Abstract
OBJECTIVE
Biobehavioral models of prenatal stress highlight the importance of the stress-related hormone cortisol. However, the association between maternal cortisol levels and length of human gestation require further investigation because most previous studies have relied on one-time cortisol measures assessed at varying gestational ages. This study assessed whether ecological momentary assessment (EMA) of cortisol sampling improves the ability to predict the length of human gestation. In addition, associations between EMA based measures of psychological state (negative affect) with cortisol levels during pregnancy were assessed.
METHODS
Over a 4-day period, 25 healthy pregnant women (mean gestational age at assessment 23.4 ± 9.1 weeks) collected 7 salivary samples per day for assessment of cortisol and provided a rating of negative affect every waking hour using an electronic diary.
RESULTS
Higher salivary cortisol concentrations at awakening and throughout the day (p=.001) as well as a flatter cortisol response to awakening (p=.005) were associated with shorter length of gestation. Women delivering at 36 weeks gestations had 13% higher salivary cortisol levels at awakening than women delivering at 41 weeks gestation. The EMA-based measure of negative affect was associated with higher cortisol throughout the day (p=.006), but not to gestational length (p=.641). The one-time measure of cortisol was not associated with length of gestation, and traditional retrospective recall measures of negative affect were not associated with cortisol.
CONCLUSION
Our findings support the ecological validity of repeated ambulatory assessments of cortisol in pregnancy and their ability to improve the prediction of adverse birth outcomes.
Keywords: cortisol, cortisol awakening response (CAR), hypothalamic-pituitary-adrenal (HPA) axis, ecological momentary assessment (EMA), pregnancy, length of gestation, negative affect
Introduction
Adverse birth outcomes resulting from shortened gestational length (preterm birth) are the leading cause of infant mortality and morbidity. However, their etiology requires further investigation (1, 2).
A substantial body of epidemiological and clinical evidence supports a significant and independent role for prenatal psychosocial stress in outcomes associated with shortened gestation and increased risk of preterm birth (3-7). A common feature across many, if not all, biobehavioral models of prenatal stress is their emphasis on the role of the stress-related hormone cortisol. Cortisol can influence developmental processes and birth outcomes. For example, cortisol regulates fetal growth and maturation in mammals to influence the length of gestation and timing of onset of labor and delivery (8, 9). In humans, maternal cortisol production increases 2- to 4-fold over the course of normal gestation (8, 10). Maternal cortisol acts on the developing fetus either directly by passing through the placenta (the placental enzyme 11β-hydroxysteroid dehydrogenase type 2 acts only as a partial barrier), or indirectly via its effects on placental production of corticotrophin-releasing hormone (CRH)(8).
Although experimental studies in animals provide strong biological plausibility for the link between maternal cortisol and the length of gestation (11, 12), findings from human clinical studies of the association between cortisol and length of gestation have been inconclusive (13-18). The majority of published papers report a negative association between maternal cortisol levels and length of gestation (reviewed recently in (18)), however, there are several published reports that do not find a significant association in humans between maternal cortisol levels during pregnancy and pregnancy duration (15, 17, 19).
Most of the previous human studies have used one-time measures of cortisol assessed in blood (plasma) collected at varying times during the day and in a clinical setting. This approach raises several methodological concerns, including the lack of control for diurnal variation (20-25), the role of short-term psychological and physical challenges in influencing cortisol levels (26), and the assessment of total (bound + free) cortisol in blood (8). Few studies have examined ambulatory cortisol levels during pregnancy (20-22). We therefore used ecological momentary assessment (EMA, (27)) of salivary (free) cortisol levels and psychological states to more precisely obtain information about maternal cortisol concentrations and their predictive ability for adverse reproductive and birth outcomes.
We hypothesized that EMA measures of maternal cortisol would exhibit a stronger and/or more precise relation with length of gestation than the conventional cortisol sampling method in the context of human pregnancy. Furthermore, we hypothesized that EMA measures of negative affect would be more closely related to cortisol levels in pregnant women than traditional recall measures, and that these EMA measures would predict length of gestation.
