Abstract
Hydroxyapatite (HA), as a bone mineral component, has been an attractive bioceramic for the reconstruction of hard tissues. However, its poor mechanical properties, including low fracture toughness and tensile strength, have been a significant challenge to the application of HA for the replacement of load-bearing and/or large bone defects. Among materials studied to reinforce HA, carbon nanotubes (CNTs: single-walled or multiwalled) have recently gained significant attention because of their unprecedented mechanical properties (high strength and toughness) and physicochemical properties (high surface area, electrical and thermal conductivity, and low weight). Here, we review recent studies of the organization of HA-CNTs at the nanoscale, with a particular emphasis on the functionalization of CNTs and their dispersion within an HA matrix and induction of HA mineralization. The organization of CNTs and HA implemented at the nanoscale can further be developed in the form of coatings, nanocomposites, and hybrid powders to enable potential applications in hard tissue reconstruction.
1. Introduction
Hydroxyapatite (HA; Ca10(PO4)6(OH)2) has long been an attractive choice for bone replacement material [1–10]. With its similarity to the mineral component of natural bone, synthetic HA has shown excellent biocompatibility in vitro and in vivo [11]. However, HA has poor mechanical properties, such as brittleness and low tensile strength, thus the clinical applications have been limited particularly in load-bearing applications or large-sized defects [12].
One of the most common approaches to overcome this weakness is to produce composites by incorporating reinforcing phases, including strong bioceramics (ZrO2 or Al2O3), tough metals (Ni3Al), or flexible biopolymers (polyethylene and poly-lactide) [13–23]. These materials have been shown to significantly affect the mechanical strength and/or toughness. Among the choices of reinforcing materials, carbon nanotubes (CNTs, including single-walled carbon nanotubes “SWCNTs” and multiwalled carbon nanotubes “MWCNTs”) have recently gained a great deal of attention [24–38]. This has mainly been due to the unprecedented mechanical properties of CNTs, such as stiffness and strength. These properties combined with the low density of CNTs indicate that they can potentially be used to reinforce HA for the development of strong and tough bone replacements. As well as the mechanical properties, CNTs also have unique properties from a structural, thermal, chemical, and electrical perspective [39–42]. Structurally, CNTs have a hollow geometry (diameter = 0.7–100 nm; length < several millimeters), large specific surface areas, and high aspect ratios (length to diameter: about 1 × 104 − 1 × 106). Moreover, due to the carbonaceous nature of the CNTs, they exhibit chemically and thermally high resistance that leads to the inhibition of oxidation by oxidative chemicals including oxygen [43–56].
Many researchers have attempted to apply CNTs as an organic phase for development of new CNT-assisted bone graft materials (HA-CNTs) in expectation of improved mechanical properties [24, 57–66] and bioactivity [67–69], respectively, because of the excellent mechanical properties of carbon nanotubes and the bioactivity of HA. Generally, the hybrid materials used for bone grafts should be osteoconductively designed to enable close integration with the surrounding bone tissue in the body. Therefore, in the case of HA-CNT nanohybrid material, HA layers formed on the surface of CNTs can be expected to provide excellent performance for complete harmony with natural bone tissue in the body since the bioactivity of HA-containing materials has been thoroughly demonstrated for dental and skeletal implants and bone-regenerative scaffolds [11].
While the applications of carbon nanotubes in human body have long been on debate due to their possible toxicity which is related to the nonbiodegradable nature [70, 71], many recent studies have started to elucidate the toxicity mechanism and to reduce toxicity by the functionalization or coating with organic and inorganic compounds that will improve their dispersibility in biological fluid [72]. For more information on the toxicity-related issues on CNTs, the readers may refer to some recent review articles [73].
In this paper, we aimed to focus on the works on HA-CNTs nanocomposites and hybrids developed for bone replacements. Several methodologies to prepare HA-CNT assembled bone graft materials such as mixing of CNTs with HA nanopowders to give rise to nanocomposites and the mineralization of HA directly on the surface of CNTs to produce hybrids have been summarized in terms of the functional groups existing on the CNTs.
2. Pristine CNTs and Conventional Composites
Pristine CNTs tend to agglomerate or form bundles due to the relatively strong π-π interaction between CNT molecules. Deagglomeration of these CNT agglomerates (or bundles) in water or organic solvents is still unsuccessful due to the persistent and high strength interaction. This problem becomes more acute when trying to disperse within solid matrices such as HA or metallic powders [74]. The high aspect ratio and stiffness of CNTs also account for the difficulty in homogeneous dispersion within matrix materials [74].
