Abstract
Neurotransmission by serotonin (5-HT) is tightly regulated by several autoreceptors that fine-tune serotonergic neurotransmission through negative feedback inhibition at the cell bodies (predominantly 5-HT1A) or at the axon terminals (predominantly 5-HT1B); however, more subtle roles for 5-HT1D and 5-HT2B autoreceptors have also been detected. This review provides an overview of 5-HT autoreceptors, focusing on their contribution in animal behavioral models of stress and emotion. Experiments targeting 5-HT autoreceptors in awake, behaving animals have generally shown that increasing autoreceptor feedback is anxiolytic and rewarding, while enhanced 5-HT function is aversive and anxiogenic; however, the role of serotonergic activity in behavioral models of helplessness is more complex. The prevailing model suggests that 5-HT autoreceptors become desensitized in response to stress exposure and antidepressant administration, two seemingly opposite manipulations. Thus there are still unresolved questions regarding the role of these receptors - and serotonin in general - in normal and pathological states.
Keywords: 5-HT1A, 5-HT1B, autoregulation, serotonergic
1 Introduction
The neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) influences a wide range of behavioral and physiological processes including anxiety, depression, aggression, sleep, memory, and reward (Lucki, 1998). There are at least 14 serotonin receptors in the mammalian nervous system. Some of these are expressed as autoreceptors, which we define as a receptor that is expressed within a serotonergic neuron that provides feedback in modulating the activity of that neuron. These receptors are also expressed on non-serotonergic neurons as heteroreceptors. Compelling evidence indicates that several 5-HT1 receptors act as inhibitory autoreceptors to provide negative feedback in serotonin neurons – functioning like a thermostat, they maintain a certain homeostatic tone in serotonergic function. There is also intriguing evidence 5-HT2B receptors may enhance serotonin release under specific circumstances. The goal of this review is to provide an overview of serotonin autoreceptor research from the perspective of the investigator interested in animal models of emotional behavior. For clarity, we shall focus on experiments in which manipulations or measurements of 5-HT receptors are restricted specifically to the autoreceptor form.
2 Molecular and pharmacological properties of 5-HT autoreceptors
For a general overview of 5-HT receptors, we refer the reader to previous reviews (Barnes and Sharp, 1999; Hannon and Hoyer, 2008; Millan et al., 2008). Because there are several reviews on the pharmacology and physiology of serotonergic autoregulation (Pineyro and Blier, 1999; Stamford et al., 2000), here we will limit our discussion to a simplified overview. The best established serotonin autoreceptors are all members of the 5-HT1 family, which consists of 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F. All members of the 5-HT1 family share high affinity for serotonin and are inhibitory in nature, coupling to Gi/o - but may also signal through additional mechanisms (Lin et al., 2002). The receptor that was originally referred to as 5-HT1C, which was placed in the 5-HT1 family on the basis of its pharmacological properties, was later found to share molecular properties of the 5-HT2 subfamily (DNA sequence, Gq coupling) and was renamed 5-HT2C. The rat 5-HT1B receptor was initially thought to be the rodent analogue of the human 5-HT1Dβ receptor (Hoyer and Middlemiss, 1989); however, it is now clear that both 5-HT1B and 5-HT1D receptors exist in every species examined and have very similar pharmacology.
The predominant somatodendritic autoreceptor is 5-HT1A. Activation of somatodendritic 5-HT1A autoreceptors leads to inhibition of action potentials via opening of potassium channels in a Gi/o-dependent manner (Bayliss et al., 1997; Innis and Aghajanian, 1987; Katayama et al., 1997; Penington et al., 1993). A recent report suggests that 5-HT1A autoreceptors may display agonist-directed trafficking to activate multiple signal transduction pathways (Valdizan et al., 2010). Terminal autoregulation occurs primarily via 5-HT1B autoreceptors, which are located on serotonergic axons adjacent to terminals (Riad et al., 2000). Activation of 5-HT1B autoreceptors reduces extracellular 5-HT concentrations in terminal regions; experiments designed to isolate mechanistic components of this have shown that 5-HT1B autoreceptor activation directly inhibits release (Hjorth and Tao, 1991) and synthesis of 5-HT (Hjorth et al., 1995) while simultaneously enhancing reuptake via the serotonin transporter (Daws et al., 2000) (Hagan et al, submitted). Though their contribution is relatively minor, there is evidence that 5-HT1D autoreceptors contribute to autoinhibition of 5-HT function at terminals and possibly also dendrites (Pineyro and Blier, 1996; Pineyro et al., 1995; Trillat et al., 1997) – see also (Stamford et al., 2000).
While less data have been reported on the 5-HT2B receptor acting as an autoreceptor, it is reported to co-express and interact with 5-HT1B receptors (Janoshazi et al., 2007). There is recent evidence that 5-HT2B autoreceptors facilitate the effects of 3,4-methylenedioxymethamphetamine (MDMA), a drug that stimulates 5-HT release via its actions on serotonin transporter (Gudelsky and Yamamoto, 2008), increases locomotion, and induces conditioned-place preference and psychomotor sensitization (Cole and Sumnall, 2003; Kalivas et al., 1998). Disruption of 5-HT2B function via antagonism or genetic knockout blocks the effects of MDMA on serotonin release and behavior (Doly et al., 2009; Doly et al., 2008). In cultured serotonergic-like cells and primary neuron cultures, 5-HT2B receptor activation phosphorylates the serotonin transporter and inhibits its function – an effect that would oppose the effects of 5-HT1B/1D autoreceptors (Launay et al., 2006). More data will be needed to fully understand the functional role of 5-HT2B autoreceptors in regulating 5-HT function. There is little or no support for any serotonin receptors ranging from 5-HT3–7 to act as autoreceptors, and the contributions of 5-HT1E and 5-HT1F receptors to physiology and behavior in general are not well understood. Because the current bulk of evidence implicates 5-HT1A, 5-HT1B, and 5-HT1D as the predominant 5-HT autoreceptors, they will be the focus of this review.
3 Neuroanatomy of 5-HT autoreceptors and heteroreceptors
The 5-HT autoreceptors and heteroreceptors have distinct anatomical distributions in the brain. Autoreceptors, by definition, are expressed on serotonergic neurons residing in the midbrain raphe nuclei. The dorsal raphe nucleus (DRN) is the largest of these nuclei, containing approximately half of the brain’s serotonergic neurons (Jacobs and Azmitia, 1992). The DRN accounts for the majority of ascending serotonergic projections, and is the focus of this review. Within the DRN, the majority of cells expressing 5-HT1A and 5-HT1B receptors are serotonergic, demonstrated by the fact that chemical lesions of serotonergic neurons destroy the majority of 5-HT1A binding (Verge et al., 1986), 5-HT1A mRNA (Miquel et al., 1992), and 5-HT1B mRNA (Doucet et al., 1995; Neumaier et al., 1996b) within the DRN. Double-label immunohistochemistry and in situ hybridization studies show that virtually all serotonergic neurons in the DRN express 5-HT1A (Day et al., 2004; Sotelo et al., 1990). The distribution of 5-HT1A and 5-HT1B mRNA densities throughout the DRN generally mirror expression of Tph2 and SERT, with peak expression of all four genes found in ventromedial DRN at mid-rostral levels (Clark et al., 2006). One study utilizing systemic 5-HT1A antagonist administration and Fos immunohistochemistry suggests that basal 5-HT1A-mediated inhibition of DRN neurons is greatest in the lateral wings and ventral caudal subregions (Commons, 2008). Expression of 5-HT1A and 5-HT1B receptors in the DRN is not purely limited to serotonergic neurons. There is expression of 5-HT1A in a modest number of GABAergic neurons throughout the rostrocaudal axis of the DRN (Beck et al., 2004; Day et al., 2004). Furthermore, at extreme caudal portions of the rat DRN (−8.5 to −9.0 bregma), there are dorsolateral “extra DRN wings” which are non-serotonergic but positive for both 5-HT1A and 5-HT1B mRNA (Clark et al., 2006).
Because 5-HT1A receptors are located on cell bodies and dendrites, there is close concordance between the distribution of 5-HT1A binding sites and mRNA throughout the brain (Chalmers and Watson, 1991; Pompeiano et al., 1992). 5-HT1A heteroreceptors are expressed widely, with most prominent expression in hippocampus (CA1, CA3, dentate gyrus), septum, and entorhinal cortex. On the other hand, because 5-HT1B and 5-HT1D receptors are trafficked to axon terminals, the distributions of their binding sites and mRNA do not correspond (Sari, 2004). Because each of these receptors is expressed as autoreceptors on terminals that are intermingled throughout the brain with terminals containing heteroreceptors, one cannot distinguish auto- versus heteroreceptor subtypes in autoradiograms. Furthermore, the large majority of total brain 5-HT1B binding reflects heteroreceptors, since lesions of serotonergic neurons do not decrease 5-HT1B binding throughout the brain (Compan et al., 1998; Manrique et al., 1993; Offord et al., 1988; Pranzatelli et al., 1996; Sexton et al., 1999; Sijbesma et al., 1991; Verge et al., 1986). With those caveats in mind, 5-HT1B binding, likely to predominantly represent 5-HT1B heteroreceptors, is reported throughout the brain with most prominent expression in the globus pallidus and substantia nigra (Bruinvels et al., 1993; Pazos and Palacios, 1985; Verge et al., 1986; Waeber et al., 1989). These data are corroborated by more recent immunohistochemical studies with antibodies demonstrated to show specificity for 5-HT1B receptors (Langlois et al., 1995; Sari et al., 1997; Sari et al., 1999). 5-HT1B heteroreceptor mRNA is expressed in a wide range of brain areas, particularly the hippocampus (CA1), caudate/putamen, and cortex (Bruinvels et al., 1994; Voigt et al., 1991); in each of these cases it is possible to associate the 5-HT1B mRNA with different neuron types based on their anatomical localization. 5-HT1D heteroreceptor mRNA, which appears to be expressed at lower densities in the brain, is found primarily in caudate/putamen and cortex (Bruinvels et al., 1994). Binding sites for 5-HT1D, while dramatically less prevalent than 5-HT1B, are most prominent in the globus pallidus and substantia nigra.
4 Methodology for studying 5-HT autoreceptors
A complication in studying autoreceptors is the fact that all genes encoding 5-HT autoreceptors are also expressed in the brain as heteroreceptors. A given environmental stimulus that alters expression and/or function of one population may not affect the other, and the interpretation of an experiment can depend critically on knowing which receptor population was affected. For example, functional desensitization of 5-HT1A autoreceptors would result in greater serotonergic cell body activity and release, and consequently greater activation of all postsynaptic 5-HT receptors - including 5-HT1A heteroreceptors. In contrast, desensitization of 5-HT1A heteroreceptors would not have any direct effect on the function of 5-HT neurons or activity at other postsynaptic 5-HT receptors. In studying serotonergic autoregulation it is important to employ methods that allow for the differentiation between 5-HT auto- and heteroreceptors. We will discuss this issue with respect to experimental designs employing measurement and manipulation of 5-HT autoreceptors.
4.1 Measurement
Measurements of 5-HT autoreceptor function were employed initially in experiments characterizing the basic physiology of these receptors. In later work, the effects of various environmental and chemical stimuli on 5-HT autoreceptor function were measured. Because 5-HT1A autoreceptors are located somatodendritically, autoreceptor function can be measured by recording electrophysiological responses on raphe neurons, or by infusing 5-HT1A ligands into the raphe nuclei and measuring electrophysiological or neurochemical responses. Because 5-HT1B and 5-HT1D autoreceptors are located axonally, alternative strategies are used. Activity of these receptors can be studied by applying drugs and measuring 5-HT efflux, which tends to select for serotonergic terminals and 5-HT autoreceptors. Because some downstream effectors of 5-HT1B and 5-HT1D autoreceptors – serotonin transporter and tryptophan hydroxylase – are only expressed in serotonergic terminals, they also provide measurement targets that selectively isolate the function of 5-HT autoreceptors. Additionally, behavioral pharmacology can be used to study autoreceptor function indirectly by measuring behaviors influenced by autoreceptor function, although it can be difficult to parse the effects of autoreceptors vs. heteroreceptors in some cases.
