Abstract
This study explores the influence of baseline factors on first-month adherence to highly active antiretroviral therapy (HAART) among adults. The study design involved a review of routinely collected patient information in the CAPRISA AIDS Treatment (CAT) programme, at a rural and an urban clinic in KwaZulu-Natal Province, South Africa. The records of 688 patients enrolled in the CAT programme between June 2004 and September 2006 were analysed. Adherence was calculated from pharmacy records (pill counts) and patients were considered adherent if they had taken at least 95% of their prescribed drugs. Logistic regression was used to analyse the data and account for confounding factors. During the first month of therapy, 79% of the patients were adherent to HAART. HAART adherence was negatively associated with a higher baseline CD4 count. Women had better adherence if they attended voluntarily testing and counselling or if they had taken an HIV test because they were unwell, while men had higher adherence if they were tested due to perceived risk of HIV infection. HAART adherence was positively associated with higher age among patients who possessed cell phones and among patients who provided a source of income in the urban setting, but not in the rural setting. Though long-term data from this cohort is required to fully evaluate the impact of non-adherence in the first month of treatment, this study identifies specific groups of patients at higher risk for whom adherence counselling should be targeted and tailored. For example, first-month HAART adherence can be improved by targeting patients initiated on treatment with a high CD4 count.
Keywords: baseline survey, compliance, CD4 count, HAART, health information, pill counts, statistical analysis
Introduction
Advances in highly active antiretroviral therapy (HAART) have dramatically reduced morbidity and mortality due to AIDS (Kalichman, Ramachandran & Catz, 1999; Berg, Demas, Howard, Schoenbaum, Gourevitch & Arnsten, 2004; Gill, Hamer, Simon, Thea & Sabin, 2005). However, HAART includes complex drug regimens, which require strict adherence to complicated schedules for the attainment of optimal long-term clinical and survival benefits. At least 95% adherence is required for adequate virological and immunological response (Paterson, Swindells, Mohr, Brester, Vergis, Squier et al., 2000). Strict adherence to all the antiretroviral (ARV) drugs prescribed is required for optimal management of HIV infection (Chen, Hoy & Lewin, 2007). This strict adherence is one of the most critical behavioural challenges in the treatment of HIV infections (Ferguson, Stewart, Funkhouser, Tolson, Westfall & Saag, 2002). Adherence appears to be the strongest predictor of both the durability of ARV medication (Esch, Klem, Kuhman, Hewitt & Morse, 2002) and the rate of cycling of the HAART regimen. Hence, it is imperative to evaluate the effect of adherence comprehensively.
Recent studies on the impact of HAART adherence on treatment outcomes in sub-Saharan Africa (in an era of treatment rollout) indicate improved immunologic response and clinical outcomes among patients with optimal adherence (Abaasa, Todd, Ekoru, Kalyango, Levin, Odeke & Karamagi, 2008; Chi, Cantrell, Zulu, Mulenga, Levy, Tambatamba et al., 2009). Even after one month of therapy, patients with optimal adherence demonstrate a significant increase in CD4 count, viral suppression and significant weight gain (Brechtl, Breitbart, Galietta, Krivo & Rosenfeld, 2001). Moreover, it is shown that optimal initial adherence is positively associated with improved long-term treatment outcomes (Carrieri, Raffi, Lewden, Sobel, Michelet, Cailleton et al., 2003). These studies highlight the need for strict adherence from the beginning of treatment in order to maintain prolonged clinical benefits. However, clinicians are not able to accurately predict individuals at risk of suboptimal HAART adherence at the beginning of treatment (Bangsberg, Hetch, Clague, Charlebois, Ciccarone, Chesney & Moss, 2002). Since these individuals do not have a prior adherence track record, patient-related factors (such as such as age, gender, income) and factors related to the disease characteristics at baseline might prove to be informative predictors of initial and future HAART adherence (Chesney, 2000; Reynolds, Testa, Marc, Chesney, Neidig, Smith et al., 2004).
There is limited data regarding factors that predict initial optimal HAART adherence to ARV medication. In particular, there are no studies that examine how patient-related factors relate to each other (interact) and their subsequent influence on initial optimal HAART adherence. The purpose of this study is to identify whether specific clinical and socio-demographic factors present at baseline (and their respective possible interactions) influenced first-month optimal adherence to HAART among HIV-positive adults. The knowledge and understanding of such factors is particularly important given the increasing number of patients enrolled on HAART who are to be maintained in therapy. Improved first-month adherence could also help to avoid switching patients to more costly second-line regimens. The findings will be useful in developing tools to assist clinicians in the identification of factors related to poor adherence prior to initiating therapy. The results can be further used to shape communication and counselling strategies, prior to treatment initiation.