In terms of surface charge, HA powder and pristine CNTs are considered to be weakly negatively charged and/or neutral because there are a number of hydroxyl groups and π-electrons on the surface of HA crystals and CNT walls, respectively, (see Table 1 and the supplementary material available online at doi:10.4061/2011/674287) and it is this surface charge analogy between both components that could also be a primary reason for their poor homogenization. To homogenize CNTs and HA, all experimental attempts can be generally categorized into the following two methodologies: CNT dispersion into HA matrices for the synthesis of HA-CNT composites (Table 2, entries 1–4) and HA mineralization onto CNT matrices for the synthesis of HA-CNT hybrids (Table 2, entry 5).
Table 1.
The Zeta (ζ) potential values of hydroxyapatitea, SWCNTb, and MWCNTb powders.
| Substrate | Particle size (nm) | Degree of purity (%) | Zeta (ζ) potentialc (mV) |
|---|---|---|---|
| Hydroxyapatite | Diameter: hundreds of nanometers | >99 | −2.4 |
| SWCNT | Diameter: 1.3–1.5 nm | 60–70 | −7.8 |
| Bundle diameter: 20–30 nm | |||
| MWCNT | Diameter: 5–20 nm | >95 | −3.6 |
| Length: 20 μm |
aPurchased from Sigma Aldrich Korea and calcined at 900°C for 3 h; bPurchased from Hanwha Nanotech Co., Ltd. (Seoul, Korea); cRecorded using a Zetasizer nano ZS90 (Malvern) at room temperature and pH = 7.0.
Table 2.
Synthesis procedures for production of HA-CNT composites and hybrids.
| Entry | Blending method | CNT content | Mechanical properties |
|---|---|---|---|
| 1 | Ball-milling/laser-alloying [74–76] | <20 wt% | Hardness: increase up to 43%Modulus: increase up to 21% |
| 2 | Jar-milling/plasma spraying [77] | 4 wt% | Toughness: increase up to 56% |
| 3 | aIn solvent mixing [78] | <20 wt% | Not measured |
| 4 | In situ synthesis via CVD [79] | 1 wt% | Not measured |
| 5 | Mineralization [80–87] | bNot measured | Not measured |
aSee Section 4.1 for details.
2.1. CNT Dispersion into HA Matrices (HA-CNT Composites)
To disperse CNT powder throughout the HA matrix material, several physicochemical blending methods have been developed, including ball milling [74–77], mixing in solvent [78], and in situ formation of CNTs in HA matrix (see Table 2 and Figure 1) [79].
Figure 1.
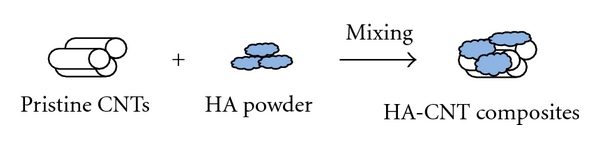
Schematic demonstration of the fabrication of HA-CNT composite powder by physicochemical blending methods.
Chen et al. [74–76] made unfunctionalized CNT-reinforced HA composite through ball-milling. In the study, pristine CNTs in different weight proportions (0–20%) were first ball-milled together with HA powder before the composite was used in surface coating by laser surface alloying. When compared with the CNT-free HA coating, the hardness and modulus of the CNT-reinforced HA coating were improved by 42.7 and 20.6%, respectively. Balani et al. [77] introduced plasma spraying as a definitive tool to coat HA-CNT powder onto metallic implants while providing uniform distribution of unfunctionalized CNTs. To accomplish this, they mixed HA powder of 10–50 μm particle in size with 4 wt% CNTs in a jar mill for 18 h to obtain HA-CNT composite powder. After this they successfully coated the powder mixture onto Ti-6Al-4V bioimplant substrate by plasma spraying. They observed improvements in fracture toughness and crystallinity of 56 and 27%, respectively, and demonstrated the nontoxicity of the HA-CNT coating by cell culture study. Lu et al. [79] reported that HA-CNT nanocomposites (about 1 wt% CNT) can be fabricated by in situ formation of CNTs in HA matrix via chemical vapor deposition (CVD). According to the in situ technique, CNT growth took place on an alumina powder containing an iron catalyst.