4.2 Manipulation
A straightforward way of selectively activating 5-HT1A autoreceptors is by infusing 5-HT1A ligands directly into the DRN, which results in decreased spike rate of serotonergic DRN neurons (Blier et al., 1989) and reduced 5-HT release in terminal regions (Bonvento et al., 1992; Hjorth and Sharp, 1991; Hutson et al., 1989). A second, less selective, method of pharmacologically manipulating 5-HT1A autoreceptors is by systemically injecting agonists/antagonists at low doses, which preferentially activate autoreceptors (Blier et al., 1993; Hjorth and Magnusson, 1988; Kennett et al., 1987; Sprouse and Aghajanian, 1988). Because this method is less selective for autoreceptors than intra-DRN infusions, results from these types of experiments will be presented in this review only when there is additional evidence demonstrating that the effects of systemic drug injections are autoreceptor-mediated.
Because 5-HT1B protein is transported to axon terminals, selectively manipulating 5-HT1B autoreceptors is technically challenging. Our laboratory has developed a system to overexpress 5-HT1B autoreceptors using herpes simplex virus that is stereotaxically injected in the DRN. These transgenic receptors are transported to axon terminals like endogenous 5-HT1B autoreceptors and possess normal 5-HT1B-like function (Clark et al., 2002; Clark et al., 2004; Riegert et al., 2008). Similar to 5-HT1A, 5-HT1B autoreceptors are preferentially activated at lower doses of agonists than 5-HT1B heteroreceptors (Sarhan and Fillion, 1999), and systemic injections of 5-HT1B agonists at low doses may activate 5-HT1B autoreceptors with partial selectivity.
Both 5-HT1A and 5-HT1B receptors have been “knocked out” using traditional mouse genetic strategies (Gardier et al., 2009). In both cases a single gene encodes both autoreceptors and heteroreceptors, making it difficult to know what aspects of behavioral phenotype to attribute to the loss of autoreceptors versus heteroreceptors. Further complicating matters, certain aspects of these phenotypes are mediated by developmental effects (Castanon et al., 2000; Gross et al., 2002). Because of these ambiguities, we will provide only limited discussion of traditional knock-out literature. Newer mouse genetics strategies allow for autoreceptor/heteroreceptor specificity and temporal control, providing an important new avenue for study of serotonin autoreceptors (Gross et al., 2002; Richardson-Jones et al., 2010).
5 Influence of 5-HT autoreceptors on behavior in unstressed, drug-free animals
5.1 Conditioned preference/aversion
Several studies have used conditioned place preference/aversion to show that 5-HT autoreceptor activation is itself inherently rewarding. Systemic injections of a 5-HT1A agonist show biphasic effects, with rats showing a serotonin-dependent (autoreceptor-mediated) preference for chambers paired with low doses and a serotonin-independent (heteroreceptor-mediated) avoidance of chambers paired with high doses (Papp and Willner, 1991). Further demonstrating that the rewarding aspects are mediated by autoreceptors, animals show a conditioned place preference for local infusion of 5-HT1A agonist into the DRN (Fletcher et al., 1993). Animals do not show a preference for low doses of systemic 5-HT1B agonists, although they do show aversion to high doses (Cervo et al., 2002; De Vry et al., 2000). Collectively these results suggest that acute inhibition of the DRN via 5-HT1A autoreceptors is rewarding, whereas stimulation of 5-HT1A and 5-HT1B heteroreceptors is aversive. This conclusion is consistent with pharmacological studies using acute administration of a serotonin-specific reuptake inhibitor or monoamine oxidase inhibitor to enhance 5-HT function, which rodents find aversive (Berendsen and Broekkamp, 1994; Buresova and Bures, 1987; Gommans et al., 1998; Prendergast et al., 1996) - but see (Olivier et al., 1999; Subhan et al., 2000). The idea of serotonin as an aversive signal has been further explored in “opponent process” theory (Boureau and Dayan, 2011; Cools et al., 2011; Daw et al., 2002).
5.2 Anxiety and conditioned fear
Stimulation of 5-HT autoreceptors is anxiolytic in a variety of tests. Intra-DRN infusions of a 5-HT1A agonist are anxiolytic in the light-dark box (Romaniuk et al., 2001), social interaction test (Higgins et al., 1992; Hogg et al., 1994), punished drinking (Higgins et al., 1988), inhibitory avoidance (Graeff et al., 1998) and shock-induced ultrasonic vocalization (Remy et al., 1996). Similarly, intra-MRN infusion of 5-HT1A agonist is anxiolytic in the elevated plus maze (De Almeida et al., 1998) and social interaction test (File et al., 1996; Picazo et al., 1995). Underscoring the difference between 5-HT1A autoreceptors and heteroreceptors, stimulation of 5-HT1A heteroreceptors in medial septum and dorsal hippocampus is anxiogenic (De Almeida et al., 1998; File et al., 1996). 5-HT1A knockout mice – which lack 5-HT1A auto- and heteroreceptors - display heightened anxiety in the open field test, elevated plus maze, elevated zero maze, and novelty-suppressed feeding (Gross et al., 2000; Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Using inducible knockout and tissue-specific rescue, it was demonstrated that these anxiety effects are due to lack of forebrain 5-HT1A heteroreceptors during early development (Gross et al., 2002). Similarly, mice with inducible suppression of 5-HT1A autoreceptor expression display normal anxiety in the open field test and elevated plus maze; however, data presented suggest that these mice may be more anxious than control mice in tests of novelty-suppressed feeding (Richardson-Jones et al., 2010). Collectively, mouse genetics literature does not strongly support a role for 5-HT1A autoreceptor function in regulating anxiety in the adult mouse – a conclusion that is inconsistent with the behavioral pharmacology literature presented above. One possibility for this discrepancy is that knocking out 5-HT1A autoreceptors results in compensatory changes in other aspects of serotonergic function that may undermine the primary effects of the genetic deletion (Ase et al., 2000, 2001; Ramboz et al., 1998). However there may also be limitations to the behavioral pharmacology literature, such as unexpected effects of 5-HT1A agonists infused into the DRN, potentially inhibiting nonserotonergic projection neurons or interneurons.
Overexpression of DRN 5-HT1B autoreceptors in unstressed rats reduces anxiety in the open field test and reduces measures of conditioned fear in both contextual fear conditioning and fear-potentiated startle (Clark et al., 2002; Clark et al., 2004; McDevitt et al., 2011). Systemic administration of the selective 5-HT1B agonist 5-propoxy-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H-pyrrolo[3,2-b]pyridine hydrochloride (CP-94,253) has a U-shaped dose-response on conditioned fear, with low doses (1 mg/kg) reducing fear similarly to 5-HT1B autoreceptor overexpression and higher having no effect at 3 mg/kg (McDevitt et al., 2011) and enhancing fear at 5 mg/kg (unpublished observations). These results suggest that activation of 5-HT1B heteroreceptors may be anxiogenic. Indeed, rats show conditioned aversion (referenced earlier) and increased anxiety in the elevated plus maze with systemic administration of this drug at 3 mg/kg or higher doses, but not at 1 mg/kg (Lin and Parsons, 2002). We have observed that endogenous expression levels of 5-HT1B autoreceptor mRNA in the rat DRN correlate inversely with anxiety (Hiroi and Neumaier, 2009; Kaiyala et al., 2003). Conclusions from traditional knockout experiments are again inconsistent with behavioral pharmacology experiments. Mice lacking the 5-HT1B receptor show reduced anxiety in certain tests, including novel object exploration and isolation-induced ultrasonic vocalization – but not in the elevated plus maze (Brunner et al., 1999; Malleret et al., 1999).
Another dimension of the relationship between serotonin and anxiety is the role of gonadal hormones such as estrogen and progesterone, which influence anxiety behavior in a variety of tests (Hiroi and Neumaier, 2006; Morgan et al., 2004). Since there are numerous suggestions that fluctuating levels of these hormones regulate serotonergic function (Bethea et al., 1998) and gonadal hormone receptors are expressed throughout raphe (Alves et al., 1998; Vanderhorst et al., 2005), the effects may be mediated in part by altered expression of 5-HT autoreceptors in the DRN. Estrogen, progesterone, and testosterone all reduce 5-HT1A mRNA in the DRN of rodents and primates (Pecins-Thompson and Bethea, 1999; Zhang et al., 1999) – but see (Hiroi and Neumaier, 2009; Sumner and Fink, 1993). These hormones reduce 5-HT1A binding in the DRN without affecting affinity or G-protein coupling efficiency (Le Saux and Di Paolo, 2005; Lu and Bethea, 2002). Additionally, estrogen decreases 5-HT1B mRNA in the DRN (Hiroi and Neumaier, 2009).
Taken together, the above data suggest that acute decreases in 5-HT function via 5-HT1A or 5-HT1B autoreceptors are anxiolytic. While this conclusion is not consistent with results from all mouse genetics experiments, it is supported by experiments demonstrating anxiogenic effects of acute serotonin-specific reuptake inhibitor (SSRI) administration in humans (Mir and Taylor, 1997; Spigset, 1999) and rodents (Burghardt et al., 2007; Dekeyne et al., 2000; Griebel et al., 1994; Matto et al., 1996; Sanchez and Meier, 1997).
5.3 Helplessness
The influence of serotonin on helplessness is less straightforward than its role in mediating anxiety or aversion. Because stress alters 5-HT autoreceptor function, and behavioral testing of helplessness and despair can induce stress, we must approach a discussion of this topic with caution. In this section we provide an overview of research focusing on the immediate expression of helplessness/despair behaviors. Later we will devote a section to discussing the role of stress in altering 5-HT autoreceptor function – which may play a mechanistic role in the development of depressive behavior. The behavioral models presented in this section all employ measurements of reactivity to a behavioral challenge, and may be considered at the most simple level to be models of stress reactivity. Additionally, the behavioral measures in these tests – particularly the forced swim test – are influenced by antidepressant drug treatment, which causes an increase in swimming in the forced swim test (Cryan et al., 2005), increased active escape in the two-way shuttlebox (Martin et al., 1990), and reduced measures of social avoidance/submission in conditioned social defeat (Marrow et al., 1999; Razzoli et al., 2011). Thus, these tests may be considered to have predictive validity in modeling “antidepressant-like” behavior. For a more in-depth discussion of validity in animal models of depression, we refer the reader elsewhere (Holmes, 2003).
The influence of 5-HT autoreceptors on behavior in the forced swim test is less straightforward than their role in anxiety and aversion. There is consistency in studies that employ acute or subchronic enhancement of autoreceptor function, showing that inhibiting 5-HT function has antidepressant-like behavioral effects. Intra-DRN infusion of a 5-HT1A agonist or overexpression of 5-HT1B receptors in the caudal DRN both result in increased swimming (McDevitt et al., 2011; Schreiber and De Vry, 1993). Findings in other behavioral models of helplessness are similar: systemic injection at low doses or intra-DRN infusion of a 5-HT1A agonist at the time of behavioral testing reverses the induction of stress-induced shuttlebox escape deficits in rats (Maier et al., 1995) and conditioned social defeat in Syrian hamsters (Cooper et al., 2008). Rats bred for congenital susceptibility to learned helplessness have reduced 5-HT1B mRNA in the DRN, suggestive of reduced inhibitory control of serotonergic neurons (Neumaier et al., 2002). From the above observations one might conclude that 5-HT exacerbates the expression of helplessness and depression. This would contradict the popular “monoamine hypothesis” of depression (Schildkraut, 1965) as well as several important findings in animal models. First, given in acute, subchronic, or chronic schedules, serotonin reuptake inhibitors increase swimming in the forced swim test (Cryan et al., 2005). Second, knockout of 5-HT1A receptors has antidepressant-like effects in the forced swim and tail suspension tests (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Similar effects are seen in mice with inducible suppression of 5-HT1A autoreceptor expression, confirming that they are not a product of heteroreceptors or developmental abnormality (Richardson-Jones et al., 2010). Curiously, however, measurements of extracellular 5-HT concentrations in several brain regions of these mice are identical to controls. This would suggest that behavior in the forced swim test is mediated by some factor(s) other than absolute concentration of 5-HT.
