Abstract
Iron deficiency (ID) anemia is associated with poor neurocognitive development in infants and children. Depending on the stage of development at the time of deficiency, these adverse effects may be reversible. Recent investigations using sensitive measurements have confirmed that the deposition of iron in the brain varies according to brain region and age, and that dopamine-dependent behaviors are among the core deficits in ID. Dr John Beard (1947–2009) has been one of the leading scientists and pioneers in the area of iron and child development. His legacy to this area of science will grow through the continuation of his work by his co-workers and colleagues.
Keywords: brain development, child development, iron
INTRODUCTION
Iron deficiency (ID) remains the most prevalent single-nutrient deficiency in developing and developed countries. The World Health Organization1 estimates that, worldwide, 1.6 billion to 2 billion people are anemic. Although ID is responsible for at least half of the cases of anemia, there are other possible causes, including genetic, infectious, and other nutritional deficiencies. It is estimated that when anemia prevalence reaches 20%, ID exists in 50% of the population, and when anemia prevalence exceeds 40%, the entire population suffers from some degree of ID.2 Infants, children, and women of reproductive age are at highest risk of developing ID and ID anemia (IDA), largely because of their high physiologic requirements associated with growth combined with greater losses and poor dietary intake. Globally, almost half of preschool-aged children and pregnant women and close to one-third of non-pregnant women have anemia.1,2
Anemia has been shown to weaken immune status and resistance to infections3 to lower work capacity,4 and to possibly repress child growth.5 Infants with anemia showed slower psychomotor and mental development, and many of these effects seem irreversible.6,7 Even ID without anemia affects cognition and motor development in children and adolescents.8,9
IRON AND CHILD DEVELOPMENT
Over the past 3 decades, substantial efforts have focused on understanding the relationship between ID and development or behavior in infants and young children. A conceptual framework for how ID may affect child development (Figure 1) suggests that the relationship between iron and development may be direct, through an effect on brain function and structure, or indirect, through changes in exploratory behavior of the anemic child, which subsequently affects caregiver behavior and the quality of parent–child interactions.10 As a result, there is strong evidence indicating that IDA is associated with poorer developmental ratings in infants11 and with lower scores on cognitive function tests and educational achievement tests in children.12 The negative effect of IDA on child development tends to be long lasting: children without anemia who had been iron deficient or anemic as infants had lower IQ scores at age 2–7 years than children who had not been anemic in the first year of life.7 In a follow-up study from Costa Rica, children who had been iron-deficient as infants scored persistently poorer on cognitive tests up to the age of 19 years, and these effects were greater in children from high-risk environments, suggesting an interaction between environment or social stimulation and ID.13
Figure 1.
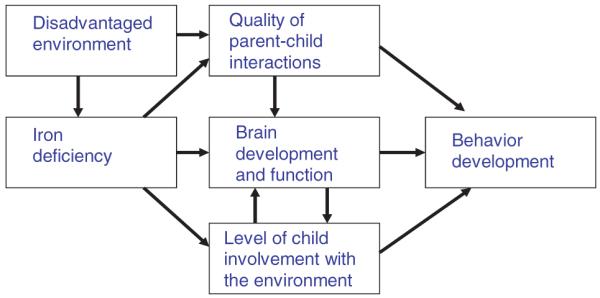
Conceptual model of iron deficiency and child behavior.
The results of intervention trials studying the effect of iron treatment on cognitive and motor development scores in iron-deficient infants have been inconsistent. Four of six recent randomized controlled trials of iron supplementation in infancy that assessed social and emotional behavior found a benefit from iron,14 but there were conflicting effects on cognitive performance tasks.12 In children aged 2 years and older, iron treatment of iron-deficient children with and without anemia led to better scores on cognitive function tests in six of eight trials.12,15–17 The differential effects of iron treatment on development in children according to age (<2 years versus ≥2 years) suggest the existence of a sensitive window in brain development during which environmental effects, such as ID, may be at least partly irreversible.