2.2. HA Mineralization onto CNT Matrices (HA-CNT Hybrids)
In fact, HA mineralization occurs in the body with the help of organic molecules for the formation and maintenance of bones and teeth. Therefore, the mineralization process can be appropriate to homogenize HA onto individual CNT molecules to form HA-CNT nanohybrids (see Figure 2). Since the first report of Abe et al. on the biomimetic HA mineralization in 1990 [80], several types of Ca-P precursor solutions including simulated body fluid (SBF) and Dulbecco's phosphate-buffered saline (DPBS) have been employed for the HA mineralization. The Ca-P solutions used for the HA mineralization are summarized in Table 3 [81–83].
Figure 2.

Schematic demonstration of HA mineralization onto pristine CNTs.
Table 3.
Type and chemical composition of Ca-P precursor solutions used for the HA mineralization onto CNTs.
| Reagent | Type and chemical composition (mg/l) of Ca-P precursor solution | |||
|---|---|---|---|---|
| SBF-1 | SBF-2 | SBF-3 | PBS | |
| NaCl | 7996 | 866 | 6547 | 8000 |
| NaHCO3 | 350 | — | 2268 | — |
| KCl | 244 | 625 | 373 | 200 |
| Na2HPO4·2H2O | — | — | 178 | 1150 |
| K2HPO4 | 174 | 803 | — | — |
| KH2PO4 | — | 326 | — | 200 |
| MgCl2·6H2O | 143 | 59 | 305 | 47 |
| CaCl2·2H2O | 278 | 125 | 368 | 100 |
| Na2SO4 | 71 | — | 71 | — |
| (CH2OH)3CNH2 | 6057 | — | 6057 | — |
| NaF | 22 | 22 | — | 22 |
| pH | 7.4 | 7.2 | 7.4 | 7.4 |
Several researchers also employed unfunctionalized CNTs as HA mineralization-inducing nanotemplates, but the mineralization efficacy was usually poor. Moreover, low levels of HA crystal formation and thus large aggregates of CNTs occurred, leading to the formation of heterogeneous composite. However, most studies claimed that the tubular structure of CNTs provides more favorable morphology for the HA crystallization when compared to other carbon materials [84–86]. A study conducted by Akasaka et al. [84] revealed that the composition and concentration of Ca-P solutions significantly affected the amount of HA crystals formed on the CNTs as well as the size and morphology of crystals. When unmodified CNTs were immersed in SBF solution, only small amount of HA was mineralized on the CNT surface even after a couple of weeks. More concentrated CaP solution such as 2x SBF facilitated a higher level of HA mineralization within the same period. The results indicate that pristine CNTs retain a low level of capacity to mineralize HA on the surface, and this was mainly due to the low number of nucleation sites which thus limit nucleation and growth of HA crystals. In a different approach, Kealley et al. [85, 86] prepared a densely compressed HA-CNT composite sheet via chemical precipitation of HA crystals from a calcium phosphate solution, using the chemicals Ca(NO3)2·4H2O and (NH4)2HPO4 followed by hot isostatic compression at 100 MPa and 900°C under Ar gas. They found some enhanced results with respect to the crystallization of HA. Liao et al. [87] employed some different types of experimental conditions such as bamboo-structured CNTs and a Ca-P solution consisting of 0.5 M CaCl2 and 0.5 M H3PO4 (Ca/P = 1.66) as a nucleation template and a calcium phosphate precursor, respectively. They observed relatively unique spindle-shaped apatite crystals precipitated on the CNTs and also obtained carbonated HA-CNT nanohybrids after treatment of the as-prepared HA-CNT hybrid with a solution of Na2CO3 (molar ratio of CO32−= PO43− = 3) for 2 h.
In conclusion, if pristine CNTs were used, a homogenous assembling of CNTs within the HA matrix is highly difficult by means of either physical mixing or mineralization route. It is likely that the following problems were responsible for this: aggregation (or bundle formation) via the well-known π-π interactions between the delocalized electron systems of the CNT surface, the limited number of nucleation sites of pristine CNTs, and the similarity of the surface charge of HA and pristine CNTs. Therefore, to overcome these difficulties related to the intrinsic physicochemical properties of CNTs, chemical modification of CNTs is the key strategy.
3. Strategies for CNT Modification
The chemical modifications of CNTs developed to date are summarized in Table 4 and schematically demonstrated in Figure 3.
Table 4.