Materials and methods
Study design
The Centre for the AIDS Programme of Research in South Africa’s (CAPRISA) AIDS Treatment (CAT) programme is an ongoing rollout programme for ART services that was started in 2004. The objective is to describe the profile of patients presenting at the CAT programme with respect to their social characteristics, clinical status, and clinical course during care, including HAART regime, HAART adherence and clinical outcomes. Adult patients who met HAART eligibility criteria were enrolled into the CAT programme. Eligibility criteria included a CD4 count below 200 cells/μL or patients with World Health Organization (WHO) stage 4 of HIV disease, regardless of CD4 count. The accrual of patients had been evenly distributed over time since initiation of the programme. All patients received three sessions of adherence education and motivation and preparedness training prior to HAART initiation. These adherence counselling sessions were held one week prior to starting treatment. The first two sessions were held the day the decision was taken to start the patient on treatment, and the third session was held two or three days subsequent to the first two sessions. All the sessions lasted 15 to 60 minutes, depending on the patient who is counselled. The CAT programme offers HIV-care services at two different sites in KwaZulu-Natal Province, namely the eThekwini Clinical Research Site located in an urban area and the Vulindlela Clinical Research Site located in a rural area (see <http://www.caprisa.org>). The programme started providing free HAART through a grant from the US President’s Emergency Plan for AIDS Relief (PEPFAR) at a time when access to HAART was limited.
All patients were on a regimen containing two nucleoside reverse transcriptase inhibitors and one non-nucleoside reverse transcriptase inhibitor. Patients in the urban clinic received efavirenz (EFV), lamivudine (3TC) and didanosine (ddI or ddI-EC). These regimens were chosen because they can be co-administered with anti-tuberculosis (TB) medication. The regimen dispensed at the rural clinic consisted of EFV, 3TC and stavudine (d4T), which is the standard government regimen in South Africa. A few patients (3.8%) received nevirapine (NVP) rather than EFV because they were pregnant.
All information for the CAT programme was recorded on data-collection sheets (administered at the treatment sites) which underwent two levels of quality control and were subsequently faxed to a data-management centre. The data used for analysis in this study consisted of a retrospective review of the records of patients in the CAT programme between June 2004 and September 2006. Only patient records with data on pill-count information for the defined study period were included in the analysis. Analysis of this data was approved by the University of KwaZulu-Natal’s biomedical research ethics committee (ref. E 248/05).
Variables of interest
Response variable
The outcome of interest is optimal adherence to HAART. Pharmacy records that contained the detailed pill-count information were used to calculate adherence rates. Patients were then classified as optimally adherent if they took at least 95% of their prescribed drugs in a given regimen; otherwise, they were considered non-adherent. Thus, the response variable is binary, indicating whether a patient was optimally adherent or not adherent.
Independent variables
Baseline socio-demographic variables included in the analysis were age (years), gender, educational status, treatment site, whether or not a patient lived with a partner, whether or not the patient was the source of household income, the patient’s access to tap water and electricity, as well as whether or not a patient owned a cell phone. The variables used to characterise the health status of the patients at baseline included World Health Organization (WHO) stage of HIV disease, CD4 count (cells/μL), and weight (kg). Information about why the HIV test was done was sought from the patients, and their responses included being unwell, attending voluntary counselling and testing (VCT), and various ‘other’ reasons. Other reasons included a partner having died of the disease, being ill or being unfaithful; thus they were classified as at risk of having been exposed to HIV.
Data analysis
The socio-demographic and clinical characteristics of the study population were summarised using the median with the inter-quartile range (IQR) for continuous variables and using proportions for the categorical variables.
The data were analysed by fitting a multivariate logistic regression model. The deviance was used to compare alternative models during model selection. Change in the deviance was used to measure the extent to which the fit of the model improved when additional variables were included. The main effects and possible combinations of two-way interaction terms were fitted, while attention was given to the hierarchic principle in statistical modelling. The selected model was the one with the smallest deviance.
The selected model was assessed for goodness of fit using the Hosmer-Lemeshow statistic (see Hosmer & Lemeshow, 1989; Collett, 2002). Influential observations were identified by plotting the Cook’s distance statistic against the observations (see Collett, 2002). The appropriateness of the link function was assessed by regressing the linear predictor and its square on the dependent variable. The link function is appropriate if the linear predictor is significant and its square is insignificant Vittinghoff, Gliden, Shiboski & McCulloch, 2005).