IRON IN THE DEVELOPING BRAIN
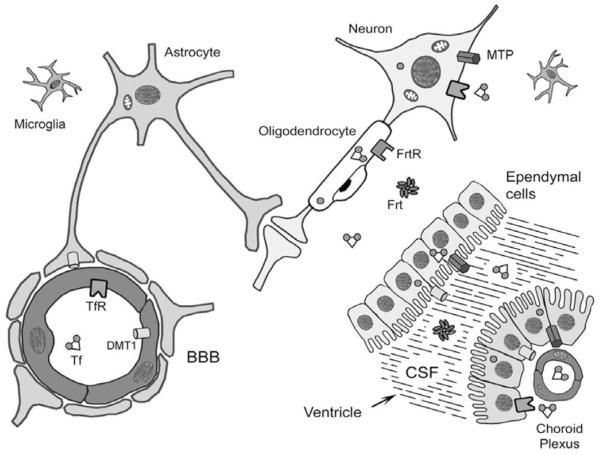
New insights are emerging from recent and ongoing investigations into the role of iron in neurocognitive and neurobehavioral development. The uptake of iron through the blood–brain barrier appears to be regulated and dependent on iron status18 such that there is a higher rate when iron status is low and a lower rate when it is high.18,19 In addition, this uptake process is highly selective and not reflective of overall blood–brain barrier permeability.18,20 The brain obtains iron primarily via Tf and Tf receptors expressed in endothelial cells on the brain microvasculature.18,21 There appears to be a regulatory role for adjacent astrocytes in the regulation of this uptake across the blood–brain barrier (Figure 2).
Figure 2. Iron uptake through the blood–brain barrier.
Reproduced from Beard (2003)18 with permission.
ID affects neurogenesis and neurochemistry during brain development. Animal studies show structural impairments of the hippocampus in ID, whereas in the striatum, less arborization and fewer affected dendrites have been observed.22 ID also results in a different location and impaired functioning of the oligodendrocytes in the rat brain, resulting in altered composition and amount of myelin in white matter.18,23 The role of iron in the production of hormones from the monoaminergic pathways, particularly dopamine and norepinephrine, illustrates the importance of iron in neurochemistry.18,24–27
The concentration of iron in the brain is highest at birth, decreases through weaning, and then begins to increase coincident with the onset of myelination and greater expression of transferrin mRNA. Noninvasive techniques are now available to measure developmental patterns of iron accumulation in the developing brain. Magnetic resonance imaging has been used to map iron distribution in the brains of children and adolescents.28,29
The deposition of iron in the brain varies according to region and age. Regions of the brain rich in iron in adulthood (e.g., the substantia nigra, globus pallidus) are not the regions that have a high iron content in early life, when the highest concentrations of iron are found in the globus pallidus, caudate nucleus, putamen, and substantia nigra.30 In addition, regions of the brain that are rich in iron are not necessarily the ones that dietary ID most affects. It might be that the different regional needs for iron in the brain during different stages of neurodevelopment impart a differential sensitivity of brain regions to nutritional deprivation of iron.
The effect of iron interventions may reverse the abnormalities in the affected brain regions depending on when in development the iron repletion occurs.In rats that had been on iron-deficient diets from mid-gestation onward, early iron treatment (at 4 days postpartum) normalized brain iron concentrations, monoamine concentrations, and monoamine transporter and receptor densities in most brain regions.Rats that had been exposed to gestational ID had lower dopamine transporter densities in caudate and substantia nigra than control animals, but these abnormalities were normalized after early iron repletion. In contrast, iron repletion later in life, at weaning, did not reverse the effect of gestational ID. The authors concluded that these findings suggested the existence of a critical window of opportunity for reversing the detrimental effects of ID in utero on brain development,at least in rats and probably also in humans.31
ID is also known to affect neurochemistry in the developing brain. ID during lactation in infant rats resulted in abnormal dopamine concentrations in the brain,18 and these abnormalities in dopamine metabolism and in behaviors that depend on striatal dopamine function were not completely restored after aggressive iron therapy.32 These findings strengthen the hypothesis that the sensitivity of a brain region to loss of iron during development is likely to be related to the regional development requirements for iron during that period.
