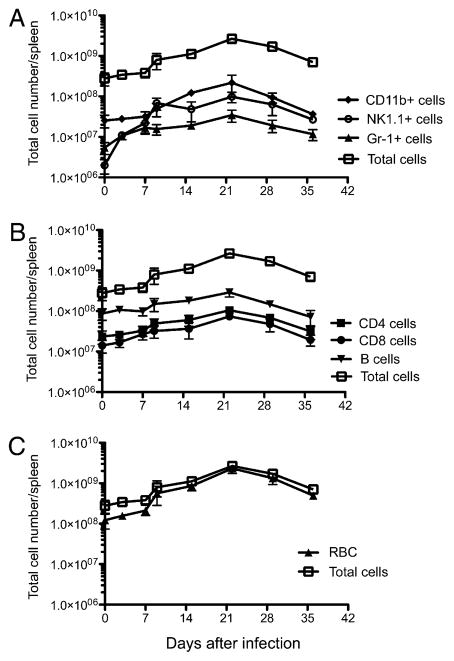
To examine which cell populations contributed to splenomegaly, spleen cells were harvested from infected mice at various time points, and the change in various cell types was examined directly by flow cytometry. As expected, a large increase in the number of splenic CD11b+ cells was detected, which peaked at ~3 wk postinfection (Fig. 2A). Most CD11b+ cells did not express the dendritic cell marker CD11c and therefore are likely to be monocytes, which are known to infiltrate infected tissues (16). A substantial increase in the number of NK1.1+ cells and Gr-1+ neutrophils was also noted over the same period (Fig. 2A). Thus, a marked increase in the number of phagocytes and NK cells was detected in the spleens of infected mice. Each of these cell populations steadily increased until bacterial clearance was eventually achieved, at ~4 wk postinfection (Fig. 1B).
FIGURE 2.
Increase in splenic leukocytes and erythroid cells during Salmonella infection. C57BL/6 mice were infected intravenously with 5 × 105 attenuated Salmonella, BRD509. Groups of infected mice were sacrificed every week and spleen cell subsets were examined by flow cytometry. Graphs shows the mean total cell number ± SD of splenic leukocytes (A), lymphocytes (B), and erythroid cells (C) following Salmonella infection. Each data point shows the mean cell number of three to four mice per time points and is representative of two separate experiments.
Similar increases were noted in the absolute number of CD4 and CD8 T cells following Salmonella infection (Fig. 2B). It has previously been reported that these expanded T cell populations display evidence of Ag-induced activation and likely represent the development of Salmonella-specific T cell responses (19–21). The number of splenic B cells also increased (Fig. 2B), although it is unclear whether this is a consequence of Ag-specific B cell expansion or polyclonal activation of splenic B cells in response to endotoxin.
Whereas each of these immune cell populations increased postinfection, the population of cells displaying the largest increase was actually cells expressing the erythroid lineage marker Ter119 (Fig. 2C). In uninfected mice Ter119+ cells represent 10–30% of spleen cells, whereas they accounted for 80–85% of all spleen cells in some mice at the peak of splenomegaly (~3 wk post-infection; Fig. 2C). Thus, an increase in erythroid cells is the largest total and proportional increase of any splenic cell population after Salmonella infection. Despite this large increase in the splenic erythrocyte population, there was only a modest reduction in circulating erythrocytes detected in the peripheral blood of infected mice (Fig. 3). Thus, splenic erythrocyte expansion is most likely initiated in the spleen, and it does not represent relocation of cells from peripheral circulation. These data are in broad agreement with early studies demonstrating that bacterial endotoxin initiates splenic erythropoiesis and a recent study reporting that Salmonella infection causes an increase in splenic erythrocytes (27–30).
FIGURE 3.
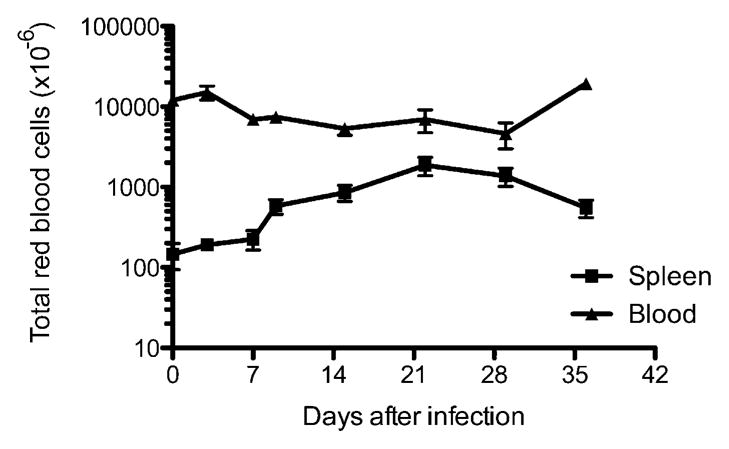
The number of erythroid cells in peripheral blood does not change substantially during the period of Salmonella-induced splenomegaly. C57BL/6 mice were infected intravenously with 5 × 105 attenuated Salmonella, BRD509. Groups of infected mice were sacrificed every week, and the total number of Ter119+ erythroid cells in the spleen and peripheral blood was determined by flow cytometry. Each data point shows the mean cell number of three to four mice per time point and is representative of two separate experiments.