Materials and Methods
Participants
All participants were pregnant women receiving prenatal care at the maternal-fetal medicine faculty practice at the University of California, Irvine Medical Center. Pregnant women were screened and approached for participation at their prenatal care visit by a research nurse. Of the total potential participants approached (n=45), 39 met eligibility criteria. Reasons for ineligibility were non-English speaking, multiple gestation, tobacco, alcohol, or other drug use in pregnancy; presence of any condition potentially associated with dysregulated neuroendocrine function such as endocrine, hepatic or renal disorders or corticosteroid medication use, and the presence of medical/obstetric risk factors for preterm birth (27) in the index pregnancy. Among the 39 eligible women, 33 women consented and completed the protocol. Women who were delivered by elective cesarean section (n=8) were excluded from the final sample because their delivery was not preceded by labor. Thus, the final sample consisted of 25 subjects.
We included pregnant women across a range of gestational ages (mean gestational age at visit: 23.4 ± 9.1 (mean ± SD) weeks, range: 10 – 35 weeks gestation) because we were interested in examining the association between maternal cortisol levels and length of gestation throughout the whole course of human pregnancy. All our analyses, however, were controlled for gestational age at visit because cortisol levels increase over the course of pregnancy. In terms of sociodemographic characteristics, the mean maternal age was 29.45 (± 5.9 SD) years, 93% of the women were married and 82% had a college degree. The majority of the sample (83%) consisted of Non-Hispanic White women.
Subjects were compensated $50/day for participation in the study, with the opportunity to get an additional $50/day if they completed >80% of the EMA protocol (electronic diary completion, saliva collection). All data were collected between July and December 2005. The study was approved by the University of California, Irvine, IRB and written informed consent was obtained from all participants.
Gestational Age Assessment
For all subjects, gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry using standard clinical criteria (28). Information on birth outcomes was abstracted from the medical record after delivery.
We assessed length of gestation as a continuous (completed weeks gestation) instead of a categorical (preterm/ term) variable, in order to assess effects across the entire distribution instead of only one tail of the distribution. Furthermore, recent evidence suggesting the effects of length of gestation on developmental and health outcomes extends continuously across the normal range of pregnancy duration instead of merely being a function of preterm birth (29, 30).
Procedures
Pregnant women collected saliva samples for cortisol assays immediately, 30, 45 and 60 min post awakening (representing the cortisol awakening response, CAR), and at 1200h, 1600h and 2000h (representing the short diurnal profile), for 4 consecutive days. The fixed sampling design for measures of salivary cortisol was chosen because it best captures the diurnal pattern of cortisol secretion (31). Since previous studies have shown there are weekend-weekday differences in the cortisol awakening response (32, 33), 2 weekend and 2 weekdays days were selected for sampling to capture a wide range of intra-individual variation in pregnant women’s cortisol levels and awakening responses. Exact time of saliva sampling was monitored using a Medication Event Monitoring System (MEMS®, Aardex group, Union City, CA, USA). Subjects were instructed to refrain from eating and drinking during the first hour after awakening. Subjects were instructed after each saliva collection to store the swab in a plastic tube labeled with the designated sampling time.
EMA data on negative affect was collected following a random sampling protocol across the same four-day period. Participants were provided with a pre-programmed hand-held computer (Tungsten E, Palm, Inc.) that gave a signal (“beep”) at an unpredictable moment in approximately 60-min time intervals following awakening until bedtime, resulting in approximately 15 measures/day over waking hours. This random time sampling protocol was chosen for the EMA measures of psychosocial state to decrease predictability and thereby increase representativeness of the measures obtained. After every beep, subjects were asked to rate their negative affect. Four adjectives describing negative affect were presented on the hand-held computer (“angry”, “nervous”, “sad”, “stressed”), and were rated on a 5-point Likert scale (0, “not at all”, 5, “very much”), and a summary score for negative affect was computed by adding up the ratings for the four adjectives for each beep. Subjects were instructed to complete their reports immediately after the beep. The device was pre-programmed in a way that it only allowed a response within a 20-minute window after the beep. At the end of the 4-day session when the subjects returned to the research suite, the same adjectives were presented and the subjects were asked to retrospectively rate their average negative affect over the previous 4-day period.