One such factor that may influence swimming behavior in the forced swim test is the relative change in extracellular 5-HT from pre-swim baseline. Extracellular 5-HT concentrations in the lateral septum are decreased during a swim session, and when individual differences are examined there is a positive correlation between extracellular 5-HT concentration during swim (as percent of baseline) and immobility behavior (Kirby and Lucki, 1997). Furthermore, pretreatment with fluoxetine – which increases baseline levels – actually magnifies the drop in 5-HT seen during the swim test. Interestingly, the lateral septum receives serotonergic input preferentially from the caudal DRN (Waselus et al., 2006), the region that we targeted in 5-HT1B overexpression (McDevitt et al., 2011). Acute 5-HT1A stimulation or 5-HT1B overexpression may serve to directly reproduce the decrease in lateral septum 5-HT seen with fluoxetine treatment.
The equivocal data on serotonin autoreceptors and rodent models of helplessness are also seen in human research. The fact that SSRI treatment alleviates symptoms of depression might lead one to predict that reduced autoreceptor function should exert antidepressant effects. However, depressed human subjects are reported to have decreased expression of 5-HT1A autoreceptors – an effect that, on its own, would be expected to enhance serotonergic function. Several PET scan studies have demonstrated reduced 5-HT1A binding in the raphe of humans with depression (Drevets et al., 1999; Meltzer et al., 2004; Sargent et al., 2000), panic disorder (Neumeister et al., 2004), and social anxiety disorder (Lanzenberger et al., 2007) - however, see (Bhagwagar et al., 2004; Parsey et al., 2006). Similar evidence of reduced 5-HT1A autoreceptor expression has been documented in post-mortem studies of suicidally depressed humans (Arango et al., 2001; Boldrini et al., 2008) – but see (Stockmeier et al., 1998). These observations and the animal model literature suggest that the relationship between 5-HT and depression is highly complex, likely to be influenced by many variables including which DRN subregion and terminal region are studied, acute versus chronic treatment, baseline extracellular 5-HT versus challenge-evoked changes, and context of stress. A great deal more research is necessary to understand how these dimensions influence the relationship between serotonin and helplessness.
5.4 Comment on DRN subregional heterogeneity
In studies employing 5-HT1B overexpression, the pattern of behavioral results is dependent on the rostrocaudal location of injection within the DRN. Because virus generally has less spread than small molecule drugs, and the exact location of infected cells can be determined ex vivo with GFP fluorescence, this method is ideal for distinguishing small subregions. While conditioned fear is influenced by 5-HT1B overexpression throughout the DRN, anxiety – or behavior in conflict tests involving innate fear - is affected only by rostral DRN injections (Clark et al., 2002; Clark et al., 2004; McDevitt et al., 2011). A selective role for the rostral DRN in anxiety is consistent with correlational data in the literature. Exposure to the open field test induces greatest Fos expression in the rostral DRN (Bouwknecht et al., 2007), and anxiety behavior in the open field test and elevated plus maze correlates inversely with endogenous expression of 5-HT1B in middle, but not caudal, DRN (Hiroi and Neumaier, 2009; Kaiyala et al., 2003). The subregions of the DRN that influence anxiety may be distinct from those regulating helplessness. We found that 5-HT1B overexpression in the caudal DRN reduced immobility in the first 5 minutes of a forced swim test (McDevitt et al., 2011). Though we did not detect effects of 5-HT1B overexression in the mid-rostral DRN (Clark et al., 2002), only the second day swim session was investigated, and it is conceivable that there were effects that went undetected. Similarly, behavior in the forced swim test correlates with measures of 5-HT release in target regions of the caudal, but not rostral, DRN (Kirby and Lucki, 1997). Furthermore, intra-DRN infusions of the stress-related peptide corticotropin-releasing factor induces helplessness behavior in an active escape task when infused in the caudal, but not rostral, DRN (Hammack et al., 2002). These studies suggest that conditioned fear is regulated by the full rostrocaudal axis of the DRN, whereas anxiety and helplessness are specifically regulated by rostral and caudal subregions, respectively.
6. Role of 5-HT autoreceptors in stress
6.1 Effects of stress on extracellular 5-HT levels
Acute stress exposure has heterogeneous effects on 5-HT release, with reports of increases, decreases, and no change in extracellular 5-HT depending on terminal region examined and stressor used (Amat et al., 1998a, b; Bland et al., 2003a; Bland et al., 2003b; Kirby et al., 1995; Rueter et al., 1997). Interestingly, some brain regions that show elevated acute 5-HT release during inescapable tailshock show persisting changes in 5-HT function. Inescapable tailshock 24 hours prior to a behavioral challenge causes enhanced 5-HT release in the basolateral amygdala, ventral hippocampus, and medial prefrontal cortex (Amat et al., 1998a, b; Bland et al., 2003a; Christianson et al., 2010; Petty et al., 1994). It has been proposed that 5-HT1A autoreceptor desensitization may account for the enhancements in 5-HT function seen 24 h post-stress (Maier and Watkins, 2005). Here we present a modified form of that theory, suggesting that both 5-HT1A and 5-HT1B autoreceptors are desensitized during uncontrollable stress. The desensitization of these receptors results in increased 5-HT activity and, consequently, greater expression of fear and anxiety behavior.
6.2 Stress and 5-HT1A autoreceptors
Studies employing various stressors have demonstrated stress-induced reduction in 5-HT1A autoreceptor function, as assessed by electrophysiological recordings (Bambico et al., 2009; Laaris et al., 1997; Lanfumey et al., 1999), receptor affinity (Flugge, 1995), 5-HT synthesis (Haleem, 1999), and hormonal responsiveness (Korte et al., 1995) - however, see (Cornelisse et al., 2007; Kirby et al., 2007). Preliminary electrophysiological evidence for stress-induced 5-HT1A autoreceptor desensitization has been recently presented by two other research groups (Lemos et al., 2010; Rozeske et al., 2010). In addition to the above effects, there is a downregulation of 5-HT1A autoreceptor expression after uncontrollable stress which has been shown via reduced DRN 5-HT1A binding (Briones-Aranda et al., 2005; Leventopoulos et al., 2009) and mRNA (Cooper et al., 2009) – however, these effects may depend on specific stress procedures or measurements, as there are several publications finding no effect on 5-HT1A autoreceptor expression (Abumaria et al., 2006; Flugge, 1995; Pare and Tejani-Butt, 1996). Interestingly, 6 weeks of exercise – which is protective against the behavioral consequences of inescapable tailshock (Greenwood et al., 2005a) has an opposite effect on DRN 5-HT1A expression, increasing 5-HT1A mRNA in the middle dorsal and dorsolateral DRN (Greenwood et al., 2005b). This may protect against the behavioral effects of stress in part by suppressing DRN activity during stressor exposure and/or providing a buffer of spare receptors to reduce the extent of autoreceptor desensitization. Puzzlingly, an increase in 5-HT1A mRNA is also seen – albeit in a different subregion, the middle ventromedial DRN – after chronic infusion of corticotropin-releasing factor, a treatment which was anxiogenic (Clark et al., 2007).
Strong activation of the DRN appears to be a critical component in the stress-induced desensitization of 5-HT1A autoreceptors. Manipulations of stressor controllability that restrict DRN activation also limit the sensitization of 5-HT release 24 h post-stress (Amat et al., 1998a, b). Furthermore, directly inhibiting the DRN during uncontrollable stress exposure via infusion of a 5-HT1A agonist (or electrolytic lesion) prevents behavioral consequences of stress in a variety of tasks including shuttlebox escape, fear conditioning, social exploration, and drug reward (Christianson et al., 2008; Maier et al., 1995; Will et al., 2004). Similarly, intra-DRN infusions of the 5-HT1A agonist flesinoxan in Syrian hamsters at the time of social defeat reduces behavioral consequences in tests carried out 24 h later (Cooper et al., 2008). Uncontrollable stress exposure results in large increases in extracellular 5-HT within the DRN (Maswood et al., 1998), and 5-HT1A autoreceptors are susceptible to internalization (Riad et al., 2001) and functional desensitization (Kennett et al., 1987) after acute activation by agonist. Stress hormones appear to be a permissive factor in this process: glucocorticoids are necessary for stress-induced desensitization (Laaris et al., 1997) but unlikely to be sufficient to explain the phenomenon, as manipulations of stressor controllability that prevent the sensitization in 5-HT function (Amat et al., 1998a, b) do not reduce plasma levels of stress hormones (Maier et al., 1986). Another possible mechanistic component is the neuropeptide galanin – the DRN contains galanin-positive fibers and cell bodies (Gundlach et al., 1990; Melander et al., 1986) and expresses galanin receptors (Melander et al., 1988) - but note important species differences in mouse (Fu et al., 2010). Galanin is capable of modulating 5-HT1A function and may play a role in stress-induced autoreceptor desensitization (Ogren et al., 2007) and psychiatric disease states (Holmes and Picciotto, 2006).