Methods of surface functionalization of CNTs.
| Entry | Method | Functional group |
|---|---|---|
| 1 | Oxidative cutting [88–92] | CNT-COOH, -OH, and -C=O |
| 2 | Covalent modifications of the carboxylated CNTs [93, 94] | CNT-C(O)OR, -C(O)NR2 |
| 3 | Covalent modification [95–98] | CNT-R |
| 4 | Organic compound-wrapping [99] | {CNTs}{R-C(O)OH, -NH3+X− and -SO3H} |
| 5 | Reductive charging [100, 101] | [CNTs]−M+ |
| 6 | Oxidative charging [100, 102] | [CNTs]+X− |
| 7 | Electrophilic alkylation [78] | [CNT-R]+X− |
R = H or organic moieties; X = inorganic anions; M = metallic cations.
Figure 3.
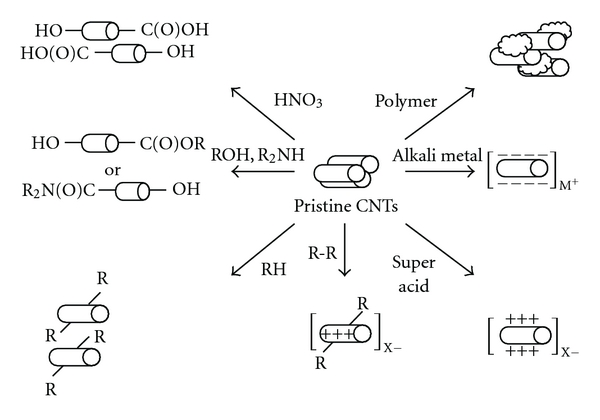
Schematic demonstration of CNT functionalization methods (R = H or organic moieties; X = inorganic anions; M = metallic cations).
Among the methods used to modify the CNT surface, oxidative cutting of CNTs using strong oxidants and acidic media has generally been regarded as a useful tool [88–91]. During functionalization of CNTs by chemical oxidation, various functional groups such as –COOH, –OH, and –C=O were created on the surface of CNTs. The functional groups generally act as nucleation sites of metallic cations for their mineralization [92]. Moreover, to increase the interaction between CNTs and surrounding matrices (liquid or solid), additional modifications of the carboxyl groups introduced by the oxidative cutting have also been studied extensively [93, 94]. Generally, during the reaction procedure for the first oxidation and the further functionalization steps, nanotube bodies lose a proportion of their original properties and morphology. However, in terms of purification, the acid-treatment process has positive effects such as the effective removal of toxic metal impurities (such as catalyst residues) in CNT powder and amorphous carbon phases on CNT surface.
It has been shown that the use of highly reactive chemicals such as alkali metals and Brønsted super acids as reductants and oxidants, respectively, leads to charge separation on the outer walls of CNTs, resulting in positively or negatively charged CNT-derivatives bearing counter ions [100–102]. The charged CNT-derivatives generally were too unstable to be applied under aqueous and ambient conditions, because nanotube bodies were ionically changed too much.
To avoid serious damage to the nanotube body, solubilizers such as polycyclic aromatics, polymers, and various surfactants were employed to provide a relatively higher solubility [99]. However, the use of solubilizers in excess and the macromolecular behavior of solubilizer-CNTs micelles, which may occur through interactions between adjacent solubilizer-wrapped CNTs, have been found to prevent their use in appropriate applications. Moreover, the survival of CNT aggregates and the restricted scope of solvent types must still be addressed. Direct sidewall functionalization will be difficult because the reactivity of the sidewall is extremely low [95]. Indeed, attempts to covalently modify the sidewall have shown that it is a time-consuming method [96, 97]. For modification in large scale, this method has a weakness. A strategy to covalently and oxidatively modify the sidewall has been accomplished via Friedel-Crafts-type alkylation of CNTs with super acid-activated tetrahydrofuran (THF) as an electrophilic species, leading to the formation of positively charged sidewalls covalently bonded with butyl alcohol moieties (or their possible oligomers) and counter anions such as SbF6− and Cl− [78]. Creating positive charges on outer walls may be useful in that it produces a bunch of nucleation sites for HA crystallization as well as those for ionic interaction with negatively charged HA powder.
4. Utilizing Modified CNTs for Nanocomposites and Hybrids
4.1. CNT Dispersion into HA Matrices (HA-CNT Nanocomposites)
4.1.1. Use of Ionically Modified CNTs
As mentioned above, pristine CNTs have several limitations that prevent their homogenous distribution into HA powder matrices. When positive charges are introduced onto the outer walls of CNTs to combine with oppositely charged HA particles, the homogeneity of dispersion of the charged CNTs within the HA matrix might be easily achieved without a harsh physical blending process.