Results
Study population
The CAT programme enrolled 1 184 patients between June 2004 and September 2006. Pill-count records were only available for 688 of these patients and all 688 were included in this study. We studied relatively equal numbers of patients from each of the treatment sites, 54% from the urban treatment site and 46% from the rural treatment site. There were no differences in the baseline characteristics between those included in the study and those excluded with regard to age (means: included = 34.1 years, excluded = 34 years; p = 0.90), gender (men: included = 30%, excluded = 31.8%; p = 0.51) and baseline CD4 count (means: included = 107.6 cells/μL, excluded = 111.5 cells/μL; p = 0.47).
Baseline socio-demographic and clinical characteristics of the patients
The baseline socio-demographic and clinical characteristics of the patients included in the analysis are presented in Table 1. The median age of the patients was 32.5 years (inter-quartile range [IQR]: 28–38 years), 70% were women, and 69% had attained a secondary-school or higher level of education. A large portion of the patients were not living with a partner (75%). Only 28% were classified as a source of their household’s income. At the time of enrolment, 64% of the patients were classified as WHO stage 3 of HIV disease; the sample’s median weight was 60 kg (IQR: 53–69 kg) and the median CD4 count was 108 cells/μL (IQR: 52–159 cells/μL). Over half (56%) reported that they had decided to take an HIV test because they were not well, while 26% reported having done so because they attended VCT. The remaining 18% said they had taken an HIV test because they felt exposed to the risk of contracting HIV. Over 90% of the patients stayed in households that had access to tap water and electricity, while only 42% of the patients owned cell phones. In the first month post-HAART initiation, 79% of the patients were at least 95% adherent to HAART.
Table 1.
Baseline socio-demographic and clinical characteristics of the HAART patients (n = 688)
| Characteristics | Median (inter-quartile range) | n (%) |
|---|---|---|
| Age (years) | 32.5 (28–38) | |
| Gender: | ||
| Men | 206 (30) | |
| Women | 482 (70) | |
| Education: | ||
| No schooling | 74 (12) | |
| Primaryschool | 116 (19) | |
| Secondary school or higher | 429 (69) | |
| Treatment site: | ||
| Urban | 369 (54) | |
| Rural | 319 (46) | |
| Living with or without a partner: | ||
| Living with a partner | 168 (25) | |
| Living without a partner | 510 (75) | |
| Contribution to household income: | ||
| Source of income | 186 (28) | |
| Not source of income | 489 (72) | |
| WHO stage of HIV disease: | ||
| Stage 1 | 71 (10) | |
| Stage 2 | 121 (16) | |
| Stage 3 | 438 (64) | |
| Stage 4 | 58 (8) | |
| Baseline CD4 count (cells/μL) | 108 (52–159) | |
| Baseline weight (kg) | 60 (53–69) | |
| Reason for taking HIV test: | ||
| Unwell | 374 (56) | |
| Attended VCT | 170 (26) | |
| Risk of exposure to HIV | 121 (18) | |
| Household access to tap water: | ||
| Yes | 611 (91) | |
| No | 59 (9) | |
| Household access to electricity: | ||
| Yes | 607 (91) | |
| No | 63 (9) | |
| Cell phone ownership: | ||
| Yes | 281 (42) | |
| No | 389 (58) | |
| First-month optimal HAART adherence: | ||
| Optimally adherent | 546 (79) | |
| Not optimally adherent | 142 (21) | |
Results of multivariate logistic regression
From a set of alternative models, a model with all the main effects and three interaction terms had the smallest deviance and was thus selected. Goodness of fit of the model was found to be satisfactory (Hosmer-Lemeshow statistic = 6.45; p = 0.60). The index plot of the Cook’s distance statistic indicated that there were no influential observations. The link function was appropriate since the linear predictor was significant (p = <0.001) while the square of it was insignificant (p = 0.50). The adjusted odds ratios (AOR) and their corresponding 95% confidence intervals (95% CI) for the selected model are presented in Table 2.
Table 2.