6.3 Stress and 5-HT1B autoreceptors
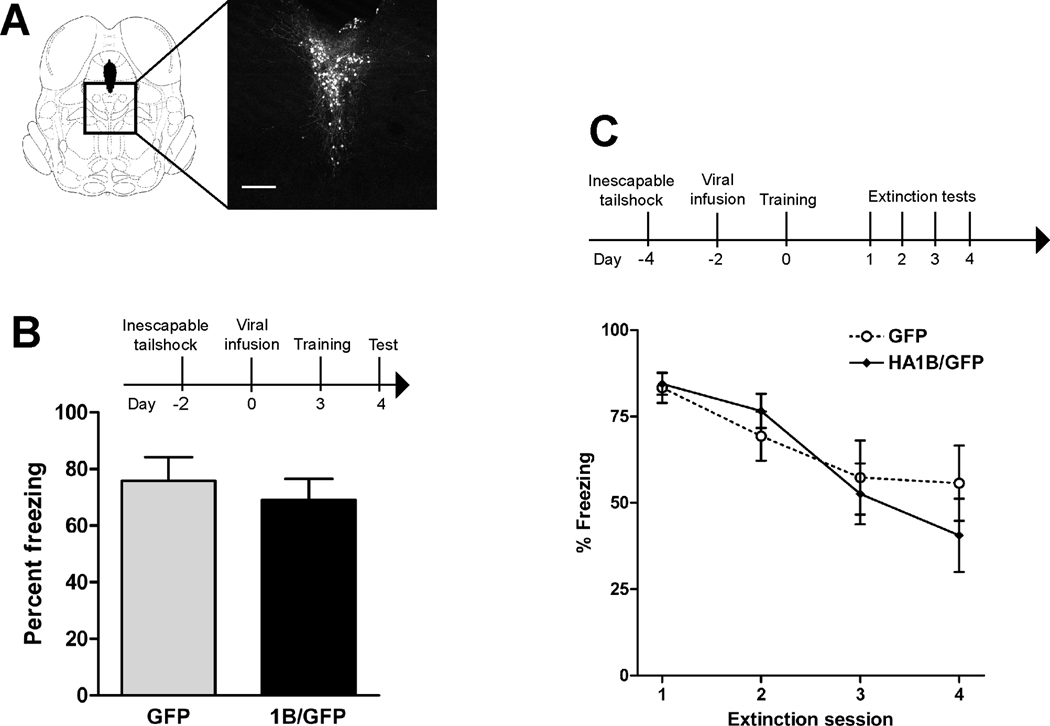
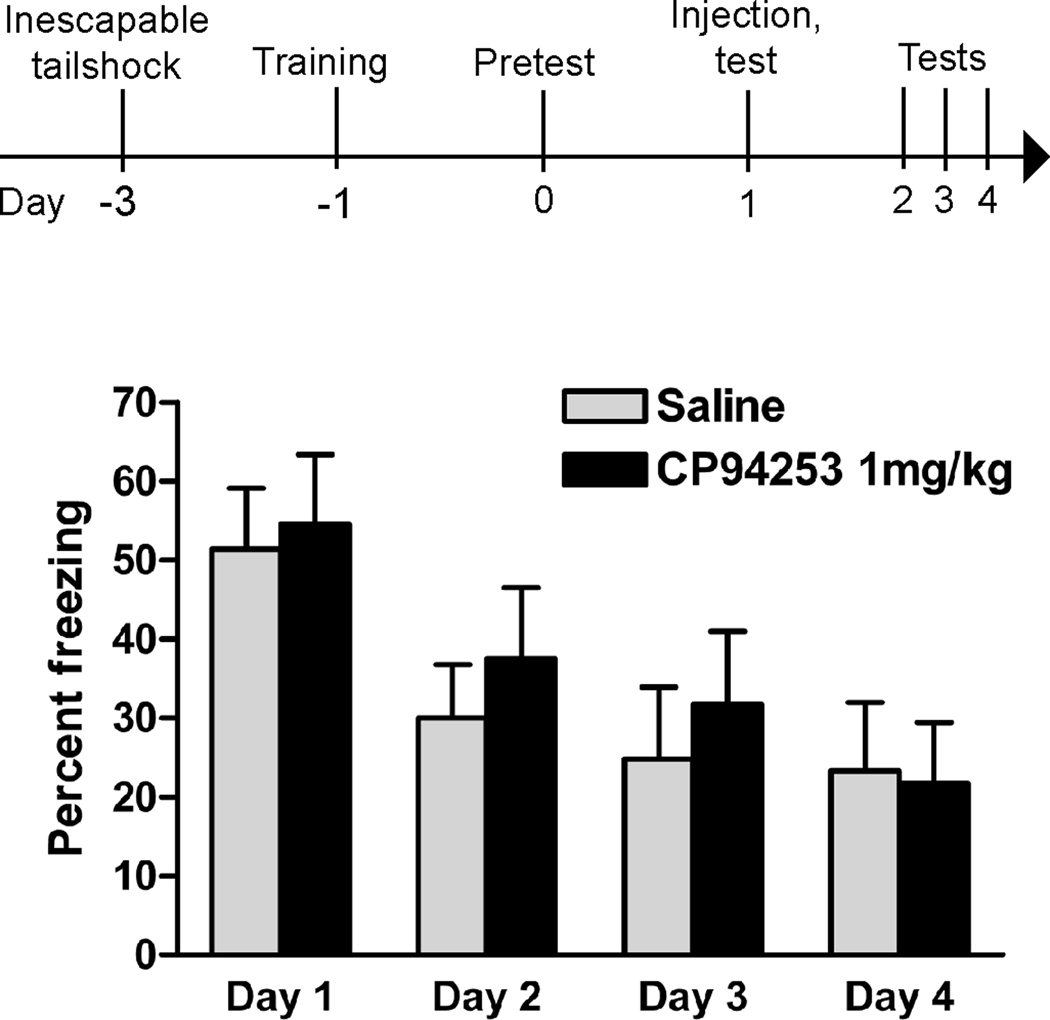
Like 5-HT1A autoreceptors, 5-HT1B autoreceptors also desensitize after stress. Using K+-stimulated release of 5-HT from synaptosomes to isolate autoreceptor-specific effects, Bolanos-Jimenez et al (Bolanos-Jimenez et al., 1995) showed that stress reduced the efficacy of 5-HT1B autoreceptors in inhibiting this effect, without altering total number of binding sites. Our laboratory has observed behavioral evidence for stress-induced 5-HT1B desensitization. First, we found that overexpression of 5-HT1B autoreceptors increased swimming in the first 5 minutes of a 15 minute forced swim session in naïve rats; however, when the same rats were tested 24 hours later in a 5 minute swim, 5-HT1B overexpression had no effect on behavior (McDevitt et al., 2011). Though interpretation of this experiment is limited by its within-subject experimental design, we have seen similar results in other studies in which either stressed or unstressed rats were tested once for a particular behavior. 5-HT1B overexpression reduced fear-potentiated startle in naïve rats, but not in rats that had been exposed to water-restraint stress 24 hours prior to testing (Clark et al., 2004). The same pattern of results emerged in experiments using inescapable tailshock as a stressor and contextual fear conditioning as a behavioral measure. In unstressed rats, 5-HT1B overexpression reduced measures of conditioned fear (McDevitt et al., 2011). When identical procedures were carried out using rats that were first exposed to inescapable tailshock, however, there was no effect of overexpression (Figure 1). We interpreted these results to reflect a lack of 5-HT1B efficacy; however, it is conceivable that stress might upregulate 5-HT1B autoreceptor expression to some maximal amount, where some other biological factor limits 5-HT1B function. Under this scenario, however, the 5-HT1B agonist CP-94,253 would be expected to have equal or greater efficacy in reducing fear in stressed, versus unstressed, rats – which it did not. In unstressed rats, systemic injections of CP-94,253 at low doses paired with exposure to a fear-conditioned context reduced the expression of fear (McDevitt et al., 2011). However, CP-94,253 had no effect in rats that had prior exposure to inescapable tailshock stress (Figure 2). Collectively, these results demonstrate that 5-HT1B autoreceptors lose their ability to regulate emotional behavior after uncontrollable stress exposure.
Figure 1.
Overexpression of 5-HT1B receptors in the caudal DRN does not reduce conditioned fear in rats with a history of inescapable tailshock stress. In contrast, unstressed rats receiving 5-HT1B overexpression demonstrate reduced expression of conditioned fear (McDevitt et al., 2011). Surgeries and behavioral testing were carried out as previously described (McDevitt et al., 2011). A, demonstration of viral-mediated gene transfer in the caudal DRN. Left, depiction of tissue targeted in stereotaxic surgery (reprinted from (Paxinos and Watson, 1986), with permission from Elsevier, copyright 1997). Right, viral-mediated expression of GFP seen in a 40 µl slice of tissue. Scale bar = 400 µm. B, Rats were exposed to inescapable tailshock, infused with GFP (n=9) or 1B/GFP (n=11) viral vector, and underwent contextual fear conditioning. Graph depicts mean (+SEM) percent of observations spent freezing during a test session. C, to ensure that negative results in the above experiment were not due to a “ceiling” of percent freezing, an extinction experiment was performed in a separate cohort of animals. Rats were exposed to inescapable tailshock, infused with GFP (n=8) or 1B/GFP (n=8) viral vector, trained in contextual fear conditioning, and then tested on four consecutive days. Data points depict mean (± SEM) percent of observations spent freezing during test sessions.
Figure 2.
Systemic injection of the 5-HT1B agonist CP-94,253 does not reduce conditioned fear in rats with a history of inescapable tailshock stress. These results are in contrast to the fear-reducing effects of this drug in unstressed rats (McDevitt et al., 2011). Drug injections and behavioral testing were carried out as previously described (McDevitt et al., 2011). Rats were exposed to inescapable tailshock stress, trained in fear conditioning, and then exposed to the context for a brief pretest session to assess conditioned fear and balance groups (no difference in pretest freezing; p = 0.85). The following day (Day 1), rats were then injected intraperitoneally with saline (n=7) or CP-94,253 (1 mg/kg, n=8) 30 min prior to a test session. For 3 subsequent days (Days 2–4), rats were retested without injections.
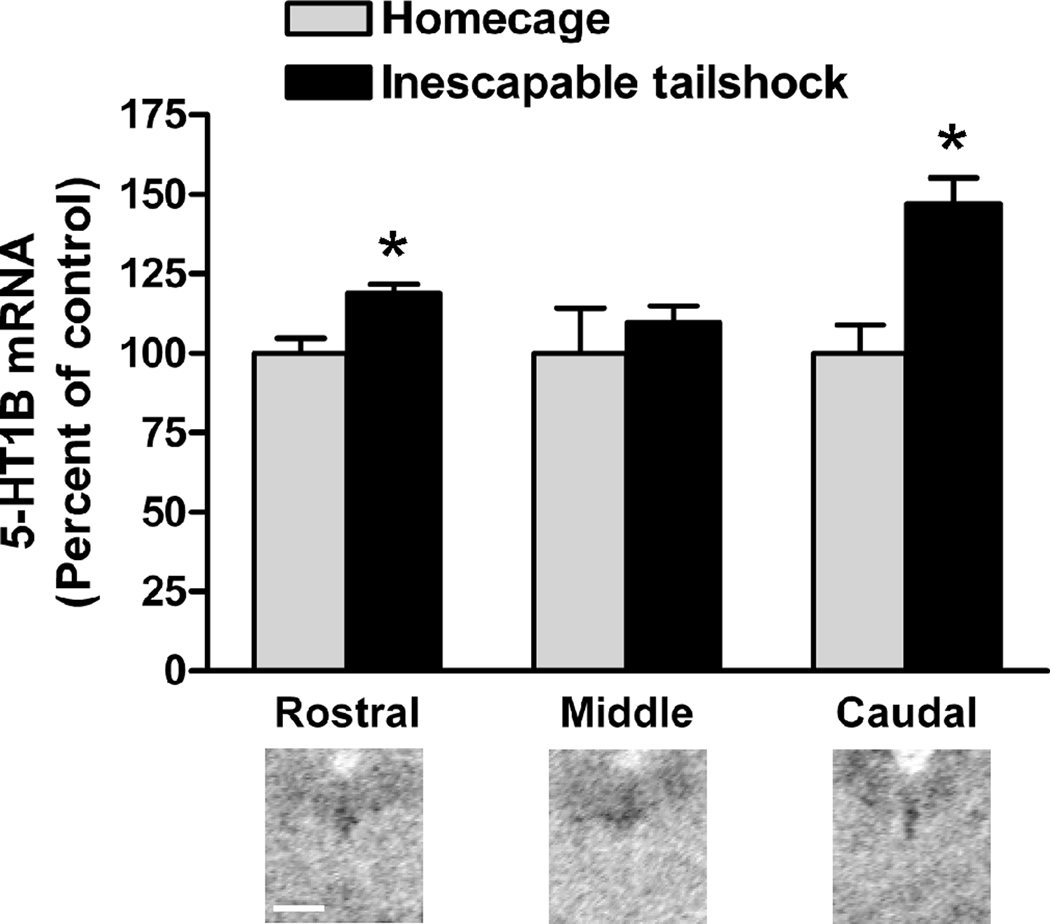
Accordingly, the correlations between anxiety and endogenous expression of 5-HT1B mRNA that are seen in unstressed animals vanish in rats with a history of uncontrollable stress exposure (Kaiyala et al., 2003) – as would be expected if these receptors are desensitized and no longer contributing to behavior. Uncontrollable stress does increase expression of 5-HT1B autoreceptor mRNA (Neumaier et al., 1997) (Figure 3). Because of the technical challenges of measuring total protein levels of autoreceptors versus heteroreceptors, it has not been possible to test whether stress increases the number of 5-HT1B autoreceptors. However, the fact that 5-HT1B autoreceptors are functionally desensitized by stress would suggest that increased mRNA expression levels may not be relevant. The increase in expression may be a direct homeostatic response to the loss of 5-HT1B function; alternatively, it could simply be a consequence of greater activity of DRN neurons. Because desensitization of 5-HT1B receptors would render their expression levels physiologically irrelevant, studying 5-HT1B autoreceptor function is critical in understanding the relationship between stress and behavior.