The majority of splenic erythroid cells are immature reticulocytes
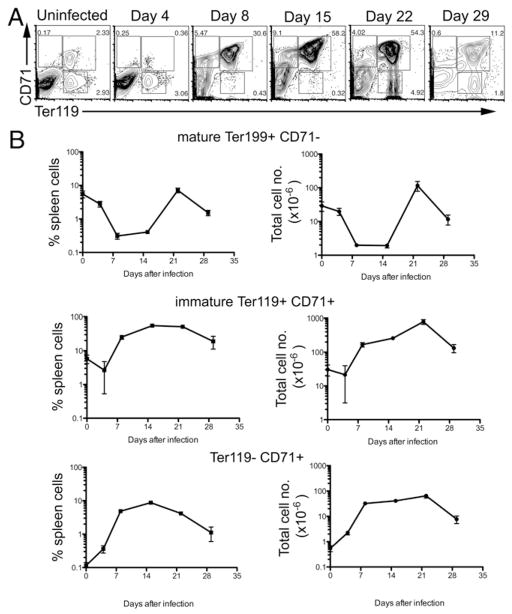
Erythrocytes develop from hematopoietic stem cells via a series of intermediate stages, starting from burst-forming unit cells, pro-erythroblasts, and then subsequent basophilic, polychromatic, and orthochromatic erythroblast stages (23, 24). In the later stages, the nucleus is shed prior to the formation of mature erythrocytes (23, 24). At these later developmental stages, the erythroid cells initially express CD71 and subsequently express Ter119. During the transition from reticulocytes to mature erythrocytes, cells eventually lose surface expression of CD71, but maintain the erythroid marker Ter119 (34, 35). Thus, surface staining with Abs specific for CD71 and Ter119 can be used to examine the stages of erythroid development. We decided to use this strategy to characterize the large increase in erythroid cells accompanying Salmonella infection.
The spleen of uninfected C57BL/6 mice contained two discrete populations of Ter119+CD71+ and Ter119+CD71− cells at relatively similar frequency (Fig. 4A, uninfected). As noted previously, Ter119+CD71− has been used to define mature erythrocytes, whereas Ter119+CD71+ represent immature reticulocytes developing into erythrocytes (34, 35). In these uninfected mice, an extremely small proportion of spleen cells expressed CD71, but did not express Ter119 (Fig. 4A). These cells could be earlier stage erythroid progenitors or simply nonerythroid cells expressing CD71, which by itself is not a specific marker for erythroid cells.
FIGURE 4.
Development of erythroid cells in the spleen during Salmonella infection. C57BL/6 mice were infected i.v. with 5 × 105 attenuated Salmonella, BRD509. Groups of infected mice were sacrificed every week, and splenic erythroid development was examined at weekly time points. A, Representative FACS plots showing changes in Ter119 and CD71 staining in the spleen during Salmonella infection. B, Graphs show the percentage and total number of spleen cell subsets expressing Ter119 and or CD71 staining at different time points postinfection. Each data point shows the mean cell number ± SD of three to five mice per time point.
Four days after Salmonella infection, the percentage of both Ter119+ populations decreased slightly (Fig. 4B). In some but not all mice, this decrease was particularly notable in the immature CD71+ population (Fig. 4A, day 4). These data suggest that Salmonella infection may initially cause erythroid depletion in the spleen, as was previously reported for the bone marrow (30). At 8 and 15 d postinfection, a massive increase in the percentage of Ter119+ cells was detected (Fig. 4A). Almost all of these cells were immature Ter119+CD71+ reticulocytes, whereas the percentage of mature erythrocytes remained significantly depressed (Fig. 4A, 4B). At 3 wk postinfection as bacterial loads were rapidly decreasing (Fig. 1), the percentage of immature Ter119+CD71+ reticulocytes remained high, but the mature erythrocyte population had recovered to levels that were detected in un-infected mice (Fig. 4). Four weeks postinfection, when bacteria were cleared from the spleen, the percentage of immature reticulocytes decreased significantly, and mature erythrocytes were also clearly detected. At every time point after 8 d, an increase in CD71+Ter119− cells was detected in Salmonella-infected mice (Fig. 4A, 4B) relative to preinfection.