Adjusted odds ratios (AOR) (95% confidence interval [CI]) for the multivariate logistic regression model on optimal HAART adherence (ref. = reference category)
| Parameter | AOR (95% CI) | p-value |
|---|---|---|
| Intercept | 0.422 (0.0444.024) | 0.454 |
| Age (years) | 1.047 (0.992–1.106) | 0.093 |
| Gender (ref. = men) | ||
| Women | 2.039 (1.066–3.900) | 0.031 |
| Education (ref. = secondary school and above) | ||
| No schooling | 0.594 (0.277–1.276) | 0.182 |
| Primary school | 1.483 (0.725–3.033) | 0.280 |
| Treatment site (ref. = rural site) | ||
| Urban site | 4.347 (2.258–8.369) | <0.001 |
| Contribution to household income (ref. = not source of income) | ||
| Source of income | 3.828 (1.311–11.17) | 0.014 |
| Partner (ref. = living with a partner) | ||
| Living without a partner | 0.908 (0.530–1.557) | 0.727 |
| WHO stage of HIV disease (ref. = stage 4) | ||
| Stage 1 | 1.575 (0.437–5.679) | 0.488 |
| Stage 2 | 0.829 (0.275–2.497) | 0.739 |
| Stage 3 | 0.860 (0.319–2.320) | 0.766 |
| Baseline CD4 count (cells μL) | 0.995 (0.992–0.999) | 0.020 |
| Baseline weight (kg) | 1.002 (0.983–1.022) | 0.834 |
| Reason for taking HIV test (ref. = unwell) | ||
| Attended VCT | 0.614 (0.271–1.389) | 0.242 |
| Risk of exposure to HIV | 3.319 (0.653–16.88) | 0.143 |
| Household access to tap water (ref. = yes) | ||
| No | 1.153 (0.473–2.811) | 0.755 |
| Household has electricity (ref. = yes) | ||
| No | 0.981 (0.441–2.184) | 0.963 |
| Cell phone ownership (ref. = yes) | ||
| No | 15.55 (1.773–136.4) | 0.013 |
| Age * cell phone ownership (ref. = own cell phone) | ||
| Age * no cell phone ownership | 0.927 (0.869–0.987) | 0.019 |
| Gender * reason for testing (ref. = men * unwell) | ||
| Women * attended VCT | 2.409 (0.767–7.566) | 0.132 |
| Women * risk of exposure to HIV | 0.147 (0.025–0.846) | 0.032 |
| Site * household income (ref. = rural site * not source of income) | ||
| Urban site * source of income | 0.173 (0.050–0.602) | 0.006 |
Gender, treatment site, contribution to household income, cell phone ownership, and baseline CD4 count were all found to be significant main effects (Table 2). There were three significant interaction terms: between age and cell phone ownership; between gender and reported reason for taking an HIV test; and between treatment site and source of household income. All significant main effects, except baseline CD4 count, were involved in significant interaction terms (Table 2). For a unit increase in CD4 count (cells/μL), the odds of HAART adherence decreased by 5% (AOR = 0.995 [0.992–0.999]; p = 0.020) (Table 2). The interaction effects are presented in the next sections. It should be noted that for the interaction terms that involved two categorical variables, the meaningful odds ratios for comparison needed to be further calculated from the table of results (i.e. Table 2). The post-hoc effects of the interactions between gender and reported reason for taking an HIV test as well as between treatment site and the patient’s contribution to household income are reported in Tables 3 and 4, respectively.
Table 3.
Post-hoc effects of the interaction between gender and reported reason for taking an HIV test (adjusted odds ratio [AOR] with 95% confidence interval [CI])
| Interaction effect | AOR (95% CI) | p-value |
|---|---|---|
| Women versus men: | ||
| Attended VCT | 4.911 (1.892–12.75) | <0.001 |
| Risk of exposure to HIV | 0.299 (0.059–1.519) | 0.145 |
| Unwell | 2.039 (1.066–3.900) | 0.031 |
| Men: | ||
| Attended VCT versus risk of exposure to HIV | 0.185 (0.035–0.993) | 0.049 |
| Attended VCT versus unwell | 0.614 (0.271–1.389) | 0.242 |
| Risk of exposure of HIV versus unwell | 3.319 (0.653–16.88) | 0.148 |
| Women: | ||
| Attended VCT versus risk of exposure to HIV | 0.446 (0.073–2.739) | 0.383 |
| Attended VCT versus unwell | 1.479 (0.657–3.330) | 0.345 |
| Risk of exposure to HIV versus unwell | 3.319 (0.653–16.88) | 0.148 |
Table 4.