Figure 3.
Inescapable tailshock stress increases 5-HT1B mRNA in rat DRN. 5-HT1B expression in DRN was examined in brains previously used for a study of stress and locus coeruleus gene expression (McDevitt et al., 2009). In situ hybridization histochemistry was carried out as previously described (Clark et al., 2006). Briefly, rats were exposed to inescapable tailshock and sacrificed at 1, 2, 4, or 24 hours after the termination of stress session. Unstressed rats remained in homecage until time of sacrifice. Within each DRN subregion, measures of 5-HT1B mRNA signal were normalized to unstressed homecage control. Comparison of stress groups by two-way ANOVA with factors stress time point and DRN subregion (not shown) did not reveal significant differences between stress time points [F(3,39)=0.805, p=0.50], therefore all stress time points were combined into a single group (n=17) and compared to homecage controls (n=6). Graph represents mean (+SEM) optical density of in situ hybridization autoradiograms, grouped by DRN subregion. Below graph is sample autoradiogram of tissue at respective rostrocaudal level. Scale bar = 1 mm. * p < 0.01 vs. homecage control.
The apparent loss of 5-HT1B autoreceptor function in stressed animals may be due to direct desensitization of 5-HT1B autoreceptors, desensitization of postsynaptic receptors in terminal regions, or increases in DRN function – via desensitization of somatodendritic 5-HT1A receptors or sensitization of afferent inputs - that result in synaptic 5-HT levels so high that 5-HT1B-mediated inhibition is unable to reduce 5-HT enough to alter behavior. While direct stress-induced desensitization of 5-HT1B receptors has been demonstrated (Bolanos-Jimenez et al., 1995), it is unknown to what degree the other proposed factors might contribute to 5-HT1B-mediated behavior. A closer look at the behavioral evidence for desensitization reveals ideas that could be explored in future experiments utilizing more direct measures of 5-HT1B autoreceptor function. In several of our experiments utilizing 5-HT1B overexpression in stressed rats, the animals were exposed to inescapable tailshock stress several days before stereotaxic surgery was performed (Figure 1). Thus, it appears that stress disrupted the function of 5-HT1B autoreceptors that did not exist until several days after termination of the stressor. It seems unlikely that stress produces a simple desensitization or internalization of existing receptors, since newly synthesized receptors should be deployed to the terminals in the several days following stress exposure. The observation that these newly-synthesized 5-HT1B autoreceptors appeared functionally impaired suggests the possibility that uncontrollable stress causes some disruption in serotonergic terminal function that is independent of 5-HT1B autoreceptors per se. Possible mechanisms could include allosteric modulators of 5-HT1B function such as the tetrapeptide Leu-Ser-Ala-Leu (LSAL; “5-HT moduline”) (Ischia et al., 1997; Massot et al., 1998), intermediary signal transduction molecules (Bolanos-Jimenez et al., 1995), downstream effectors of 5-HT1B autoreceptors, or other proteins known to interact with 5-HT1B receptors such as glycogen synthase kinase-3 (Chen et al., 2009) or p11/S100A10 (Svenningsson et al., 2006).
6.4 Differences in 5-HT1A and 5-HT1B reactivity to stress
5-HT1B autoreceptors may be more susceptible to stress-induced desensitization than 5-HT1A autoreceptors. While caution must be employed in comparing across studies, there are two separate lines of evidence supporting this idea. First, there is a disparity in the severity of stressor required to desensitize 5-HT1A and 5-HT1B autoreceptors. A single session of restraint stress is sufficient to reduce physiological measurements of 5-HT1B (Bolanos-Jimenez et al., 1995) but not 5-HT1A function, which requires more severe or prolonged stressors for functional desensitization (Bambico et al., 2009; Laaris et al., 1997). Similarly, while a single 15-minute forced swim session completely disrupts the behavioral effects of 5-HT1B autoreceptor overexpression in a subsequent 5-minute test (McDevitt et al., 2011), it has no effect on electrophysiological measures of 5-HT1A autoreceptor function (Kirby et al., 2007). A preliminary electrophysiological study in mice suggests that chronic, but not acute, exposure to forced swim is required for desensitization of 5-HT1A autoreceptors (Lemos et al., 2010). A second line of evidence for differences in stress susceptibility of 5-HT1A and 5-HT1B autoreceptors is suggested in behavioral pharmacology experiments. In rats with a history of inescapable tailshock exposure, overexpression of 5-HT1B autoreceptors or systemic low doses of 5-HT1B agonist – treatments that reduce fear in unstressed rats - fail to affect conditioned fear, even when tested several days post-stress (Figure 1, Figure 2). In contrast, 5-HT1A agonists administered intra-DRN or systemically at low doses do alter helplessness behavior (Maier et al., 1995) and social exploration (Christianson et al., 2010) in rats exposed to inescapable tailshock 24 h prior to testing. Thus, 5-HT1A and 5-HT1B autoreceptors may have different thresholds for desensitization, may desensitize to stress in varying degrees.
The differences in 5-HT1A and 5-HT1B autoreceptor desensitization may have behavioral relevance. Inescapable tailshock stress has been shown to induce a variety of behavioral consequences – interestingly, however, these effects appear to segregate neatly into two groups on the basis of their duration. While some effects last about 1–2 days post-stress, such as anxiety and shuttlebox deficits (Christianson et al., 2008; Maier, 1990), sensitization of subsequent fear conditioning lasts at least a week post-stress (Baratta et al., 2007; Maier, 1990). In a similar stress model, enhanced fear conditioning effects were seen even at three months post-stress (Rau and Fanselow, 2009). One possible explanation for the short- and long-lasting behavioral effects of stress is that they are mediated by desensitization of autoreceptors with different rates of recovery. Our laboratory has seen behavioral evidence for stress-induced desensitization of overexpressed 5-HT1B autoreceptors as early as 24 hours post-stress (Clark et al., 2004; McDevitt et al., 2011) and as late as 6–8 days post-stress (Figures 1B, 1C). Additionally, experiments using the 5-HT1B agonist CP-94,253 yielded similar results, suggesting desensitization at 4–7 days post-stress (Figure 2). These experiments show a relatively long-lasting time course of 5-HT1B desensitization that appears to mirror the behavioral effects of stress on conditioned fear. There is a paucity of published data on the duration of desensitization of 5-HT1A autoreceptors induced by acute stress. More detailed information on the duration of 5-HT1A and 5-HT1B desensitization would be valuable in further supporting or denying the theory that these effects mediate different behavioral consequences of stress.
7. Role of 5-HT autoreceptors in antidepressant treatment
A mystery in 5-HT research is the delayed onset of antidepressant drug efficacy. One theory invokes autoreceptor desensitization (Hjorth et al., 2000; Pineyro and Blier, 1999). Because autoreceptors provide negative feedback that serves to drive 5-HT function towards equilibrium, they may initially provide homeostatic opposition to the effects of antidepressant drugs. However, as the autoreceptors are chronically exposed to high levels of 5-HT, they gradually desensitize and allow 5-HT levels to reach an asymptotic maximum. This theory posits that therapeutic effects of antidepressant drugs do not occur until these maximal levels of extracellular 5-HT are achieved.
There is extensive evidence that antidepressants drugs desensitize 5-HT1A autoreceptors - reviewed in greater detail in (Pineyro and Blier, 1999). Functional desensitization following chronic antidepressant treatment has been demonstrated via electrophysiology (Blier and De Montigny, 1983; Blier et al., 1984; Chaput et al., 1986; Jolas et al., 1994; Le Poul et al., 1995; Rueter et al., 1997), adenylate cyclase activity (Newman et al., 1993) and microdialysis (Cremers et al., 2000; Dawson et al., 2002; Invernizzi et al., 1994; Kreiss and Lucki, 1995). Acute antidepressant treatment results in internalization of 5-HT1A autoreceptors, however this effect is abolished in rats treated chronically with antidepressant drugs (Riad et al., 2008; Riad et al., 2001). Thus antidepressant drugs may cause repeated activation and internalization of 5-HT1A autoreceptors, which are gradually replaced on the plasma membrane by receptors in an inactivated state. This idea is supported by observations that chronic antidepressant treatment decreases agonist-stimulated binding of GTPγS to 5-HT1A autoreceptors, despite unchanged total receptor binding (Castro et al., 2003; Hensler, 2002; Pejchal et al., 2002; Shen et al., 2002). The above evidence would predict that pharmacological blockade of 5-HT1A receptors would accelerate antidepressant onset. Indeed, in eight out of ten published placebo-controlled clinical trials, the 5-HT1A/β-adrenergic antagonist pindolol was found to accelerate SSRI onset - reviewed in (Blier, 2003). While the human 5-HT1B (formerly 5-HT1Dβ) is pindolol-insensitive like 5-HT1D receptors, a single amino acid substitution in the rat 5-HT1B sequence confers high affinity binding of pindolol (Metcalf et al., 1992; Oksenberg et al., 1992; Parker et al., 1993). A recent animal study showed that mice with genetically reduced expression of 5-HT1A autoreceptors responded more quickly to antidepressants in terms of extracellular 5-HT concentrations (Richardson-Jones et al., 2010). However, behavioral responding to the drug treatment did not correspond with neurochemical changes: although four weeks of antidepressant treatment resulted in similar 5-HT concentrations in multiple terminal brain regions of control and transgenic mice, it produced an anxiolytic behavioral response in the novelty-suppressed feeding test in transgenic mice only.
Independently of electrophysiological activity at cell bodies, chronic antidepressants also result in greater evoked terminal release of 5-HT (Blier et al., 1988; Chaput et al., 1986; Maura and Raiteri, 1984; Moret and Briley, 1990). Studies attempting to identify the receptor(s) mediating this effect have yielded equivocal results. For example, studies testing sensitivity of 5-HT release to nonselective 5-HT1B/1D drugs have reported both functional desensitization (Pineyro and Blier, 1996) and a lack of desensitization (Cremers et al., 2000; Jongsma et al., 2005; Moret and Briley, 1996). Studies employing selective drugs suggest that there is desensitization of 5-HT1B, not 5-HT1D autoreceptors (Davidson and Stamford, 2000; el Mansari et al., 1995; O'Connor and Kruk, 1994; Sayer et al., 1999) – but see (Bosker et al., 1995). Detection of 5-HT1B autoreceptor desensitization by antidepressant is dependent upon circadian phase (Sayer et al., 1999), specific terminal region examined (el Mansari et al., 1995), and blockade of masking effects of 5-HT1A autoreceptors (Davidson and Stamford, 2000). Conservatively, we might conclude that chronic antidepressants do reduce the sensitivity of 5-HT1B autoreceptors, but this effect does not appear to be as robust as the effect on 5-HT1A autoreceptors. In addition, chronic antidepressants also reduce expression of 5-HT1B autoreceptor mRNA in the DRN, but this effect is rapidly reversed by discontinuation of drug (Anthony et al., 2000; Neumaier et al., 1996a). This result also raises the methodological concern that many of these studies have investigated 5-HT autoreceptors after acute withdrawal from the antidepressant, which may be a serious confound limiting their interpretability. Nevertheless, the preponderance of the data indicates that antidepressants desensitize both somatodendritic and terminal 5-HT autoreceptors, thereby increasing overall activity of the 5-HT system and resulting extracellular concentrations of 5-HT.