Given these substantial changes in splenic erythroid cell populations, we also examined whether immature Ter119+CD71+ reticulocytes were elevated at other systemic sites of infection—the bone marrow and liver. Whereas a large increase in Ter119+CD71+ reticulocytes was found in the spleen, this population remained unchanged or slightly decreased in the bone marrow (Fig. 5). A small but statistically significant increase in Ter119+CD71+ reticulocytes was also detected in the liver of infected mice (Fig. 5). These data demonstrate that Salmonella infection induces massive splenic erythropoiesis and that this erythroid population is composed almost completely of immature reticulocytes. Furthermore, few mature erythrocytes were detected developing from this immature pool until bacteria started to be cleared from the spleen.
FIGURE 5.
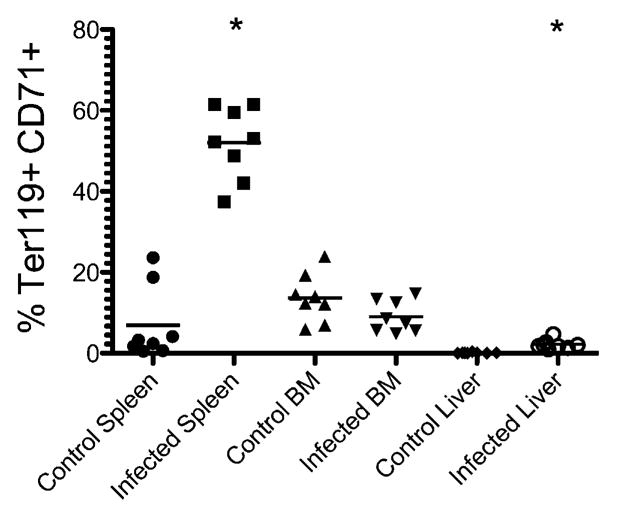
Immature erythroid cells in the spleen, bone marrow, and liver during Salmonella infection. C57BL/6 mice were infected i.v. with 5 × 105 attenuated Salmonella, BRD509. Groups of mice were sacrificed 15 d postinfection, and immature erythroid cells were examined. The graph shows the percentage of cells expressing Ter119 and CD71 from a single cell suspension from the spleen, bone marrow, or liver. Each data point shows an individual mouse, and data are pooled from two separate experiments. The mean percentage of Ter119+CD71+ cells was significantly higher in the spleen and liver of Salmonella-infected mice compared with uninfected mice by Student t test; *p < 0.05.
Salmonella innate activation induces the production of EPO
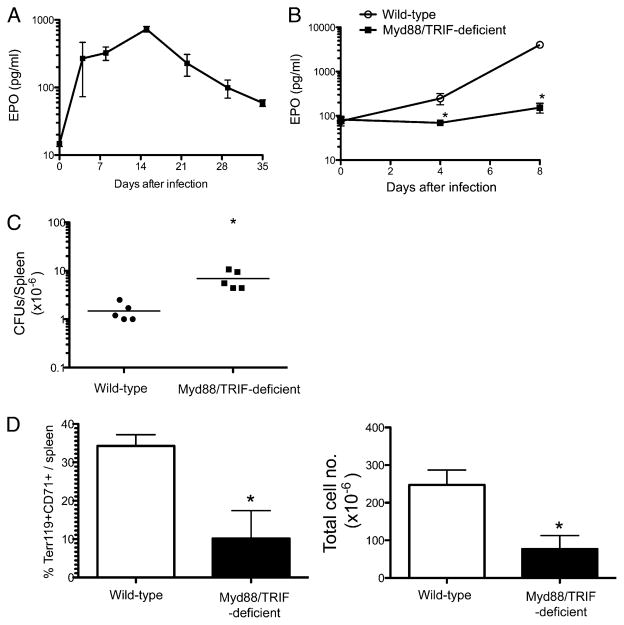
Given the large increase in immature erythroid cell development, it seemed likely that EPO production was induced by Salmonella infection. However, previous studies in a viral model have also demonstrated direct viral stimulation of erythropoiesis that was EPO-independent (26). Therefore, we measured the level of EPO in the serum of mice after Salmonella infection. Indeed, Salmonella infection caused a substantial increase in the serum EPO concentration, which peaked at ~2 wk postinfection before steadily declining thereafter (Fig. 6A).