Post-hoc effects of the interaction between HAART treatment site and patient’s contribution to household income (adjusted odds ratio [AOR] with 95% confidence interval [CI])
| Interaction effect | AOR (95% CI) | p-value |
|---|---|---|
| Urban versus rural treatment site: | ||
| Source of household income | 0.751 (0.237–2.385) | 0.628 |
| Not source of household income | 4.347 (2.258–8.369) | <0.001 |
| Source versus not source of household income: | ||
| Urban site | 0.662 (0.342–1.278) | 0.219 |
| Rural site | 3.828 (1.311–11.17) | 0.014 |
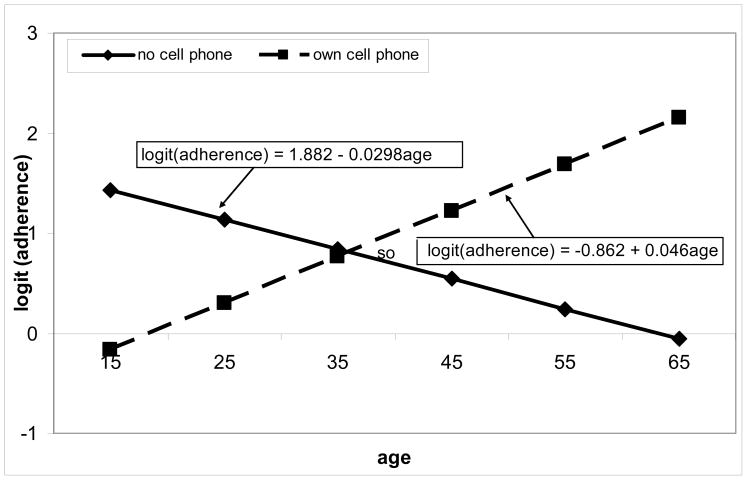
Interactions between patient’s age and cell phone ownership
As age increased, optimal HAART adherence was less likely for patients without cell phone ownership than those with cell phone ownership (AOR = 0.927 [0.869–0.987]; p = 0.019) (Table 2). More specifically, the rate of change in optimal HAART adherence increased with age for patients with cell phones, whereas it decreased as age increased for patients without cell phones (Figure 1). Figure 1 shows that the gap in optimal HAART adherence between groups of patients with and without cell phone ownership widened with increasing age.
Figure 1.
Log odds ratio associated with optimal HAART adherence and age for patients with and without cell phones (based on the estimates of the coefficients from the fitted multivariate logistic regression model).
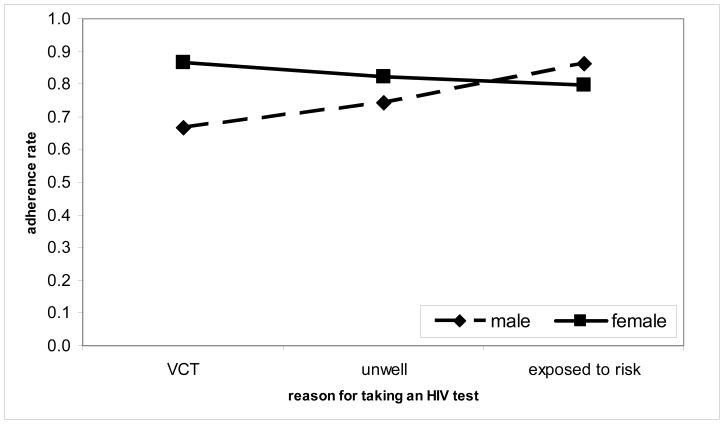
Interactions between gender and patient’s reason for taking an HIV test
Optimal HAART adherence was significantly higher for women than for men among patients who reported having attended VCT as a reason for taking an HIV test (AOR = 4.911 [1.892–12.75]; p = 0.001) as well as those who reported having tested because they were not well (AOR = 2.039 [1.066–3.900]; p = 0.031) (Table 3). There was, however, no significant difference in optimal HAART adherence between women and men who reported having tested for HIV because they felt exposed to the risk of contracting HIV (AOR = 0.299 [0.059–1.519]; p = 0.145) (Table 3). It is also shown that for men, HAART adherence was significantly lower for patients who reported VCT as a reason for taking an HIV test than for those who tested because they felt exposed to the risk of contracting HIV [AOR = 0.185 [0.035–0.993]; p = 0.049) (Table 3). These results confirm the observed proportions of optimal HAART adherence for gender classified by reported reason for taking an HIV test as depicted in Figure 2.
Figure 2.