CAN “NEW” TECHNIQUES HELP GAIN MORE INSIGHTS?
New emerging insights into the role of iron during brain development have allowed researchers to design intervention studies with sensitive measurements of child development, focusing on specific functions in areas of the brain known to be most sensitive to ID. The advantage of a combination of techniques, measuring responses in the brain and performance on cognitive tasks, enabled scientists to better define the role of iron in child development.
Rocanglio et al.33 found differences between the maturation of central conduction time of the auditory brain stem in infants who were anemic at 6 months of age and that of infants who were not anemic. At 12 and 18 months, the infants who were initially anemic showed longer conduction times than the infants who were not anemic, even though their iron status had been restored to normal and they were no longer anemic. Burden et al.34 used event-related potential techniques to locate the corresponding brain areas involved during a delayed-processing-information task in iron-deficient infants. In a delayed-processing-information task, iron-deficient infants aged 9–12 months were less able to distinguish a familiar face from a stranger’s face than nondeficient infants, as confirmed according to event-related potential graphs, perhaps because of alterations in the striatum and hippocampus in the iron-deficient infants. Changes in the hippocampus are of particular interest because of this brain region’s role in the discrimination between novel and familiar stimuli in recognition memory.
The role of iron in dopamine production allowed researchers to better design tests of the effect of ID on socioemotional development by focusing on behaviors that are known to be regulated by dopamine. In a cohort study, 77 African American infants aged 9–10 months were tested on shyness, orientation and engagement, and response to unfamiliar pictures; the scores of iron-sufficient infants for socioemotional development were the highest, while those of iron-deficient infants with anemia were the worst, and those of iron-deficient infants without anemia were intermediate.35 These findings are consistent with early disruption of the dopamine system, due to ID, and they contribute to a growing understanding that altered affect and response to novelty are among the core deficits in early ID.35
CONCLUSION
A series of articles in The Lancet36 estimated that, world-wide, 200 million children do not reach their full developmental potential because of preventable causes. ID was identified as one of the leading nutrition-related causes of impaired child development, along with stunting and iodine deficiency. Correction of ID is likely to contribute to better child development, particularly in geographical regions in which ID is endemic. However, ID is not the only cause of impaired development in the world, and with an estimated 187 million stunted children37 who are presently suffering from lack of food, poverty, and poor social environments, iron interventions alone will not alleviate all disparities.
Nevertheless, micronutrient interventions for children, including iron fortification, were considered the most cost-effective solution for solving some of the world’s biggest challenges, including malnutrition.38 It is estimated that the effect of ID on cognitive losses during childhood results in an estimated 2.5% loss of earnings during adulthood.39 Given the high proportion of the population with ID or anemia in most developing countries, interventions to improve the iron status of young children should be given high priority. New insights into the biology of early development and the role of iron in neurodevelopment will eventually allow public health professionals to better design and target these interventions.
John Lawrence Beard (1947–2009) was one of the leading scientists and pioneers in the area of iron and children’s development. Dr Beard’s contributions to the field of ID epitomize translational research. In his laboratory at Pennsylvania State University, he and his colleagues conducted basic research using animal models to study the brain mechanisms underlying ID.24,40 In addition, he often stepped outside the laboratory to examine how ID operated in humans. In a series of landmark studies performed in South Africa, Beard and his colleagues conducted a randomized controlled trial in mothers of infants aged 10 weeks to 9 months who varied in iron status.41–43 These studies showed that iron-deficient mothers experienced significant improvements in mental health (depression and stress), cognition, and interactions with their children after receiving iron supplementation. In addition, infants of mothers with anemia had lower scores on standardized measures of development than infants of iron-sufficient mothers. These studies suggest that the deficits in maternal mental health, cognition, and mother–child interaction associated with maternal ID may negatively affect infant development. The beneficial effects of maternal iron treatment have significant public health importance for the health and well-being of mothers and their young children.