FIGURE 6.
Salmonella infection-induced EPO production and erythroid expansion is dependent on Myd88/TRIF expression. A, C57BL/6 mice were infected i.v. with 5 × 105 attenuated Salmonella, BRD509, and blood was collected at weekly intervals. EPO concentrations were measured at weekly time points. The graph shows the mean serum EPO ± SD for three to five mice per time point and is representative of three individual experiments. B–D, C57BL/6 WT and Myd88/TRIF-deficient mice were infected i.v. with 5 × 105 attenuated Salmonella. Spleens and/or peripheral blood were harvested at weekly time points. EPO concentrations were measured in blood (B), bacterial CFUs were determined (C), and splenic erythroid cell populations were examined by flow cytometry (D). B, The graph shows the mean serum EPO ± SD for three to five mice per time point and is representative of two individual experiments. The mean EPO concentration was significantly lower in the blood of Salmonella-infected Myd88/TRIF-deficient mice at days 4 and 8. C, The graph shows bacterial CFUs at day 8 postinfection and is representative of two individual experiments. The mean bacterial counts were significantly higher in Myd88/TRIF-deficient mice. D, The graphs show mean percentage and total number of Ter119+/CD71+ cells in the spleen ± SD for three to five mice per group and are representative of two individual experiments. The mean percentage and total number of Ter119+/CD71+ cells was significantly lower in Salmonella-infected Myd88/TRIF-deficient mice. *p < 0.05, as determined by Student t test.
The kinetics of EPO production closely mirrored the bacterial load detected in the spleen during infection (Fig. 1), suggesting that recognition of bacteria by the innate immune system was responsible for stimulating EPO production. Alternatively, the production of EPO could simply be a natural consequence of erythropoietic stress accompanying infection. To examine this issue, we measured EPO production in Salmonella-infected, wild type (WT) and Myd88/TRIF-deficient mice, lacking the major adaptor proteins downstream of TLR ligation. As expected, WT mice displayed a substantial increase in serum EPO concentration after Salmonella infection (Fig. 6B). In marked contrast, Myd88/TRIF-deficient mice had no significant increase in serum EPO concentration up to 8 d after Salmonella infection (Fig. 6B), despite the fact that elevated numbers of bacteria were detected in the spleen of these mice (Fig. 6C). Furthermore, the expansion of immature reticulocytes (Ter119+CD71+) was substantially reduced in Salmonella-infected Myd88/TRIF-deficient mice (Fig. 6D). Therefore, both EPO production and the expansion of Ter119+CD71+ immature reticulocytes required recognition of bacteria by innate immune receptors and signaling through their associated adaptor molecules.
Effect of EPO on immunity to Salmonella infection
It seemed plausible that massive expansion of erythroid precursors and the resulting splenomegaly was advantageous for bacterial growth, either because immune architecture is substantially disrupted during erythroid expansion or because of the direct effects of increased numbers of erythroid cells on dendritic cell or T cell populations. It has been reported that erythroid cells can inhibit dendritic cell function in vitro, probably via CD47 interactions with SIRPα (36). However, we infected CD47-deficient mice with Salmonella and found no difference in the ability of these mice to resolve infection (data not shown). Next, we directly examined whether erythroid expansion was detrimental to Salmonella-specific CD4 T cell activation using SM1 TCR transgenic T cells. Mice were adoptively transferred with Salmonella-specific SM1 CD4 T cells, and T clonal expansion was examined in the presence or absence of recombinant EPO injections. As expected, injection of rEPO caused transient expansion of Ter119+CD71+ erythroid cells in the spleen, but did not affect the activation or clonal expansion of Salmonella-specific SM1 T cells after immunization (data not shown). Thus, we were unable to detect a direct effect of erythroid expansion on the initiation of an adaptive immune response in vivo.
To examine whether erythroid expansion was inhibiting bacterial clearance, we injected Salmonella-infected mice with a neutralizing Ab against EPO. As expected, mice injected with anti-EPO had a much lower frequency of Ter119+CD71+ cells in the spleen after Salmonella infection, conclusively demonstrating that the expansion of these precursors is due to increased EPO concentration (Fig. 7A). Furthermore, anti-EPO mice with lower percentages of erythroid cells also had a modest but significant reduction in bacterial burden (Fig. 7B). Thus, although we were unable to detect a direct effect of erythroid cells on T cell responses, the rapid expansion of immature erythroid cells appears to be advantageous for bacterial growth.