Percentage adherence associated with gender and reported reason for taking an HIV test (based on observed proportions of optimal HAART adherence)
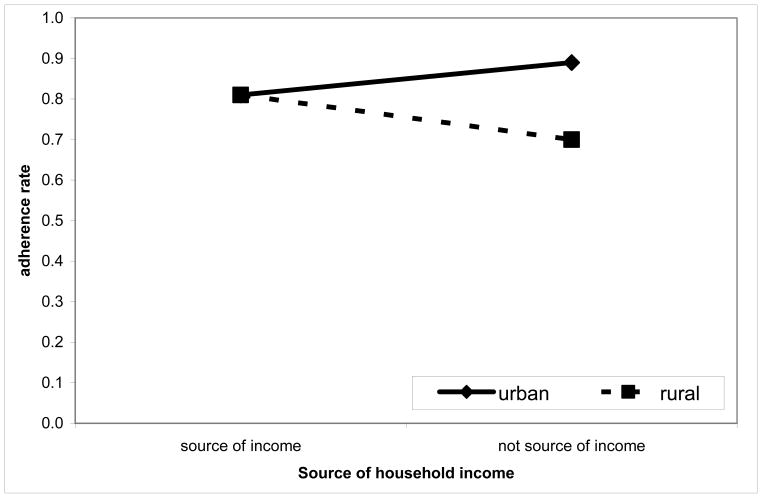
Interactions between treatment site and patient’s contribution to household income
For patients who were not sources of their household’s income, optimal HAART adherence was significantly higher for patients at the urban treatment site than at the rural treatment site [AOR = 4.347 [2.258–8.369]; p = <0.001) (Table 4), whereas there was no difference between treatment sites in regard to patients who were sources of household income [AOR = 0.751 [0.237–2.385]; p = 0.628) (Table 4). For the rural treatment site, optimal HAART adherence was significantly higher for patients who were a source of household income than those who were not a source of household income (AOR = 3.828 [1.311–11.17]; p = 0.014) (Table 4; Figure 3).
Figure 3.
Percentage adherence associated with treatment site and whether a patient is a source of household income (based on observed proportions of optimal HAART adherence)
Discussion and conclusions
First-month adherence to HAART decreased with increasing baseline CD4 count. It has been previously established that healthier patients tend to have significantly greater rates of missing appointments than immuno-compromised patients (Esch et al., 2002). Patients with a higher CD4 count may not have experienced debilitating opportunistic infections (despite laboratory evidence of the need to start HAART) at the time they start HAART, and this seems to have an adverse effect on adherence (Sarna, Pujari, Sengar, Garg, Gupta & Dam, 2008). Lack of experience of severe opportunistic infections may influence the patient’s perceptions about the severity of the disease and the need to maintain a high level of adherence.
Forgetfulness has been found to be the most frequently mentioned reason for missed doses among patients on HAART (e.g. Chesney, 2000; Bartlett, 2002; Barfod, Sorensen, Nielsen, Rodkjaer & Obel, 2006). As a result, many interventions consist of providing memory aids for dosing times. These include the use of new technologies such as reminders through cell phones (Bartlett, 2002; Ickovics & Meade, 2002; Abel & Painter, 2003; Osterberg & Blaschke, 2005). Our results seem to indicate that older patients who have cell phones use them effectively as reminders to preempt forgetfulness and thus will have higher adherence than older patients without cell phones.
Women who had sought VCT were more likely to have adhered to their medication than were their male counterparts. The expectation is that patients in a VCT setting are ready for behaviour change, therefore better compliance can be expected. Lower adherence levels for men than women who attended VCT might be attributed to the suggestion that men are less likely to adopt positive behaviour change (Laforge, Velicer & Owen, 1999). Men who tested for HIV because there were compelling reasons to take an HIV test tended to adhere to medication significantly better than those who had attended VCT. In view of the general reluctance of men to seek healthcare (Macintyre, Hunt & Sweeting, 1996; Laforge et al., 1999), the attitudes and behaviour of the men who admitted that they had been exposed to the risk of HIV and consequently sought healthcare might have led to higher adherence than would be expected generally.
A major challenge facing rural communities is food insecurity, which has a negative relationship with income (Laforge et al., 1999; Nord & Winicki, 2000). In the rural setting we found that adherence was significantly lower among patients who were not sources of their household’s income as compared to patients who were sources of income. Lack of food and hunger following HAART introduction and has been a regularly cited reason for non-adherence to HAART among patients (Marston & De Cock, 2004).
It has been shown that demographic factors are not consistently associated with adherence to HAART (e.g. Haubrich, Little, Currier, Forthal, Kemper, Beall et al., 1999; Fong, Ho, Fung, Lee, Tse, Yuen et al., 2003). The results from this study demonstrate that age, gender, baseline CD4 count and contribution to household income have an effect on first-month HAART adherence through significantly interacting with other variables, which include cell phone ownership, reasons given by patients for taking an HIV test, and whether a patient resides in an urban or a rural setting. We found urban versus rural differences with regard to some of the factors that might affect first-month adherence. Due attention should be paid to address the specific needs in each setting. It has been established that non-adherence to treatment is associated with faster disease progression, even for those who start HAART at a relatively high baseline CD4 count (Wood & Hogg, 2003). This, combined with lower adherence among patients with higher CD4 counts, implies that pre-treatment counselling interventions should also be targeted at people initiated on HAART with a relatively high CD4 count, and should be tailored to the specific needs of these patients.