In addition, before the beginning of the 4-day EMA assessments, a blood sample for cortisol assays was obtained from each subject at our clinical research laboratory in the afternoon, and a single saliva sample also was collected at the same time. Maternal venous blood was collected in siliconized EDTA vacutainers. Aprotinin was added at 500 KIU/ml blood (to arrest enzymatic degradation; Sigma Chemical). Chilled tubes were centrifuged at 2000 ×g (15 min) and plasma was decanted into polypropylene tubes for storage at −70°C until assayed.
Cortisol Assay
Saliva samples were collected using a Salivette sampling device (Sarstedt, Numbrecht, Germany). Samples were clarified by depressing the plunger, spun and stored at −70°C degrees until assayed. Thawed samples were centrifuged at 3,000 rpm for 15 minutes before assay. Salivary cortisol levels were determined by a competitive luminescence immunoassay (LIA; IBL-America, Minneapolis, MN) with reported detection limits of 0.015 μg/dl. All samples were assayed in duplicate and averaged.
Total plasma cortisol levels were determined by immunofluorescence using an automated procedure on an Abbott TDx Analyzer (Abbott Laboratories). The assay was less than 5% cross-reactive with 11-deoxycortisol, corticosterone, and less than 1% cross-reactive with ten other naturally occurring steroids. The inter- and intra-assay CVs were less than 9%with a minimum detectable level (95% confidence) of 0.45 mg/dl. All samples were assayed in duplicates and averaged.
Statistical Analysis
Cortisol was log-transfomred by LnCort = ln (Cort + 1) to yield an unskewed response variable.
Cortisol concentrations from blood and from the saliva sample taken with the blood draw were residualized for time of day of collection and gestational age at the study visit.
Hierarchical linear modeling (HLM) growth curve analyses (34, 35) were used to evaluate the cortisol concentrations over the day and their association with gestational age at birth. HLM allows precise measures of timing (i.e. gestational age at assessment; time of day of sample collection) of data collection rather than nominal estimates of assessment intervals. The HLM analysis proceeded in three major steps (36):
Step 1 - Modeling diurnal cortisol patterns. Time-of-day values were expressed as number of hours since awakening for each subject over each of the 4 days. Time was centered at awakening so that the model intercept represents the mean log transformed cortisol levels at awakening. Because the cortisol awakening response (CAR, assessed at 0, 30, 45 and 60 min after awakening) and the slope over the day (day time profile, assessed at awakening,1100h, 1600h and 2000h) are considered two discrete characteristics of HPA axis function (37-39), level 1 included two time parameters, one for the cortisol awakening response (CAR), and the second parameter for the slope over the (DAY) to capture within-the-day changes in cortisol, including linear and quadratic effects of time for the CAR slope.
Step 2 - Associations between awakening time and diurnal cortisol. The influence of time of awakening on diurnal cortisol rhythms for each of the four days was then tested by conducting analyses between time of awakening and each of the level 1 coefficients.
Step 3 - Associations between length of gestation and cortisol levels. Between-individual differences predictors (gestational age at delivery as well as gestational age at cortisol testing) were introduced at level 3. Only the significant level 3 predictors were retained in the complete HLM model.
Associations between EMA measures of negative affect and cortisol were modeled similarly, with daily averages of negative affect entered on level 2 (day-to-day). A separate model was conducted to examine the association between the 4-day recall measure of negative affect entered at level 3. Furthermore, an HLM model with negative affect at level 1, assessment day entered on level 2 (day-to-day), and length of gestation and gestational age at test at level 3 was set up to examine the association between repeated EMA measures of negative affect and length of gestation.