FIGURE 7.
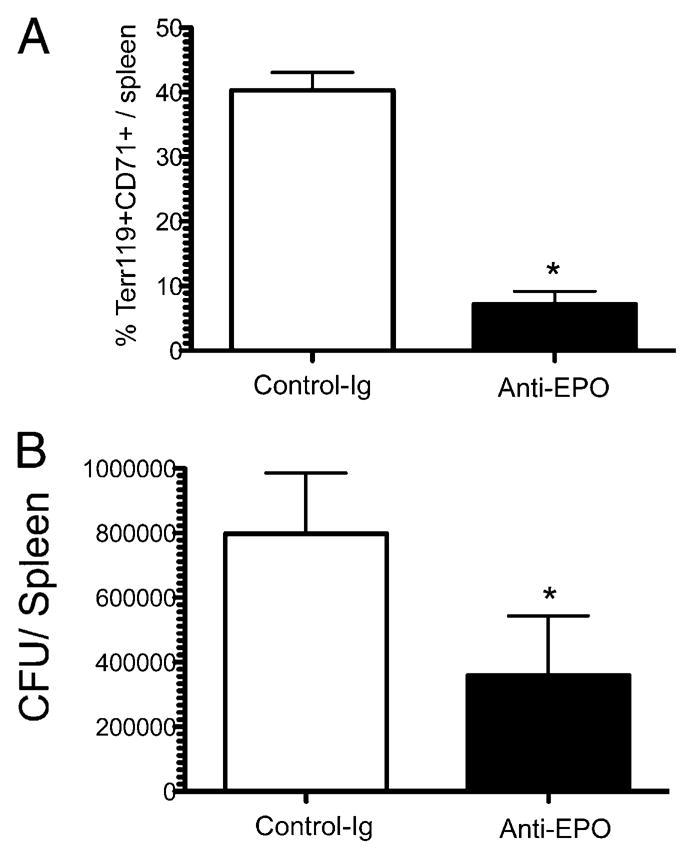
In vivo neutralization of EPO reduces splenic erythroid expansion and bacterial counts. C57BL/6 mice were infected i.v. with 5 × 105 attenuated Salmonella, and 250 μg monoclonal anti-EPO or isotype control-Ig was injected 2 and 4 d later. Seven days postinfection, spleens were harvested, splenic erythroid cell populations were examined by flow cytometry, and bacterial counts determined. Bar graphs show (A) mean Ter119+/CD71+ cells in the spleen ± SD and (B) mean bacterial counts ± SD for five mice per group. The mean percentage of Ter119+/CD71+ cells in the spleen and mean bacterial counts were significantly lower in anti-EPO–treated mice, as assessed by Student t test; *p < 0.05.
Discussion
Although Salmonella is a mucosal pathogen and enters the host via the intestine, the major anatomical sites of bacterial replication are the spleen, liver, and bone marrow—all systemic tissues that contain a large population of resident macrophages (37). The capacity of Salmonella to evade killing and replicate within tissue phagocytes means that infection can quickly overwhelm local host defenses at the site of infection. Thus, inflammatory signals produced by cells in the infected tissue rapidly recruit neutrophils and inflammatory monocytes to engulf replicating bacteria (16). One consequence of rapid phagocyte recruitment to infected secondary lymphoid tissues, such as the spleen, is that the well-defined architecture of T cell and B cell zones becomes severely disrupted (16, 31, 38). In addition, Salmonella LPS signaling via TLR4 can disrupt homeostatic chemokine production, preventing cellular trafficking and enhancing bacterial virulence (39). Furthermore, expansion of the CD4 and CD8 T cell pool is rapidly induced, as the adaptive immune system attempts to control the infection (19–21). These rapid changes to the splenic architecture, cellular composition, and cell trafficking are also accompanied by a profound change in the overall size of the organ, expanding to more than 10-fold its normal size (Fig. 1).
Our data confirm these previous observations and show that the number of NK cells, neutrophils, monocytes, T cells, and B cells increase markedly in the spleen of infected mice, and the kinetics of this expansion correlate closely with the detection of bacteria in the tissue. Once bacterial numbers are reduced, each of these leukocyte populations slowly decreases in number as the corresponding inflammatory response subsides. However, our data also demonstrate that the biggest change in splenic cellular composition is actually found within the erythroid compartment, a cell population that has not been well studied in the context of Salmonella infection. Indeed, at the peak of the host response to infection, Ter119+ cells can account for ~80% of the total cell number in the spleen. Expansion of the erythroid compartment therefore represents the main cause of Salmonella-induced splenomegaly.