Owing to the considerable amount of information collected from the patients at the two treatment sites for the CAT programme, as well as the quality-control measures undertaken before the data was faxed to the data management centre, the pill-count information was available for 688 out of 1 184 patients enrolled at the time of the analysis. However, we established that there were no major demographic differences between those included and those excluded in the analysis, thus not biasing the results.
One limitation of this study is that the interactions between variables were identified using the data and model fit techniques. The interactions were not pre-specified or expected during data collection. Therefore, detailed information on why these interactions influenced HAART adherence were not collected and the reason for some of these findings cannot be explained. For example, we can only speculate about why older, but not younger, patients who claimed cell phone ownership were found to adhere better.
Furthermore, this study focused on first-month HAART adherence and there was no evidence that first-month HAART adherence was indicative of longer-term adherence in this cohort. Early non-adherence to HAART has been associated with initial attrition of patients from HIV-treatment programmes (Mocroft, Youle, Moore, Sabin, Madge, Lepri et al., 2001; O’Brien, Clark, Besch, Myers & Kissinger, 2003) and is therefore important to understand. An understanding of underlying factors that may contribute to non-adherence has the potential to improve the effective scaling up of HAART programmes. The researchers intend to further study the factors influencing long-term adherence to HAART. It would also be interesting to see whether factors that influence first-month adherence would also influence adherence over a longer period of time using the same dataset.
The power of this study lies in the fact that it suggests possible interactions between certain characteristics of the patients, which merit further research. In addition, it identifies specific groups of patients at higher risk for whom ARV adherence counselling should be targeted and tailored.
Acknowledgments
CAPRISA was established in 2002 through a Comprehensive International Program of Research on AIDS (CIPRA) grant (AI51794) from the American National Institutes of Health (NIH), as a multi-institutional collaboration, incorporated as an independent non-profit AIDS research organisation. The NIH funded the development of the research infrastructure, including the data management, laboratory and pharmacy cores established through the CIPRA grant. A PEPFAR grant (1U2GPS001350) funded the care of the patients in the CAT programme. Dikokole Maqutu was supported by the Columbia University–Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center (grant D43TW00231). We gratefully acknowledge all the patients in the CAT programme. We also thank all the staff who worked on treating patients in the programme and who helped with the data collection. Special thanks are extended to the pharmacists for collection of the pill-count data.
References
- Abaasa MA, Todd J, Ekoru K, Kalyango JN, Levin J, Odeke E, Karamagi CAS. Good adherence to HAART and improved survival in a community HIV/AIDS treatment and care programme: the experience of The AIDS Support Organisation (TASO), Kampala, Uganda. BMC Health Service Research. 2008;8:241. doi: 10.1186/1472-6963-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel E, Painter L. Factors that influence adherence to HIV medications: perceptions of women and health care providers. Journal of the Association of Nurses in AIDS Care. 2003;14(4):61–69. doi: 10.1177/1055329003252879. [DOI] [PubMed] [Google Scholar]
- Bangsberg D, Hetch FM, Clague H, Charlebois ED, Ciccarone D, Chesney M, Moss A. Provider assessment of adherence to HIV antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2002;26(5):435–442. doi: 10.1097/00126334-200104150-00005. [DOI] [PubMed] [Google Scholar]
- Barfod TS, Sorensen HT, Nielsen H, Rodkjaer L, Obel N. ‘Simply forgot’ is the most frequently stated reason for missed doses of HAART irrespective of degree of adherence. HIV Medicine. 2006;7(5):285–290. doi: 10.1111/j.1468-1293.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Bartlett JA. Addressing the challenges of adherence. Journal of Acquired Immune Deficiency Syndromes. 2002;29(supplement 1):S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. Journal of General Internal Medicine. 2004;19(11):1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechtl JR, Breitbart W, Galietta M, Krivo S, Rosenfeld B. The use of highly active antiretroviral therapy (HAART) in patients with advanced HIV infections: impact on medical, palliative care and quality of life outcomes. Journal of Pain and Symptom Management. 2001;21(1):41–51. doi: 10.1016/s0885-3924(00)00245-1. [DOI] [PubMed] [Google Scholar]