Results
Compliance with the EMA sampling
For the 25 subjects included in the final analysis, from a total of 700 expected samples (7 saliva samples/ day × 4 days × 25 subjects =700 samples), only 16 (2.3 %) of the 700 samples were not collected. Comparison of the time stamps from MEMS caps readings with scheduled times yielded a very high degree of convergence. The discrepancy between the time recorded by the electronic cap and scheduled time of collection was less than 5 minutes in 43% of all samples, less than 15 minutes in 85% of all samples, and less than 20 minutes in 92% of all samples. Only 3.9% and 1.6% of samples were discrepant by greater than 30 min and 1 hour, respectively. Compliance with filling out the electronic EMA questionnaire regarding negative affect was also very high. On average, participants answered 13 ± 2.16 (SD) prompts per day (range: 7-20), and 89% of the prompts were answered within the stipulated 20-min time window.
Association between length of gestation and salivary and plasma cortisol from one-time assessments
The mean pregnancy duration (gestational age at birth) was 39.26 ± 1.96 (SD) weeks (range: 36.5 - 42 weeks).
Cortisol concentrations obtained from the clinic blood sample and the single saliva sample that was assessed at the clinical visit did not correlate with gestational age at birth (r = .06, p = .78 for serum cortisol and r = .15, p= .48 for saliva cortisol, respectively).
The correlation between blood-based cortisol levels and EMA-based cortisol (averaged over all ambulatory cortisol levels) was r = .71, p < .001.
Association between length of gestation and salivary cortisol from repeated 4-day ambulatory (EMA) assessments
As expected, there was a significant increase and subsequent decrease in cortisol concentrations in response to awakening, as well as a decline over the course of the day, as indicated by the significant linear and quadratic time slopes (CAR, CAR2 and DAY, see Table 1).
Table 1.
Hierarchical linear model estimates for log-transformed salivary cortisol concentrations during the first hour after awakening (CAR) and over the course of the day (DAY), and the effects of length of gestation and gestational age at testing for N=25 pregnant women; estimates are presented for the final, most parsimonious model.
| Coefficient* | SE | p-value | |
|---|---|---|---|
| Cortisol intercept (awakening) | |||
| Intercept | 2.746 | .041 | < .001 |
| Length of gestation | −.071 | .030 | .030 |
| Gestational age at test | .018 | .003 | < .001 |
| Cortisol awakening response (CAR) |
|||
| Intercept | .684 | .112 | < .001 |
| Length of gestation | .255 | .087 | .008 |
| Cortisol awakening response (CAR)2 |
|||
| Intercept | −.542 | .094 | < .001 |
| Length of gestation | −.242 | .093 | .016 |
| Time since awakening (DAY) | |||
| Intercept | −.108 | .005 | < .001 |
| Gestational age at test | .002 | .001 | .001 |
| Awakening time | −.011 | .002 | < .001 |
unstandardized regression coefficient ‘B’
Also, as expected, cortisol levels at awakening were higher later in pregnancy, as suggested by the significant interaction between the intercept and gestational age at testing.
Awakening time was significantly associated with the decline of cortisol over the day. The negative coefficient for the interaction between the DAY slope and awakening time suggests that participants who woke up later had a steeper decline of cortisol over the day.
Higher cortisol levels at awakening were associated with shorter duration of pregnancy, as indicated by a significant interaction between the cortisol intercept and length of gestation; specifically, a 2.6 % increase in cortisol levels at awakening was associated with a one week shortening of pregnancy duration. This indicates that women delivering at 36 weeks gestation had 13% higher salivary cortisol levels at awakening compared to women delivering at 41 weeks gestation. Furthermore, a less steep and more platykurtic cortisol increase in response to awakening was associated with earlier delivery, as indicated by the interaction between the linear and quadratic CAR slopes and length of gestation.
The rate of decline of cortisol concentrations over the course of the day was not associated with length of gestation (as reflected by the non-significant interaction between length of gestation and the DAY slope). This indicates that the slopes over the day were parallel for women delivering earlier and later, and since cortisol levels at awakening were significantly higher in women delivering earlier, this suggests higher cortisol levels were present throughout the day in women who subsequently delivered earlier. Thus, more cumulative exposure to cortisol was associated with a shorter length of gestation.