It is known that microbes or bacterial products can have major effects on erythroid cell development (26). Indeed, erythroid colony-forming cells are induced in the spleen after injection of LPS or other bacterial cell wall components (27–29). Our data show that these erythroid cells are primarily CD71+Ter119+ reticulocytes, which likely represent an immature erythroid population produced in the spleen, because no increase in this population was detected in the bone marrow. However, we also detected a small increase in CD71+ Ter119+ reticulocytes in the infected liver; therefore, we cannot exclude the possibility that immature erythroid cells initially develop in the bone marrow in response to infection, but then develop further at infected sites such as the spleen and liver. Indeed, previous studies have documented the expansion of hematopoietic stem cells in the context of infection (40, 41). This expansion can occur as a consequence of TLR signaling in hematopoietic stems cells themselves (42), or it can be completely independent of TLR signaling (43), and therefore could be a contributing factor to the extramedullary erythropoiesis observed in our study.
During erythroid differentiation, cells move through sequential developmental stages including, proerythroblasts (CD71medTer119low), early basophilic erythroblasts (CD71HiTer119low), early and late basophilic erythroblasts (CD71HiTer119Hi), chromatophilic and orthochromatic erythroblasts (CD71medTer119Hi), to late orthochromatic erythroblasts and reticulocytes (CD71lowTer119Hi). It is interesting that Salmonella infection caused a large increase in CD71Hi Ter119Hi erythroid cells while simultaneously decreasing the percentage of mature RBCs. It has been suggested that increased erythroid development could simply be initiated because of a greater host requirement for oxygen in infected tissues, perhaps corresponding with oxidative burst as a microbicidal mechanism in infected phagocytes (30). However, our data suggest that very few of these immature cells will actually become mature RBCs in the spleen, and no increase in peripheral red cell numbers was observed. One possibility is that enhanced red cell destruction also occurs, effectively offsetting the increased production of red cells in the spleen. However, we suggest that enhanced erythroid development in the infected spleen is simply a direct consequence of the vastly elevated concentrations of EPO induced by Salmonella infection, and few of these cells will actually mature to become erythroid cells in circulation. Our data show that Salmonella spp. induce a massive, but transient, increase in serum EPO production that would account for extramedullary erythropoiesis in the spleen.
EPO is primarily produced by kidney fibroblasts and is induced under hypoxic conditions. It is possible that Salmonella infection induces EPO production from a cell type in the spleen itself, although we have not yet been able to examine this experimentally. Although Salmonella infection will likely induce hypoxia, it seems unlikely that this alone could explain the increase in circulating EPO concentrations, because Myd88/TRIF-deficient mice have greater bacterial loads than do WT mice, but they do not display increased EPO production or extramedullary erythropoiesis. Instead, our data suggest that EPO is induced as a direct or indirect consequence of TLR signaling. Thus, EPO production is increased following detection of circulating LPS in the kidney or detection of inflammatory mediators secreted by other cells in a TLR-dependent manner.
Previous studies have suggested that EPO can have direct effects on the immune responses and enhance dendritic cell activation, Ab production, and cellular immunity (44–48). However, our studies using SM1 TCR transgenic mice failed to detect a prominent effect of recombinant EPO on CD4 T cell responses in vivo, although it should be noted that we used slightly lower concentrations of EPO than these previous studies, because of the prohibitive cost of using larger concentrations. Other studies suggest that CD47-expressing RBCs can specifically inhibit dendritic cells by binding to SIRPα (36), an inhibitory receptor containing two ITIM motifs. However, we failed to detect any difference in bacterial clearance, splenomegaly, or erythroid expansion in Salmonella-infected or WT mice. However, blocking EPO in vivo using a neutralizing Ab reduced erythroid expansion and also lowered bacterial loads in the spleen. Thus, in the context of Salmonella infection, increased EPO production and the expansion of erythroid progenitors had a modest role in promoting bacterial growth. Thus, we suggest that one effect of increased EPO production and erythroid expansion is the inhibition of immune responses in the spleen. However, because we were unable to detect any direct effect of EPO or CD47 expression on immune function, we propose that this may simply be an indirect consequence of the major disruption in splenic architecture that occurs when more than 80% of the spleen is made of erythroid cells. As a strategy for evasion of host defenses, this effect could complement more direct disruption of splenic architecture via modulation of homeostatic chemokine production via TLR4 ligation, as described previously (39). An alternative possibility is that increased erythrophagocytosis will result in increased iron availability within infected macrophages and allow for increased bacterial growth (49).