8. Conclusions
Serotonin function is regulated primarily by 5-HT1A, 5-HT1B, and 5-HT1D autoreceptors. Although investigating these autoreceptors is technically challenging because they are also expressed widely in CNS as heteroreceptors, their central role as key regulators of the pattern of serotonin neuron firing and transmitter release makes them an important focus of attention. Manipulations of these autoreceptors reveal roles in regulating aversion, anxiety, and helplessness behaviors. Acute stimulation of 5-HT1A and 5-HT1B autoreceptors generally exert anxiolytic and antidepressant-like effects on behavior. There are discrepancies in the conclusions of literature using behavioral pharmacology versus genetic approaches, perhaps due in part to limitations of traditional “knockout” approaches. New genetic approaches allowing cell type and temporal specificity are critical in exploring the role of 5-HT autoreceptors. Numerous studies show that exposure to stress, sex hormones, and both therapeutic and illicit drugs modulate the expression and function of these 5-HT autoreceptors, thereby conferring changes in 5-HT functioning and behavior that can last from days to weeks. The contradiction that 5-HT autoreceptor desensitization has been demonstrated following both uncontrollable stress exposure and chronic SSRI administration, stimuli that have seemingly opposing effects on behavior, remains perplexing. Greater understanding of autoreceptor desensitization is needed to reconcile these disparate findings. Further study will help reveal the utility and limitations of 5-HT autoreceptors as a class of therapeutic target in the treatment of anxiety disorders and depression.
Highlight.
In this review of serotonin autoreceptors, we primarily cover the role of 5-HT1A and 5-HT1B receptors in regulating behavior.
Overall, activation of these autoreceptors is rewarding and anxiolytic, but has more complex effects in models of helplessness.
Both stress and antidepressant treatment appear to desensitize these autoreceptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abumaria N, Rygula R, Havemann-Reinecke U, Ruther E, Bodemer W, Roos C, Flugge G. Identification of genes regulated by chronic social stress in the rat dorsal raphe nucleus. Cell Mol Neurobiol. 2006;26:145–162. doi: 10.1007/s10571-006-9024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998a;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998b;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Anthony JP, Sexton TJ, Neumaier JF. Antidepressant-induced regulation of 5-HT(1b) mRNA in rat dorsal raphe nucleus reverses rapidly after drug discontinuation. J Neurosci Res. 2000;61:82–87. doi: 10.1002/1097-4547(20000701)61:1<82::AID-JNR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. J Neurochem. 2000;75:2415–2426. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Regional changes in density of serotonin transporter in the brain of 5-HT1A and 5-HT1B knockout mice, and of serotonin innervation in the 5-HT1B knockout. J Neurochem. 2001;78:619–630. doi: 10.1046/j.1471-4159.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur Neuropsychopharmacol. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J Neurophysiol. 1997;77:1349–1361. doi: 10.1152/jn.1997.77.3.1349. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HH, Broekkamp CL. Comparison of stimulus properties of fluoxetine and 5-HT receptor agonists in a conditioned taste aversion procedure. Eur J Pharmacol. 1994;253:83–89. doi: 10.1016/0014-2999(94)90760-9. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Mol Neurobiol. 1998;18:87–123. doi: 10.1007/BF02914268. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003a;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Bland ST, Twining C, Watkins LR, Maier SF. Stressor controllability modulates stress-induced serotonin but not dopamine efflux in the nucleus accumbens shell. Synapse. 2003b;49:206–208. doi: 10.1002/syn.10229. [DOI] [PubMed] [Google Scholar]
- Blier P. The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol. 2003;13:57–66. doi: 10.1016/s0924-977x(02)00173-6. [DOI] [PubMed] [Google Scholar]
- Blier P, Chaput Y, de Montigny C. Long-term 5-HT reuptake blockade, but not monoamine oxidase inhibition, decreases the function of terminal 5-HT autoreceptors: an electrophysiological study in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:246–254. doi: 10.1007/BF00168834. [DOI] [PubMed] [Google Scholar]
- Blier P, De Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J Neurosci. 1983;3:1270–1278. doi: 10.1523/JNEUROSCI.03-06-01270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Tardif D. Effects of the two antidepressant drugs mianserin and indalpine on the serotonergic system: single-cell studies in the rat. Psychopharmacology (Berl) 1984;84:242–249. doi: 10.1007/BF00427453. [DOI] [PubMed] [Google Scholar]
- Blier P, Lista A, De Montigny C. Differential properties of pre- and postsynaptic 5-hydroxytryptamine1A receptors in the dorsal raphe and hippocampus: I. Effect of spiperone. J Pharmacol Exp Ther. 1993;265:7–15. [PubMed] [Google Scholar]
- Blier P, Steinberg S, Chaput Y, de Montigny C. Electrophysiological assessment of putative antagonists of 5-hydroxytryptamine receptors: a single-cell study in the rat dorsal raphe nucleus. Can J Physiol Pharmacol. 1989;67:98–105. doi: 10.1139/y89-017. [DOI] [PubMed] [Google Scholar]
- Bolanos-Jimenez F, Manhaes de Castro RM, Seguin L, Cloez-Tayarani I, Monneret V, Drieu K, Fillion G. Effects of stress on the functional properties of pre- and postsynaptic 5-HT1B receptors in the rat brain. Eur J Pharmacol. 1995;294:531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvento G, Scatton B, Claustre Y, Rouquier L. Effect of local injection of 8-OH-DPAT into the dorsal or median raphe nuclei on extracellular levels of serotonin in serotonergic projection areas in the rat brain. Neurosci Lett. 1992;137:101–104. doi: 10.1016/0304-3940(92)90308-t. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Klompmakers AA, Westenberg HG. Effects of single and repeated oral administration of fluvoxamine on extracellular serotonin in the median raphe nucleus and dorsal hippocampus of the rat. Neuropharmacology. 1995;34:501–508. doi: 10.1016/0028-3908(95)00023-y. [DOI] [PubMed] [Google Scholar]
- Boureau YL, Dayan P. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones-Aranda A, Rocha L, Picazo O. Influence of forced swimming stress on 5-HT1A receptors and serotonin levels in mouse brain. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:275–281. doi: 10.1016/j.pnpbp.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- Buresova O, Bures J. Conditioned taste aversion induced in rats by intracerebral or systemic administration of monoamine oxidase inhibitors. Psychopharmacology (Berl) 1987;91:209–212. doi: 10.1007/BF00217064. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R. Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol Biochem Behav. 2000;67:559–566. doi: 10.1016/s0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Castro ME, Diaz A, del Olmo E, Pazos A. Chronic fluoxetine induces opposite changes in G protein coupling at pre and postsynaptic 5-HT1A receptors in rat brain. Neuropharmacology. 2003;44:93–101. doi: 10.1016/s0028-3908(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Cervo L, Rozio M, Ekalle-Soppo CB, Carnovali F, Santangelo E, Samanin R. Stimulation of serotonin1B receptors induces conditioned place aversion and facilitates cocaine place conditioning in rats. Psychopharmacology (Berl) 2002;163:142–150. doi: 10.1007/s00213-002-1145-8. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain--a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Chen L, Salinas GD, Li X. Regulation of serotonin 1B receptor by glycogen synthase kinase-3. Mol Pharmacol. 2009;76:1150–1161. doi: 10.1124/mol.109.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Hoplight BJ, Neumaier JF. Chronic low dose ovine corticotropin releasing factor or urocortin II into the rostral dorsal raphe alters exploratory behavior and serotonergic gene expression in specific subregions of the dorsal raphe. Neuroscience. 2007;146:1888–1905. doi: 10.1016/j.neuroscience.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007:86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Commons KG. Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system. Eur J Neurosci. 2008;27:2611–2618. doi: 10.1111/j.1460-9568.2008.06235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan V, Segu L, Buhot MC, Daszuta A. Selective increases in serotonin 5-HT1B/1D and 5-HT2A/2C binding sites in adult rat basal ganglia following lesions of serotonergic neurons. Brain Res. 1998;793:103–111. doi: 10.1016/s0006-8993(98)00168-1. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelisse LN, Van der Harst JE, Lodder JC, Baarendse PJ, Timmerman AJ, Mansvelder HD, Spruijt BM, Brussaard AB. Reduced 5-HT1A- and GABAB receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J Neurophysiol. 2007;98:196–204. doi: 10.1152/jn.00109.2007. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Spoelstra EN, de Boer P, Bosker FJ, Mork A, den Boer JA, Westerink BH, Wikstrom HV. Desensitisation of 5-HT autoreceptors upon pharmacokinetically monitored chronic treatment with citalopram. Eur J Pharmacol. 2000;397:351–357. doi: 10.1016/s0014-2999(00)00308-3. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson C, Stamford JA. Effect of chronic paroxetine treatment on 5-HT1B and 5-HT1D autoreceptors in rat dorsal raphe nucleus. Neurochem Int. 2000;36:91–96. doi: 10.1016/s0197-0186(99)00115-1. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Smith DL, Schechter LE. Effect of chronic fluoxetine and WAY-100635 treatment on serotonergic neurotransmission in the frontal cortex. J Psychopharmacol. 2002;16:145–152. doi: 10.1177/026988110201600205. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida RM, Giovenardi M, Charchat H, Lucion AB. 8-OH-DPAT in the median raphe nucleus decreases while in the medial septal area it may increase anxiety in female rats. Neurosci Biobehav Rev. 1998;23:259–264. doi: 10.1016/s0149-7634(98)00026-8. [DOI] [PubMed] [Google Scholar]
- De Vry J, Eckel G, Kuhl E, Schreiber R. Effects of serotonin 5-HT(1) and 5-HT(2) receptor agonists in a conditioned taste aversion paradigm in the rat. Pharmacol Biochem Behav. 2000;66:797–802. doi: 10.1016/s0091-3057(00)00248-3. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Denorme B, Monneyron S, Millan MJ. Citalopram reduces social interaction in rats by activation of serotonin (5-HT)(2C) receptors. Neuropharmacology. 2000;39:1114–1117. doi: 10.1016/s0028-3908(99)00268-3. [DOI] [PubMed] [Google Scholar]
- Doly S, Bertran-Gonzalez J, Callebert J, Bruneau A, Banas SM, Belmer A, Boutourlinsky K, Herve D, Launay JM, Maroteaux L. Role of serotonin via 5-HT2B receptors in the reinforcing effects of MDMA in mice. PLoS One. 2009;4:e7952. doi: 10.1371/journal.pone.0007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly S, Valjent E, Setola V, Callebert J, Herve D, Launay JM, Maroteaux L. Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J Neurosci. 2008;28:2933–2940. doi: 10.1523/JNEUROSCI.5723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet E, Pohl M, Fattaccini CM, Adrien J, Mestikawy SE, Hamon M. In situ hybridization evidence for the synthesis of 5-HT1B receptor in serotoninergic neurons of anterior raphe nuclei in the rat brain. Synapse. 1995;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- el Mansari M, Bouchard C, Blier P. Alteration of serotonin release in the guinea pig orbito-frontal cortex by selective serotonin reuptake inhibitors. Relevance to treatment of obsessive-compulsive disorder. Neuropsychopharmacology. 1995;13:117–127. doi: 10.1016/0893-133X(95)00045-F. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Ming ZH, Higgins GA. Conditioned place preference induced by microinjection of 8-OH-DPAT into the dorsal or median raphe nucleus. Psychopharmacology (Berl) 1993;113:31–36. doi: 10.1007/BF02244330. [DOI] [PubMed] [Google Scholar]