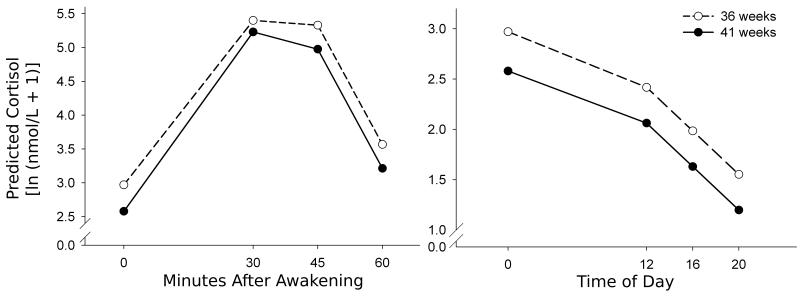
Figure 1 shows the predicted log-transformed cortisol concentrations for women who delivered at 36 weeks gestation vs. women who delivered at 41 weeks gestation based on the applied hierarchical linear model.
Figure 1.
Predicted log-transformed cortisol concentrations during the first hour after awakening (left) and throughout the day (right). The cortisol concentrations were predicted for women whowere delivered of an infant at 36 weeks of gestation versus women who were delivered of an infant at 41 weeks of gestation, based on the applied hierarchical linear model, and were controlled for the time of sampling in reference to awakening, gestational age at assessment, and awakening time.
Association between salivary cortisol and negative affect from repeated 4-day ambulatory (EMA) assessments
Higher cortisol levels at awakening and throughout the day were associated with higher daily EMA negative affect values. This finding is supported by a significant interaction between the cortisol intercept and negative affect and a non-significant interaction between length of gestation and the DAY slope (see Table 2). The rate of change of cortisol immediately after awakening was also not influenced by negative affect (indicated by a non significant interaction between the linear and quadratic CAR slopes and negative affect).
Table 2.
Hierarchical linear model estimates for log-transformed salivary cortisol concentrations during the first hour after awakening (CAR) and over the course of the day (DAY), and the effects of negative affect for N=25 pregnant women; estimates are presented for the final, most parsimonious model.
| Coefficient* | SE | p-value | |
|---|---|---|---|
| Cortisol intercept (awakening) | |||
| Intercept | 2.746 | .041 | < .001 |
| Negative affect | .079 | .026 | .006 |
| Gestational age at test | .026. | 003 | < .001 |
| Cortisol awakening response (CAR) |
|||
| Intercept | .649 | .125 | < .001 |
| Gestational age at test | −0.009 | .004 | .028 |
| Cortisol awakening response (CAR)2 |
|||
| Intercept | −.542 | .094 | < .001 |
| Time since awakening (DAY) | |||
| Intercept | −.109 | .006 | < .001 |
| Awakening time | −.014 | .003 | < .001 |
unstandardized regression coefficient ‘B’
There was no association between the four-day recall measurement of negative affect with cortisol levels at awakening, nor the CAR, or DAY slopes.
Association between negative affect and length of gestation
Levels of negative affect did not change significantly over the course of the day (see Table 3, effect for “Time since awakening (DAY)”). Furthermore, negative affect was not associated with gestational age at test (indicated by a non-significant interaction between negative affect and gestational age at test), or with length of gestation (indicated by a non-significant interaction between negative affect and length of gestation).
Table 3.