In conclusion, we report that Salmonella infection induces Myd88/TRIF-dependent production of EPO and expansion of immature erythroid cells, and this response causes splenomegaly and interferes with the host’s ability to clear bacteria. Greater understanding of the interaction of host immunity and erythroid development could therefore allow for improved therapy and vaccines against Salmonella infection.
Acknowledgments
This work was supported by Grants AI055743, AI56172, and AI076278 from the National Institutes of Health.
Abbreviations used in this paper
- EPO
erythropoietin
- WT
wild type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 2.Rabsch W, Tschäpe H, Bäumler AJ. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 2001;3:237–247. doi: 10.1016/s1286-4579(01)01375-2. [DOI] [PubMed] [Google Scholar]
- 3.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker D, Selbach M, Rollenhagen C, Ballmaier M, Meyer TF, Mann M, Bumann D. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature. 2006;440:303–307. doi: 10.1038/nature04616. [DOI] [PubMed] [Google Scholar]
- 5.Bumann D. Has nature already identified all useful antibacterial targets? Curr Opin Microbiol. 2008;11:387–392. doi: 10.1016/j.mib.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Valdez Y, Ferreira RB, Finlay BB. Molecular mechanisms of Salmonella virulence and host resistance. Curr Top Microbiol Immunol. 2009;337:93–127. doi: 10.1007/978-3-642-01846-6_4. [DOI] [PubMed] [Google Scholar]
- 7.Ibarra JA, Steele-Mortimer O. Salmonella—the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Micro-biol. 2009;11:1579–1586. doi: 10.1111/j.1462-5822.2009.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 9.Ramarathinam L, Niesel DW, Klimpel GR. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 10.Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002;169:4450–4459. doi: 10.4049/jimmunol.169.8.4450. [DOI] [PubMed] [Google Scholar]
- 11.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin A, Baraho-Hassan D, McSorley SJ. Successful treatment of bacterial infection hinders development of acquired immunity. J Immunol. 2009;183:1263–1270. doi: 10.4049/jimmunol.0900772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VanCott JL, Chatfield SN, Roberts M, Hone DM, Hohmann EL, Pascual DW, Yamamoto M, Kiyono H, McGhee JR. Regulation of host immune responses by modification of Salmonella virulence genes. Nat Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 14.Ravindran R, Foley J, Stoklasek T, Glimcher LH, McSorley SJ. Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J Immunol. 2005;175:4603–4610. doi: 10.4049/jimmunol.175.7.4603. [DOI] [PubMed] [Google Scholar]
- 15.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam MA, Rydström A, Sundquist M, Wick MJ. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev. 2008;225:140–162. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 17.Rydström A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 18.Wijburg OL, Simmons CP, van Rooijen N, Strugnell RA. Dual role for macrophages in vivo in pathogenesis and control of murine Salmonella enterica var. Typhimurium infections. Eur J Immunol. 2000;30:944–953. doi: 10.1002/1521-4141(200003)30:3<944::AID-IMMU944>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Mittrücker HW, Köhler A, Kaufmann SH. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan A, Foley J, McSorley SJ. Massive number of antigen-specific CD4 T cells during vaccination with live attenuated Salmonella causes interclonal competition. J Immunol. 2004;172:6884–6893. doi: 10.4049/jimmunol.172.11.6884. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan A, Salazar-Gonzalez RM, Jarcho M, Sandau MM, Lefrancois L, McSorley SJ. Innate immune activation of CD4 T cells in salmonella-infected mice is dependent on IL-18. J Immunol. 2007;178:6342–6349. doi: 10.4049/jimmunol.178.10.6342. [DOI] [PubMed] [Google Scholar]
- 22.Ravindran R, McSorley SJ. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology. 2005;114:450–458. doi: 10.1111/j.1365-2567.2005.02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9:93–100. doi: 10.1097/00062752-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36:1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Kam HY, Ou LC, Thron CD, Smith RP, Leiter JC. Role of the spleen in the exaggerated polycythemic response to hypoxia in chronic mountain sickness in rats. J Appl Physiol. 1999;87:1901–1908. doi: 10.1152/jappl.1999.87.5.1901. [DOI] [PubMed] [Google Scholar]