- Carrieri MP, Raffi F, Lewden C, Sobel A, Michelet C, Cailleton V, Chene G, Leport C, Moatti JP, Spire B APROCO Study Group. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virological response: a 3-year follow-up study. Antiviral Therapy. 2003;8(6):585–594. doi: 10.1177/135965350300800606. [DOI] [PubMed] [Google Scholar]
- Chen LF, Hoy J, Lewin SR. Ten years of highly active antiretroviral therapy for HIV infection. Medical Journal of Australia. 2007;186(3):146–151. doi: 10.5694/j.1326-5377.2007.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Chesney MA. Factors affecting adherence to antiretroviral therapy. Clinical Infectious Diseases. 2000;30(supplement 2):S171–S176. doi: 10.1086/313849. [DOI] [PubMed] [Google Scholar]
- Chi HB, Cantrell RA, Zulu I, Mulenga LB, Levy JW, Tambatamba BC, Reid S, Mwango A, Mwinga A, Bulterys M, Saag MS, Stringer JSA. Adherence to first-line antiretroviral therapy effects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. International Journal of Epidemiology. 2009;38(3):746–756. doi: 10.1093/ije/dyp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett D. Modelling Binary Data. 2. New York: Chapman & Hall/CRC; 2002. [Google Scholar]
- Esch L, Klem K, Kuhman L, Hewitt R, Morse G. Intensive adherence interventions improve virologic response to antiretroviral therapy (ART) in treatment-naive patients. Poster [#MoPeB3301] at the 14th International AIDS Conference; Barcelona, Spain. 7–12 July 2002.2002. [Google Scholar]
- Ferguson TF, Stewart KE, Funkhouser E, Tolson J, Westfall AO, Saag MS. Patient-perceived barriers to antiretroviral adherence: associations with race. AIDS Care. 2002;14(5):607–617. doi: 10.1080/0954012021000005434. [DOI] [PubMed] [Google Scholar]
- Fong OW, Ho CF, Fung LY, Lee FK, Tse WH, Yuen CY, Sin KP, Wong KH. Determinants of adherence to highly active antiretroviral therapy (HAART) in Chinese HIV/AIDS patients. HIV Medicine. 2003;4(2):133–138. doi: 10.1046/j.1468-1293.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005;19(12):1243–1249. doi: 10.1097/01.aids.0000180094.04652.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich RH, Little SJ, Currier JS, Forthal DN, Kemper CA, Beall G, Johnson ND, Dube MP, Hwang JY, McCutchan JA. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response: California Collaborative Treatment Group. AIDS. 1999;13(9):1099–1107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- Hosmer D, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care. 2002;14(3):309–318. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine. 1999;14(5):267–273. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge RG, Velicer WF, Owen N. Stage distributions for five health behaviors in the United States and Australia. Preventive Medicine. 1999;28(1):61–74. doi: 10.1006/pmed.1998.0384. [DOI] [PubMed] [Google Scholar]
- Macintyre S, Hunt K, Sweeting H. Gender differences in health: Are things really as simple as they seem? Social Science and Medicine. 1996;42(4):617–624. doi: 10.1016/0277-9536(95)00335-5. [DOI] [PubMed] [Google Scholar]
- Marston B, De Cock K. Multivitamins, nutrition and antiretroviral therapy for HIV disease in Africa. New England Journal of Medicine. 2004;351(1):78–80. doi: 10.1056/NEJMe048134. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Youle M, Moore A, Sabin CA, Madge S, Lepri AC, Tyrer M, Chaloner C, Wilson D, Loveday C, Johnson MA, Phillips AN. Reasons for modification and discontinuation of antiretrovirals: results from a single-treatment centre. AIDS. 2001;15(2):185–194. doi: 10.1097/00002030-200101260-00007. [DOI] [PubMed] [Google Scholar]
- Nord M, Winicki J. Prevalence of household hunger declines in rural households. Rural Conditions and Trends. 2000;11(5):80–86. [Google Scholar]
- O’Brien M, Clark RA, Besch CL, Myers L, Kissinger P. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. Journal of Acquired Immune Deficiency Syndromes. 2003;34(4):407–414. doi: 10.1097/00126334-200312010-00008. [DOI] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T. Adherence to medication. New England Journal of Medicine. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Reynolds NR, Testa MA, Marc LG, Chesney MA, Neidig JL, Smith SR, Vella S, Robbins GK. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS and Behaviour. 2004;18(2):141–150. doi: 10.1023/B:AIBE.0000030245.52406.bb. [DOI] [PubMed] [Google Scholar]
- Sarna A, Pujari S, Sengar AK, Garg R, Gupta I, Dam J. Adherence to antiretroviral therapy and its determinants amongst HIV patients in India. Indian Journal of Medical Research. 2008;127(1):28–36. [PubMed] [Google Scholar]
- Vittinghoff E, Gliden DV, Shiboski SC, McCulloch SE. Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated-Measures Models. New York: Springer; 2005. [Google Scholar]
- Wood E, Hogg RS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4 cell count is 0.200 to 0.350 × 109 cells/L. Annals of Internal Medicine. 2003;139(10):810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]