- Flugge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Le Maitre E, Fabre V, Bernard JF, David Xu ZQ, Hokfelt T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518:3464–3494. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- Gardier AM, Guiard BP, Guilloux JP, Reperant C, Coudore F, David DJ. Interest of using genetically manipulated mice as models of depression to evaluate antidepressant drugs activity: a review. Fundam Clin Pharmacol. 2009;23:23–42. doi: 10.1111/j.1472-8206.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- Gommans J, Bouwknecht JA, Hijzen TH, Berendsen HH, Broekkamp CL, Maes RA, Olivier B. Stimulus properties of fluvoxamine in a conditioned taste aversion procedure. Psychopharmacology (Berl) 1998;140:496–502. doi: 10.1007/s002130050794. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Netto CF, Zangrossi H., Jr The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev. 1998;23:237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005a;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005b;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology (Berl) 1994;113:463–470. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach AL, Wisden W, Morris BJ, Hunt SP. Localization of preprogalanin mRNA in rat brain: in situ hybridization study with a synthetic oligonucleotide probe. Neurosci Lett. 1990;114:241–247. doi: 10.1016/0304-3940(90)90570-y. [DOI] [PubMed] [Google Scholar]
- Haleem DJ. Attenuation of 8-OH-DPAT-induced decreases in 5-Ht synthesis in brain regions of rats adapted to a repeated stress schedule. Stress. 1999;3:123–129. doi: 10.3109/10253899909001117. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Bradbury AJ, Jones BJ, Oakley NR. Behavioural and biochemical consequences following activation of 5HT1-like and GABA receptors in the dorsal raphe nucleus of the rat. Neuropharmacology. 1988;27:993–1001. doi: 10.1016/0028-3908(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Jones BJ, Oakley NR. Effect of 5-HT1A receptor agonists in two models of anxiety after dorsal raphe injection. Psychopharmacology (Berl) 1992;106:261–267. doi: 10.1007/BF02801982. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res. 2006;166:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience. 2009;158:456–464. doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol. 2000;14:177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Magnusson T. The 5-HT 1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:463–471. doi: 10.1007/BF00179315. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 1991;48:1779–1786. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19:170–176. doi: 10.1002/syn.890190304. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Tao R. The putative 5-HT1B receptor agonist CP-93,129 suppresses rat hippocampal 5-HT release in vivo: comparison with RU 24969. Eur J Pharmacol. 1991;209:249–252. doi: 10.1016/0014-2999(91)90177-r. [DOI] [PubMed] [Google Scholar]
- Hogg S, Andrews N, File SE. Contrasting behavioural effects of 8-OH DPAT in the dorsal raphe nucleus and ventral hippocampus. Neuropharmacology. 1994;33:343–348. doi: 10.1016/0028-3908(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Holmes A, Picciotto MR. Galanin: a novel therapeutic target for depression, anxiety disorders and drug addiction? CNS Neurol Disord Drug Targets. 2006;5:225–232. doi: 10.2174/187152706776359600. [DOI] [PubMed] [Google Scholar]
- Holmes PV. Rodent models of depression: reexamining validity without anthropomorphic inference. Crit Rev Neurobiol. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Middlemiss DN. Species differences in the pharmacology of terminal 5-HT autoreceptors in mammalian brain. Trends Pharmacol Sci. 1989;10:130–132. doi: 10.1016/0165-6147(89)90159-4. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Sarna GS, O'Connell MT, Curzon G. Hippocampal 5-HT synthesis and release in vivo is decreased by infusion of 8-OHDPAT into the nucleus raphe dorsalis. Neurosci Lett. 1989;100:276–280. doi: 10.1016/0304-3940(89)90698-8. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Pertussis toxin blocks 5-HT1A and GABAB receptormediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Bramante M, Samanin R. Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical serotonin output: role of presynaptic 5-HT1A receptors. Eur J Pharmacol. 1994;260:243–246. doi: 10.1016/0014-2999(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Ischia R, Lovisetti-Scamihorn P, Hogue-Angeletti R, Wolkersdorfer M, Winkler H, Fischer-Colbrie R. Molecular cloning and characterization of NESP55, a novel chromogranin-like precursor of a peptide with 5-HT1B receptor antagonist activity. J Biol Chem. 1997;272:11657–11662. doi: 10.1074/jbc.272.17.11657. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, Manivet P, Launay JM, Maroteaux L. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol. 2007;71:1463–1474. doi: 10.1124/mol.106.032656. [DOI] [PubMed] [Google Scholar]
- Jolas T, Haj-Dahmane S, Kidd EJ, Langlois X, Lanfumey L, Fattaccini CM, Vantalon V, Laporte AM, Adrien J, Gozlan H, et al. Central pre- and postsynaptic 5-HT1A receptors in rats treated chronically with a novel antidepressant, cericlamine. J Pharmacol Exp Ther. 1994;268:1432–1443. [PubMed] [Google Scholar]
- Jongsma ME, Bosker FJ, Cremers TI, Westerink BH, den Boer JA. The effect of chronic selective serotonin reuptake inhibitor treatment on serotonin 1B receptor sensitivity and HPA axis activity. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:738–744. doi: 10.1016/j.pnpbp.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Vincow ES, Sexton TJ, Neumaier JF. 5-HT1B receptor mRNA levels in dorsal raphe nucleus: inverse association with anxiety behavior in the elevated plus maze. Pharmacol Biochem Behav. 2003;75:769–776. doi: 10.1016/s0091-3057(03)00152-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology. 1998;18:469–479. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Katayama J, Yakushiji T, Akaike N. Characterization of the K+ current mediated by 5-HT1A receptor in the acutely dissociated rat dorsal raphe neurons. Brain Res. 1997;745:283–292. doi: 10.1016/s0006-8993(96)01141-9. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Marcou M, Dourish CT, Curzon G. Single administration of 5-HT1A agonists decreases 5-HT1A presynaptic, but not postsynaptic receptor-mediated responses: relationship to antidepressant-like action. Eur J Pharmacol. 1987;138:53–60. doi: 10.1016/0014-2999(87)90336-0. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther. 1997;282:967–976. [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Buwalda B, Meijer O, De Kloet ER, Bohus B. Socially defeated male rats display a blunted adrenocortical response to a low dose of 8-OH-DPAT. Eur J Pharmacol. 1995;272:45–50. doi: 10.1016/0014-2999(94)00621-d. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharmacol Exp Ther. 1995;274:866–876. [PubMed] [Google Scholar]
- Laaris N, Le Poul E, Hamon M, Lanfumey L. Stress-induced alterations of somatodendritic 5-HT1A autoreceptor sensitivity in the rat dorsal raphe nucleus--in vitro electrophysiological evidence. Fundam Clin Pharmacol. 1997;11:206–214. doi: 10.1111/j.1472-8206.1997.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Pardon MC, Laaris N, Joubert C, Hanoun N, Hamon M, Cohen-Salmon C. 5-HT1A autoreceptor desensitization by chronic ultramild stress in mice. Neuroreport. 1999;10:3369–3374. doi: 10.1097/00001756-199911080-00021. [DOI] [PubMed] [Google Scholar]
- Langlois X, Gerard C, Darmon M, Chauveau J, Hamon M, el Mestikawy S. Immunolabeling of central serotonin 5-HT1D beta receptors in the rat, mouse, and guinea pig with a specific anti-peptide antiserum. J Neurochem. 1995;65:2671–2681. doi: 10.1046/j.1471-4159.1995.65062671.x. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Launay JM, Schneider B, Loric S, Da Prada M, Kellermann O. Serotonin transport and serotonin transporter-mediated antidepressant recognition are controlled by 5-HT2B receptor signaling in serotonergic neuronal cells. Faseb J. 2006;20:1843–1854. doi: 10.1096/fj.06-5724com. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Changes in 5-HT1A receptor binding and G-protein activation in the rat brain after estrogen treatment: comparison with tamoxifen and raloxifene. J Psychiatry Neurosci. 2005;30:110–117. [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Phillips PE, Chavkin CC. Stress-induced dysregulation of kappa opioid modulation of serotonergic DRN neuronal excitability; San Diego, CA: Society for Neuroscience Annual Meeting; 2010. [Google Scholar]
- Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology. 2009;56:692–701. doi: 10.1016/j.neuropharm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lin D, Parsons LH. Anxiogenic-like effect of serotonin(1B) receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav. 2002;71:581–587. doi: 10.1016/s0091-3057(01)00712-2. [DOI] [PubMed] [Google Scholar]
- Lin SL, Setya S, Johnson-Farley NN, Cowen DS. Differential coupling of 5-HT(1) receptors to G proteins of the G(i) family. Br J Pharmacol. 2002;136:1072–1078. doi: 10.1038/sj.bjp.0704809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–149. [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behav Neurosci. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique C, Segu L, Hery F, Hery M, Faudon M, Francois-Bellan AM. Increase of central 5-HT1B binding sites following 5,7-dihydroxytryptamine axotomy in the adult rat. Brain Res. 1993;623:345–348. doi: 10.1016/0006-8993(93)91452-x. [DOI] [PubMed] [Google Scholar]
- Marrow LP, Overton PG, Brain PF. A re-evaluation of social defeat as an animal model of depression. J Psychopharmacol. 1999;13:115–121. doi: 10.1177/026988119901300201. [DOI] [PubMed] [Google Scholar]
- Martin P, Soubrie P, Puech AJ. Reversal of helpless behavior by serotonin uptake blockers in rats. Psychopharmacology (Berl) 1990;101:403–407. doi: 10.1007/BF02244061. [DOI] [PubMed] [Google Scholar]
- Massot O, Rousselle JC, Grimaldi B, Cloez-Tayarani I, Fillion MP, Plantefol M, Bonnin A, Prudhomme N, Fillion G. Molecular, cellular and physiological characteristics of 5-HT-moduline, a novel endogenous modulator of 5-HT1B receptor subtype. Ann N Y Acad Sci. 1998;861:174–182. doi: 10.1111/j.1749-6632.1998.tb10189.x. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Matto V, Harro J, Allikmets L. The effects of cholecystokinin A and B receptor antagonists, devazepide and L 365260, on citalopram-induced decrease of exploratory behaviour in rat. J Physiol Pharmacol. 1996;47:661–669. [PubMed] [Google Scholar]
- Maura G, Raiteri M. Functional evidence that chronic drugs induce adaptive changes of central autoreceptors regulating serotonin release. Eur J Pharmacol. 1984;97:309–313. doi: 10.1016/0014-2999(84)90466-7. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Hiroi R, Mackenzie SM, Robin NC, Cohn A, Kim JJ, Neumaier JF. Serotonin 1B Autoreceptors Originating in the Caudal Dorsal Raphe Nucleus Reduce Expression of Fear and Depression-Like Behavior. Biol Psychiatry. 2011;69:780–787. doi: 10.1016/j.biopsych.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, Szot P, Baratta MV, Bland ST, White SS, Maier SF, Neumaier JF. Stress-induced activity in the locus coeruleus is not sensitive to stressor controllability. Brain Res. 2009;1285:109–118. doi: 10.1016/j.brainres.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986;248:475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- Melander T, Kohler C, Nilsson S, Hokfelt T, Brodin E, Theodorsson E, Bartfai T. Autoradiographic quantitation and anatomical mapping of 125I-galanin binding sites in the rat central nervous system. J Chem Neuroanat. 1988;1:213–233. [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Metcalf MA, McGuffin RW, Hamblin MW. Conversion of the human 5-HT1D beta serotonin receptor to the rat 5-HT1B ligand-binding phenotype by Thr355Asn site directed mutagenesis. Biochem Pharmacol. 1992;44:1917–1920. doi: 10.1016/0006-2952(92)90092-w. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Doucet E, Riad M, Adrien J, Verge D, Hamon M. Effect of the selective lesion of serotoninergic neurons on the regional distribution of 5-HT1A receptor mRNA in the rat brain. Brain Res Mol Brain Res. 1992;14:357–362. doi: 10.1016/0169-328x(92)90104-j. [DOI] [PubMed] [Google Scholar]