Hierarchical linear model estimates for negative affect over the course of the day (DAY), and the effects of length of gestation and gestational age at testing for N=25 pregnant women.
| Coefficient* | SE | p-value | |
|---|---|---|---|
| Negative affect intercept (awakening) |
|||
| Intercept | 1.055 | .272 | .001 |
| Length of gestation | −.054 | .113 | .641 |
| Gestational age at test | .001 | .001 | .630 |
| Time since awakening (DAY) | |||
| Intercept | .649 | .125 | <.001 |
unstandardized regression coefficient ‘B’
Discussion
In the present study we compared the ability of two different methods - the traditional approach (measuring maternal cortisol from a one-time blood/ saliva sample assessed in a clinical setting) and EMA sampling of maternal cortisol – in predicting the length of human gestation. While maternal cortisol levels assessed with the conventional one-time plasma or saliva sample in a laboratory setting were not associated with length of gestation, we found that EMA assessments of maternal salivary cortisol samples significantly predicted pregnancy duration. Higher cortisol concentrations at awakening and throughout the day, as well as a flatter cortisol response to awakening, were associated with shorter length of gestation, after controlling for gestational age at assessment. Our model predicted that a 2.6% increase in cortisol levels at awakening was associated with a one week shortening of pregnancy duration. This indicates that women delivering at 36 weeks gestation had 13% higher salivary cortisol levels at awakening compared to women delivering at 41 weeks gestation. Although EMA measures of negative affect were not directly related to length of gestation, we did find that EMA-based measures of negative affect were associated with higher cortisol levels during pregnancy.
In the current study we employed a standardized EMA sampling method of HPA axis function in the context of human pregnancy. Subjects were instructed to collect saliva at fixed time intervals after awakening (time of sampling was monitored electronically) and throughout the day over four days in their natural, every-day environment. This method has several advantages over one-time measures of plasma cortisol obtained at varying times during the day in a laboratory setting (the protocol employed by previous studies of the association between maternal cortisol in pregnancy and birth outcomes related to the length of gestation (13-15)).
By assessing several samples after awakening and over the course of the day, we were able to model discrete aspects of diurnal variation in cortisol levels and test their association with length of gestation. Since each saliva sample collection was time-stamped electronically, this allowed for greater precision in estimating cortisol levels over the course of the day after controlling for time of awakening at each assessment day and time of collection of each sample.
One important biological pathway in human parturition involves the activation of the maternal, fetal, and placental neuroendocrine systems (40, 41) with placental CRH as one of the major contributing factors in humans (42, 43). Women in preterm labor have significantly elevated levels of placental CRH compared to gestational age-matched controls, and these elevations of CRH precede the onset of spontaneous preterm labor (16, 42-44). Studies have shown that CRH is released from cultured human placental cells in a dose-response manner in response to all the major biological effectors of stress, including cortisol (45-47). In vivo studies have reported significant correlations among maternal pituitary-adrenal stress-related hormones, including cortisol, and placental CRH levels (16, 48).
Thus, our results suggest that if salivary cortisol is assessed over several consecutive days in women’s natural, every-day setting, deviations from the expected diurnal cortisol activity (i.e., higher cortisol levels after awakening and a lower increase in response to awakening) may represent a non-invasive marker of a dysregulated maternal-fetal-placental neuroendocrine system. Higher cortisol levels throughout the day may stimulate placental CRH, and thereby lead to earlier onset of labor. Consistent with this finding, in a recent longitudinal study we serially assessed the CAR in pregnant women in early and late gestation and reported that a reduced attenuation of the CAR from early to late gestation was associated with significantly shorter gestational length (22). In the present study, in which we assessed the CAR in women across different gestational ages, a flatter cortisol awakening response in the presence of higher baseline values was associated with earlier delivery. This may reflect a function of the law of initial values, suggesting that the predicted lower increase in response to awakening is a function of the elevated baseline.
We also found that EMA-based measures of negative affect are inversely related to women’s cortisol levels during pregnancy, despite the fact that the state of gestation produces profound alterations in several maternal systems, including the neuroendocrine system. However, traditional recall measures of negative affect were not related to HPA axis function.