- 26.Ruscetti SK. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int J Biochem Cell Biol. 1999;31:1089–1109. doi: 10.1016/s1357-2725(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 27.Fruhman GJ. Bacterial endotoxin: effects on erythropoiesis. Blood. 1966;27:363–370. [PubMed] [Google Scholar]
- 28.Reissmann KR, Udupa KB, Labedzki L. Induction of erythroid colony forming cells (CFU-E) in murine spleen by endotoxin. Proc Soc Exp Biol Med. 1976;153:98–101. doi: 10.3181/00379727-153-39488. [DOI] [PubMed] [Google Scholar]
- 29.Staber FG, Johnson GR. The responses of hemopoietic precursor cells in mice to bacterial cell-wall components. J Cell Physiol. 1980;105:143–152. doi: 10.1002/jcp.1041050116. [DOI] [PubMed] [Google Scholar]
- 30.Hyland L, Villarreal-Ramos B, Clarke B, Baaten B, Hou S. Bone marrow immunosuppression in Salmonella-infected mice is prolonged following influenza virus infection. Exp Hematol. 2005;33:1477–1485. doi: 10.1016/j.exphem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan A, Foley J, Ravindran R, McSorley SJ. Low-dose Salmonella infection evades activation of flagellin-specific CD4 T cells. J Immunol. 2004;173:4091–4099. doi: 10.4049/jimmunol.173.6.4091. [DOI] [PubMed] [Google Scholar]
- 33.Chatfield SN, Charles IG, Makoff AJ, Oxer MD, Dougan G, Pickard D, Slater D, Fairweather NF. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Biotechnology (N Y) 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 35.Gordon AR, Outram SV, Keramatipour M, Goddard CA, Colledge WH, Metcalfe JC, Hager-Theodorides AL, Crompton T, Kemp PR. Splenomegaly and modified erythropoiesis in KLF13−/− mice. J Biol Chem. 2008;283:11897–11904. doi: 10.1074/jbc.M709569200. [DOI] [PubMed] [Google Scholar]
- 36.Schäkel K, von Kietzell M, Hänsel A, Ebling A, Schulze L, Haase M, Semmler C, Sarfati M, Barclay AN, Randolph GJ, et al. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–777. doi: 10.1016/j.immuni.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Jones BD, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 38.Kirby AC, Yrlid U, Svensson M, Wick MJ. Differential involvement of dendritic cell subsets during acute Salmonella infection. J Immunol. 2001;166:6802–6811. doi: 10.4049/jimmunol.166.11.6802. [DOI] [PubMed] [Google Scholar]
- 39.St John AL, Abraham SN. Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines. Nat Med. 2009;15:1259–1265. doi: 10.1038/nm.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh P, Yao Y, Weliver A, Broxmeyer HE, Hong SC, Chang CH. Vaccinia virus infection modulates the hematopoietic cell compartments in the bone marrow. Stem Cells. 2008;26:1009–1016. doi: 10.1634/stemcells.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P, Nelson S, Bagby GJ, Siggins R, II, Shellito JE, Welsh DA. The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells. 2008;26:1778–1786. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184:2247–2251. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittelman M, Neumann D, Peled A, Kanter P, Haran-Ghera N. Erythropoietin induces tumor regression and antitumor immune responses in murine myeloma models. Proc Natl Acad Sci USA. 2001;98:5181–5186. doi: 10.1073/pnas.081275298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz O, Gil L, Lifshitz L, Prutchi-Sagiv S, Gassmann M, Mittelman M, Neumann D. Erythropoietin enhances immune responses in mice. Eur J Immunol. 2007;37:1584–1593. doi: 10.1002/eji.200637025. [DOI] [PubMed] [Google Scholar]
- 46.Prutchi Sagiv S, Lifshitz L, Orkin R, Mittelman M, Neumann D. Erythropoietin effects on dendritic cells: potential mediators in its function as an immunomodulator? Exp Hematol. 2008;36:1682–1690. doi: 10.1016/j.exphem.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Lifshitz L, Prutchi-Sagiv S, Avneon M, Gassmann M, Mittelman M, Neumann D. Non-erythroid activities of erythropoietin: Functional effects on murine dendritic cells. Mol Immunol. 2009;46:713–721. doi: 10.1016/j.molimm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Avneon M, Lifshitz L, Katz O, Prutchi-Sagiv S, Gassmann M, Mittelman M, Neumann D. Non-erythroid effects of erythropoietin: are neutrophils a target? Leuk Res. 2009;33:1430–1432. doi: 10.1016/j.leukres.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790:682–693. doi: 10.1016/j.bbagen.2008.08.006. [DOI] [PubMed] [Google Scholar]