Professor Beard’s legacy to the science will grow through the continuation of his work by his coworkers and colleagues. With a goal of better understanding the link between ID and altered cognitive functioning, further research by Professor John Beard’s colleagues and students is underway to examine the effect of ID and iron treatment on sensitive elements of neural functioning. Future research is expected to shed more light on the nature of the relationship between systemic iron status and brain iron status (Murray-Kolb, personal communication 2009).
John Lawrence Beard was a passionate, talented, and amiable person who is dearly missed by colleagues, friends, and family.
Acknowledgments
Declaration of interest. This work was commissioned by the Nutrition and Mental Performance Task Force of the European branch of the International Life Sciences Institute (ILSI Europe). Industry members of this task force are Abbott Nutrition, Barilla G. & R. Fratelli, Coca-Cola Europe, Danone, Dr Willmar Schwabe, DSM, FrieslandCampina, Kellogg Europe, Kraft Foods, Martek Biosciences Corporation, Naturex, Nestlé, PepsiCo International, Pfizer, Roquette, Soremartec – Ferrero Group, Südzucker/BENEO Group, Unilever. For further information about ILSI Europe, please call + 32 2 771.00.14 or info@ilsieurope.be. The opinions expressed herein are those of the authors and do not necessarily represent the views of ILSI Europe. Dr S.J.M. Osendarp is an employee of Unilever. Drs L.E. Murray-Kolb and M. M. Black have no conflict of interest to declare. The coordinator for this supplement was Ms Agnes Meheust, ILSI Europe.
Contributor Information
Saskia JM Osendarp, Unilever Research & Development, Vlaardingen, the Netherlands, and Division of Human Nutrition, Wageningen University, the Netherlands..
Laura E Murray-Kolb, Department of Nutritional Sciences, The Pennsylvania State University, University Park, Pennsylvania, USA..
Maureen M Black, Department of Pediatrics, University of Maryland, School of Medicine, Baltimore, Maryland, USA..
REFERENCES
- 1.World Health Organisation . In: Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. de Benoist B, McLean E, Egli I, Cogswell M, editors. WHO Press; Geneva, Switzerland: 2008. [Google Scholar]
- 2.McLean E, Egli I, de Benoist B, Wojdyla D, Cogswell M. World-wide prevalence of anemia in pre-school aged children, pregnant women and non-pregnant women of reproductive age. In: Kraemer K, Zimmermann MB, editors. Nutritional Anemia. Sight and Life Press; Basel, Switzerland: 2007. pp. 1–12. [Google Scholar]
- 3.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131(Suppl):S616–633. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 4.Haas JD, Brownlie IV. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131(Suppl):S676–690. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev HPS, Gera T, Nestel P. Effect of iron supplementation on physical growth in children: systematic review of randomised controlled trials. Public Health Nutr. 2006;9:904–920. doi: 10.1017/phn2005918. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 7.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(Suppl):S34–43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, et al. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ. 2001;323:1389–1393. doi: 10.1136/bmj.323.7326.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137(Suppl):S524–530. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 10.Black MM, Lozoff B. Nutrition and child development. In: Haith MM, Benson JB, editors. Encyclopedia of Infant and Child Development. Elsevier Inc.; Oxford, UK: 2008. pp. 449–459. [Google Scholar]
- 11.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131(Suppl):S649–666. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 13.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2006;160:1108–1113. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28(Suppl):S560–571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 15.Sachdev HPS, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 2005;8:117–132. doi: 10.1079/phn2004677. [DOI] [PubMed] [Google Scholar]