It would be interesting to test the differential predictive value of EMA assessed psychological state versus cortisol, and to test whether cortisol mediates the effects of psychological state on birth outcomes. However, the current study design and sample size does not permit these tests of the combined effects of EMA psychological state and EMA cortisol. We have previously reported that an optimal approach to testing of the effects of psychological states on pregnancy and birth outcomes would incorporate a longitudinal design (as opposed to the cross-sectional design in the current study) because the state of pregnancy progressively alters maternal psychological appraisals and individual differences in the rate of change over time of psychological state predicts birth outcomes (49-52). Moreover, in addition to outcomes related to the length of gestation and timing of birth (the primary outcome in the current study), it would be interesting and relevant to examine the effects of EMA vs. traditional measures on other birth outcomes related to the rate of fetal growth (e.g., birth weight adjusted for length of gestation, fetal sex, parity and race/ethnicity) and to subsequent newborn, infant and child developmental and health outcomes.
The finding that negative affect was associated with cortisol, but not with length of gestation can potentially be explained by multiple sources of cortisol production, including clinical, nutritional, behavioral and psychosocial factors. Therefore, stress-related endocrine factors, including cortisol, may serve as a common physiological pathway that mediates the effects of a host of intrauterine perturbations - including but not limited to psychological distress - on birth outcomes (53).
The present sample size was small (25 subjects), precluding the evaluation of both ambulatory cortisol and mood simultaneously. This limitation is outweighed to some extent by the advantages of the EMA approach resulting in a total of 28 assessments per subject across all four assessment days.
In conclusion, the findings of the present study are promising and support the ecological validity of repeated ambulatory assessments of salivary cortisol during pregnancy and their ability to improve the prediction of adverse birth outcomes that constitute one of the major problems in maternal-child health in our society.
Acknowledgments
This study was supported by US PHS (NIH) grants RO1 HD-06028 and PO1 HD-047609 to PDW.
Acronyms
- CAR
cortisol awakening response
- CBG
cortisol binding globulin
- CRH
corticotrophin-releasing hormone
- EMA
ecological momentary assessment
- HLM
hierarchical linear modeling
- HPA
hypothalamic-pituitary-adrenal
- LIA
luminescence immunoassay
- MEMS
medication event monitoring system
- OLS
ordinary least squares
- SD
standard deviation
- SEM
standard error of the mean
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.IOM . Preterm Birth: Causes, Consequences, and Prevention. The National Academy of Science Press; 2007. [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong X, Harville EW, Mattison DR, Elkind-Hirsch K, Pridjian G, Buekens P. Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. Am J Med Sci. 2008;336:111–5. doi: 10.1097/MAJ.0b013e318180f21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, Tordjman S. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68:938–46. doi: 10.1097/01.psy.0000244025.20549.bd. [DOI] [PubMed] [Google Scholar]
- 5.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 6.Misra DP, O’Campo P, Strobino D. Testing a sociomedical model for preterm delivery. Paediatr Perinat Epidemiol. 2001;15:110–22. doi: 10.1046/j.1365-3016.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 7.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–15. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 8.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–49. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 9.Murphy AJ, Wells JC, Williams JE, Fewtrell MS, Davies PS, Webb DK. Body composition in children in remission from acute lymphoblastic leukemia. The American journal of clinical nutrition. 2006;83:70–4. doi: 10.1093/ajcn/83.1.70. [DOI] [PubMed] [Google Scholar]
- 10.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Chan EC, Falconer J, Madsen G, Rice KC, Webster EL, Chrousos GP, Smith R. A corticotropin-releasing hormone type I receptor antagonist delays parturition in sheep. Endocrinology. 1998;139:3357–60. doi: 10.1210/endo.139.7.6189. [DOI] [PubMed] [Google Scholar]
- 12.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35:730–43. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 13.Erickson K, Thorsen P, Chrousos G, Grigoriadis DE, Khongsaly O, McGregor J, Schulkin J. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. The Journal of clinical endocrinology and metabolism. 2001;86:2544–52. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 14.Mazor M, Chaim W, Hershkowitz R, Levy J, Leiberman JR, Glezerman M. Association between preterm birth and increased maternal plasma cortisol concentrations. Obstet Gynecol. 1994;84:521–4. [PubMed] [Google Scholar]
- 15.Ruiz RJ, Fullerton J, Brown CE, Schoolfield J. Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biol Res Nurs. 2001;3:39–48. doi: 10.1177/109980040100300106. [DOI] [PubMed] [Google Scholar]