- Mir S, Taylor D. The adverse effects of antidepressants. Curr Opin Psychiatr. 1997;10:88–94. [Google Scholar]
- Moret C, Briley M. Serotonin autoreceptor subsensitivity and antidepressant activity. Eur J Pharmacol. 1990;180:351–356. doi: 10.1016/0014-2999(90)90320-6. [DOI] [PubMed] [Google Scholar]
- Moret C, Briley M. Effects of acute and repeated administration of citalopram on extracellular levels of serotonin in rat brain. Eur J Pharmacol. 1996;295:189–197. doi: 10.1016/0014-2999(95)00646-x. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neurosci Biobehav Rev. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mrna regulation in two animal models of altered stress reactivity. Biol Psychiatry. 2002;51:902–908. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Petty F, Kramer GL, Szot P, Hamblin MW. Learned helplessness increases 5-hydroxytryptamine1B receptor mRNA levels in the rat dorsal raphe nucleus. Biol Psychiatry. 1997;41:668–674. doi: 10.1016/S0006-3223(96)00114-X. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Root DC, Hamblin MW. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology. 1996a;15:515–522. doi: 10.1016/S0893-133X(96)00095-4. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Szot P, Peskind ER, Dorsa DM, Hamblin MW. Serotonergic lesioning differentially affects presynaptic and postsynaptic 5-HT1B receptor mRNA levels in rat brain. Brain Res. 1996b;722:50–58. doi: 10.1016/0006-8993(96)00178-3. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME, Lerer B, Shapira B. 5-HT-1A receptor-mediated effects of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:1–19. doi: 10.1016/0278-5846(93)90031-m. [DOI] [PubMed] [Google Scholar]
- O'Connor JJ, Kruk ZL. Effects of 21 days treatment with fluoxetine on stimulated endogenous 5-hydroxytryptamine overflow in the rat dorsal raphe and suprachiasmatic nucleus studied using fast cyclic voltammetry in vitro. Brain Res. 1994;640:328–335. doi: 10.1016/0006-8993(94)91889-9. [DOI] [PubMed] [Google Scholar]
- Offord SJ, Ordway GA, Frazer A. Application of [125I]iodocyanopindolol to measure 5-hydroxytryptamine1B receptors in the brain of the rat. J Pharmacol Exp Ther. 1988;244:144–153. [PubMed] [Google Scholar]
- Ogren SO, Razani H, Elvander-Tottie E, Kehr J. The neuropeptide galanin as an in vivo modulator of brain 5-HT1A receptors: possible relevance for affective disorders. Physiol Behav. 2007;92:172–179. doi: 10.1016/j.physbeh.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Oksenberg D, Marsters SA, O'Dowd BF, Jin H, Havlik S, Peroutka SJ, Ashkenazi A. A single amino-acid difference confers major pharmacological variation between human and rodent 5-HT1B receptors. Nature. 1992;360:161–163. doi: 10.1038/360161a0. [DOI] [PubMed] [Google Scholar]
- Olivier B, Gommans J, van der Gugten J, Bouwknecht JA, Herremans AH, Patty T, Hijzen TH. Stimulus properties of the selective 5-HT reuptake inhibitor fluvoxamine in conditioned taste aversion procedures. Pharmacol Biochem Behav. 1999;64:213–220. doi: 10.1016/s0091-3057(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P. 8-OH-DPAT-induced place preference and place aversion: effects of PCPA and dopamine antagonists. Psychopharmacology (Berl) 1991;103:99–102. doi: 10.1007/BF02244082. [DOI] [PubMed] [Google Scholar]
- Pare WP, Tejani-Butt SM. Effect of stress on the behavior and 5-HT system in Sprague-Dawley and Wistar Kyoto rat strains. Integr Physiol Behav Sci. 1996;31:112–121. doi: 10.1007/BF02699783. [DOI] [PubMed] [Google Scholar]
- Parker EM, Grisel DA, Iben LG, Shapiro RA. A single amino acid difference accounts for the pharmacological distinctions between the rat and human 5-hydroxytryptamine1B receptors. J Neurochem. 1993;60:380–383. doi: 10.1111/j.1471-4159.1993.tb05865.x. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Sydney; Orlando: Academic Press; 1986. [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Pejchal T, Foley MA, Kosofsky BE, Waeber C. Chronic fluoxetine treatment selectively uncouples raphe 5-HT(1A) receptors as measured by [(35)S]-GTP gamma S autoradiography. Br J Pharmacol. 2002;135:1115–1122. doi: 10.1038/sj.bjp.0704555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J Physiol. 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty F, Kramer G, Wilson L, Jordan S. In vivo serotonin release and learned helplessness. Psychiatry Res. 1994;52:285–293. doi: 10.1016/0165-1781(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Picazo O, Lopez-Rubalcava C, Fernandez-Guasti A. Anxiolytic effect of the 5-HT1A compounds 8-hydroxy-2-(di-n-propylamino) tetralin and ipsapirone in the social interaction paradigm: evidence of a presynaptic action. Brain Res Bull. 1995;37:169–175. doi: 10.1016/0361-9230(94)00273-4. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Regulation of 5-hydroxytryptamine release from rat midbrain raphe nuclei by 5-hydroxytryptamine1D receptors: effect of tetrodotoxin, G protein inactivation and long-term antidepressant administration. J Pharmacol Exp Ther. 1996;276:697–707. [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- Pineyro G, Castanon N, Hen R, Blier P. Regulation of [3H]5-HT release in raphe, frontal cortex and hippocampus of 5-HT1B knock-out mice. Neuroreport. 1995;7:353–359. [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli MR, Durkin MM, Farmer M. Plastic responses of neonatal 5-hydroxytryptamine1B receptors to 5,7-dihydroxytryptamine lesions mapped by quantitative autoradiography. Int J Dev Neurosci. 1996;14:621–629. [PubMed] [Google Scholar]
- Prendergast MA, Hendricks SE, Yells DP, Balogh S. Conditioned taste aversion induced by fluoxetine. Physiol Behav. 1996;60:311–315. doi: 10.1016/0031-9384(95)02234-1. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Carboni L, Andreoli M, Michielin F, Ballottari A, Arban R. Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol Biochem Behav. 2011;97:566–576. doi: 10.1016/j.pbb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Remy SM, Schreiber R, Dalmus M, De Vry J. Somatodendritic 5-HT1A receptors are critically involved in the anxiolytic effects of 8-OH-DPAT. Psychopharmacology (Berl) 1996;125:89–91. doi: 10.1007/BF02247397. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Riad M, Rbah L, Verdurand M, Aznavour N, Zimmer L, Descarries L. Unchanged density of 5-HT(1A) autoreceptors on the plasma membrane of nucleus raphe dorsalis neurons in rats chronically treated with fluoxetine. Neuroscience. 2008;151:692–700. doi: 10.1016/j.neuroscience.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J Neurosci. 2001;21:8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegert C, Rothmaier AK, Leemhuis J, Sexton TJ, Neumaier JF, Cassel JC, Jackisch R. Increased expression of 5-HT(1B) receptors by Herpes simplex virus gene transfer in septal neurons: New in vitro and in vivo models to study 5-HT(1B) receptor function. Brain Res Bull. 2008;76:439–453. doi: 10.1016/j.brainresbull.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk A, Koprowska M, Krotewicz M, Strzelczuk M, Wieczorek M. Effects of 8-OHDPAT administration into the dorsal raphe nucleus and dorsal hippocampus on fear behavior and regional brain monoamines distribution in rats. Behav Brain Res. 2001;120:47–57. doi: 10.1016/s0166-4328(00)00357-0. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Watkins LR, Lowry CA, Maier SF. The role of the medial prefrontal cortex in dorsal raphe nucleus 5-HT1A receptor adaptation following escapable and inescapable stress; San Diego, CA: Society for Neuroscience Annual Meeting; 2010. [Google Scholar]
- Rueter LE, Fornal CA, Jacobs BL. A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci. 1997;8:117–137. doi: 10.1515/revneuro.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl) 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sarhan H, Fillion G. Differential sensitivity of 5-HT1B auto and heteroreceptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:382–390. doi: 10.1007/s002109900067. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sari Y, Lefevre K, Bancila M, Quignon M, Miquel MC, Langlois X, Hamon M, Verge D. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Res. 1997;760:281–286. doi: 10.1016/s0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Verge D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Sayer TJ, Hannon SD, Redfern PH, Martin KF. Diurnal variation in 5-HT1B autoreceptor function in the anterior hypothalamus in vivo: effect of chronic antidepressant drug treatment. Br J Pharmacol. 1999;126:1777–1784. doi: 10.1038/sj.bjp.0702535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schreiber R, De Vry J. Neuroanatomical basis for the antidepressant-like effects of the 5-HT(1A) receptor agonists 8-OH-DPAT and ipsapirone in the rat forced swimming test. Behav Pharmacol. 1993;4:625–636. [PubMed] [Google Scholar]
- Sexton TJ, McEvoy C, Neumaier JF. (+) 3,4-methylenedioxymethamphetamine ('ecstasy') transiently increases striatal 5-HT1B binding sites without altering 5-HT1B mRNA in rat brain. Mol Psychiatry. 1999;4:572–579. doi: 10.1038/sj.mp.4000574. [DOI] [PubMed] [Google Scholar]
- Shen C, Li H, Meller E. Repeated treatment with antidepressants differentially alters 5-HT1A agonist-stimulated [35S]GTP gamma S binding in rat brain regions. Neuropharmacology. 2002;42:1031–1038. doi: 10.1016/s0028-3908(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Sijbesma H, Schipper J, de Kloet ER, Mos J, van Aken H, Olivier B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav. 1991;38:447–458. doi: 10.1016/0091-3057(91)90305-l. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct Immunohistochemical Evidence of the Existence of 5-HT1A Autoreceptors on Serotoninergic Neurons in the Midbrain Raphe Nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Spigset O. Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf. 1999;20:277–287. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988;27:707–715. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Davidson C, McLaughlin DP, Hopwood SE. Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: parallel purposes or pointless plurality? Trends Neurosci. 2000;23:459–465. doi: 10.1016/s0166-2236(00)01631-3. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhan F, Deslandes PN, Pache DM, Sewell RD. Do antidepressants affect motivation in conditioned place preference? Eur J Pharmacol. 2000;408:257–263. doi: 10.1016/s0014-2999(00)00771-8. [DOI] [PubMed] [Google Scholar]
- Sumner BE, Fink G. Effects of acute estradiol on 5-hydroxytryptamine and dopamine receptor subtype mRNA expression in female rat brain. Mol Cell Neurosci. 1993;4:83–92. doi: 10.1006/mcne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Trillat AC, Malagie I, Scearce K, Pons D, Anmella MC, Jacquot C, Hen R, Gardier AM. Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: in vivo microdialysis studies. J Neurochem. 1997;69:2019–2025. doi: 10.1046/j.1471-4159.1997.69052019.x. [DOI] [PubMed] [Google Scholar]
- Valdizan EM, Castro E, Pazos A. Agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int J Neuropsychopharmacol. 2010;13:835–843. doi: 10.1017/S1461145709990940. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt MM, Laurie DJ, Seeburg PH, Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. Embo J. 1991;10:4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C, Dietl MM, Hoyer D, Palacios JM. 5.HT1 receptors in the vertebrate brain. Regional distribution examined by autoradiography. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:486–494. doi: 10.1007/BF00260602. [DOI] [PubMed] [Google Scholar]
- Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat. 2006;31:233–242. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Will MJ, Der-Avakian A, Bland ST, Grahn RE, Hammack SE, Sparks PD, Pepin JL, Watkins LR, Maier SF. Electrolytic lesions and pharmacological inhibition of the dorsal raphe nucleus prevent stressor potentiation of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2004;171:191–198. doi: 10.1007/s00213-003-1572-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]