- 16.Lynn R, Harland EP. A positive effect of iron supplementation on the IQs of iron deficient children. Pers Individ Dif. 1998;24:883–885. [Google Scholar]
- 17.Seshadri S, Gopaldas T. Impact of iron supplementation on cognitive functions in preschool and school-aged children – the Indian experience. Am J Clin Nutr. 1989;50:675–686. doi: 10.1093/ajcn/50.3.675. [DOI] [PubMed] [Google Scholar]
- 18.Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133(Suppl):S1468–S1472. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 19.Taylor EM, Crowe A, Morgan EH. Transferrin and iron uptake by the brain: effects of altered iron status. J Neurochem. 1991;57:1584–1592. doi: 10.1111/j.1471-4159.1991.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 20.Crowe A, Morgan EH. Iron and transferrin uptake by brain and cerebrospinal fluid in the rat. Brain Res. 1992;592:8–16. doi: 10.1016/0006-8993(92)91652-u. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Day JR, Connor JR, Beard JL. Gene expression of transferrin and transferrin receptor in brains of control vs. iron-deficient rats. Nutr Neurosci. 2003;6:1–10. [PubMed] [Google Scholar]
- 22.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 23.Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138:2534–2536. doi: 10.1093/jn/138.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci. 2003;25:308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- 25.Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J Neurochem. 2008;106:205–215. doi: 10.1111/j.1471-4159.2008.05358.x. [DOI] [PubMed] [Google Scholar]
- 26.Burhans MS, Dailey C, Beard Z, et al. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- 27.Burhans MS, Dailey C, Wiesinger J, Murray-Kolb LE, Jones BC, Beard JL. Iron deficiency affects acoustic startle response and latency, but not prepulse inhibition in young adult rats. Physiol Behav. 2006;87:917–924. doi: 10.1016/j.physbeh.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Dwork AJ, Lawler G, Zybert PA, et al. An autoradiographic study of the uptake and distribution of iron by the brain of the young rat. Brain Res. 1990;518:31–39. doi: 10.1016/0006-8993(90)90950-g. [DOI] [PubMed] [Google Scholar]
- 29.Aoki S, Okada Y, Nishimura K, et al. Normal deposition of brain iron in childhood and adolescence: MR imaging at 1.5 T. Radiology. 1989;172:381–385. doi: 10.1148/radiology.172.2.2748819. [DOI] [PubMed] [Google Scholar]
- 30.Beard JL, Connor JD, Jones BC. Brain iron: location and function. Prog Food Nutr Sci. 1993;17:183–221. [PubMed] [Google Scholar]
- 31.Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. J Nutr. 2007;137:1176–1182. doi: 10.1093/jn/137.5.1176. [DOI] [PubMed] [Google Scholar]
- 32.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 33.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68:683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 34.Burden MJ, Westerlund AJ, Armony-Sivan R, et al. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child under-nutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 38.Copenhagen Business School . Global Crises, Global Solutions: Costs and Benefits. Cambridge University Press; Cambridge: 2009. [Google Scholar]
- 39.Horton S, Alderman H. The economics of addressing nutritional anemia. In: Kraemer K, Zimmermann MB, editors. Nutritional Anemia. Sight and Life Press; Zurich: 2007. pp. 19–35. [Google Scholar]
- 40.Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr Rev. 1993;51:157–170. doi: 10.1111/j.1753-4887.1993.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 41.Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267–272. doi: 10.1093/jn/135.2.267. [DOI] [PubMed] [Google Scholar]
- 42.Perez EM, Hendricks MK, Beard JL, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850–855. doi: 10.1093/jn/135.4.850. [DOI] [PubMed] [Google Scholar]
- 43.Murray-Kolb LE, Beard JL. Iron deficiency and child and maternal health. Am J Clin Nutr. 2009;89(Suppl):S946–S950. doi: 10.3945/ajcn.2008.26692D. [DOI] [PubMed] [Google Scholar]