- 16.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–63. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 17.Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, Park JS. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;179:1107–14. doi: 10.1016/s0002-9378(98)70114-0. [DOI] [PubMed] [Google Scholar]
- 18.Giurgescu C. Are maternal cortisol levels related to preterm birth? J Obstet Gynecol Neonatal Nurs. 2009;38:377–90. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 19.McCool WF, Dorn LD, Susman EJ. The relation of cortisol reactivity and anxiety to perinatal outcome in primiparous adolescents. Res Nurs Health. 1994;17:411–20. doi: 10.1002/nur.4770170604. [DOI] [PubMed] [Google Scholar]
- 20.de Weerth C, Buitelaar JK. Cortisol awakening response in pregnant women. Psychoneuroendocrinology. 2005;30:902–7. doi: 10.1016/j.psyneuen.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Jones NM, Holzman CB, Zanella AJ, Leece CM, Rahbar MH. Assessing mid-trimester salivary cortisol levels across three consecutive days in pregnant women using an at-home collection protocol. Paediatr Perinat Epidemiol. 2006;20:425–37. doi: 10.1111/j.1365-3016.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 22.Buss C, Entringer S, Reyes JF, Chicz-Demet A, Sandman CA, Waffarn F, Wadhwa PD. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009;201:398, e1–8. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 23.Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–22. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 24.Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 25.Stalder T, Hucklebridge F, Evans P, Clow A. Use of a single case study design to examine state variation in the cortisol awakening response: Relationship with time of awakening. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–6. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Hobel CJ, Arora CP, Korst LM. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Ann N Y Acad Sci. 1999;897:54–65. doi: 10.1111/j.1749-6632.1999.tb07878.x. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–5. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- 29.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, Moawad AH, Caritis SN, Meis PJ, Wapner RJ, Sorokin Y, Miodovnik M, Carpenter M, Peaceman AM, O’Sullivan MJ, Sibai BM, Langer O, Thorp JM, Ramin SM, Mercer BM. Timing of elective repeat cesarean delivery at term and neonatal outcomes. The New England journal of medicine. 2009;360:111–20. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Kramer MS. Variations in Mortality and Morbidity by Gestational Age among Infants Born at Term. J Pediatr. 2008 doi: 10.1016/j.jpeds.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Schlotz W, Schulz P, Hellhammer J, Stone AA, Hellhammer DH. Trait anxiety moderates the impact of performance pressure on salivary cortisol in everyday life. Psychoneuroendocrinology. 2006;31:459–72. doi: 10.1016/j.psyneuen.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Schlotz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med. 2004;66:207–14. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- 33.Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–18. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage Publications; Thousand Oaks: 2002. [Google Scholar]
- 35.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 36.Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–79. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–49. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–66. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–20. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- 40.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–43. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–68. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–3. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 43.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–9. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 44.Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079–85. doi: 10.1016/s0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 45.Petraglia F, Calza L, Garuti GC, Giardino L, De Ramundo BM, Angioni S. New aspects of placental endocrinology. J Endocrinol Invest. 1990;13:353–71. doi: 10.1007/BF03349579. [DOI] [PubMed] [Google Scholar]
- 46.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160:247–51. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 47.Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987;328:717–9. doi: 10.1038/328717a0. [DOI] [PubMed] [Google Scholar]
- 48.Chan EC, Smith R, Lewin T, Brinsmead MW, Zhang HP, Cubis J, Thornton K, Hurt D. Plasma corticotropin-releasing hormone, beta-endorphin and cortisol inter-relationships during human pregnancy. Acta Endocrinol (Copenh) 1993;128:339–44. doi: 10.1530/acta.0.1280339. [DOI] [PubMed] [Google Scholar]
- 49.Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–42. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 50.Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 51.Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, Sandman CA, Wadhwa PD. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13:258–68. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 53.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:507–16. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]