Our recent work [78] successfully prepared HA-CNT composite nanopowders in an organic solvent (see Figure 4). As a first step, the surface of the CNTs was positively modified, and then negatively charged HA nanopowders were added within the positively charged CNT derivative solution in an organic solvent (THF or ethanol), resulting in rapid precipitation of the composite HA-CNT nanopowders. The report also showed that the positively charged CNT powder could be combined in greater quantities with HA powder. High-resolution SEM revealed that individual CNTs were evenly distributed within the clusters of HA nanoparticulates.
Figure 4.
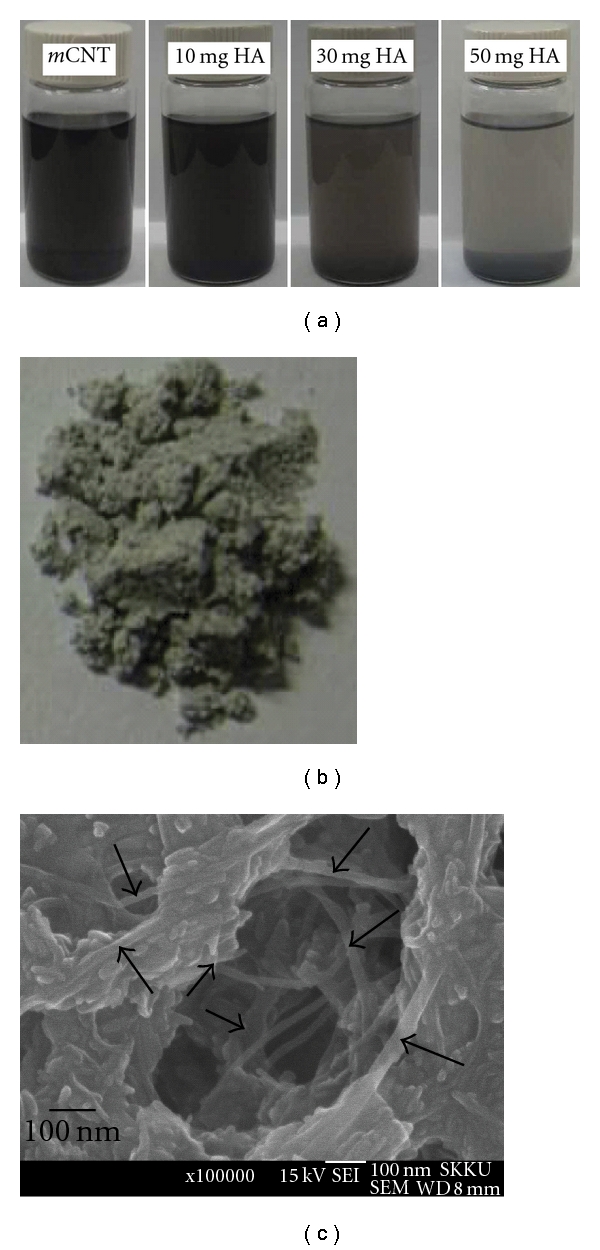
(a) Images showing the addition of negatively charged HA nanopowders to form precipitates with positively charged CNTs (mCNT) within THF solvent and (b) photographs and (c) high-resolution SEM morphology of the HA-CNTs composite nanopowders.
4.2. HA Mineralization onto CNT Matrices (HA-CNT Hybrids)
HA mineralization is a process that involves the controlled nucleation and growth of HA crystals from a confined Ca-P solution. When the CNTs surface was tailored with some ionic groups, either of the calcium or phosphate ions are guided on the surface which subsequently induces opposite charged ions to form nuclei which accompanies by clustering and crystallization and further crystal growth. Therefore, the initially functionalized groups should be well controlled in terms of their types of groups, interspacing distance and numbers, and so forth. Moreover, the ionic composition of the mineralization medium should also be importantly considered.
4.2.1. Use of Organic Molecule-Wrapped CNTs
As mentioned above, the simplest approach to functionalize CNT walls is wrapping the nanotubes with organic molecules such as surfactants and polymers. The noncovalent functionalization can allow the dispersion of CNTs in water while creating charged groups on the tube surfaces to provide nucleation sites. Several experiments of HA mineralization conducted on the organic molecule-wrapped CNTs are shown in Table 5.
Table 5.
HA mineralization on organic molecule-wrapped CNTs.
Zhao and Gao reported the synthesis of HA-CNT composites using sodium dodecyl sulfate- (SDS-) treated CNTs and Ca(NO3)2 and (NH4)2HPO4 solutions. The preparation process was conducted in an autoclave at 118°C and pH > 10, which resulted in the production of HA nanopowders containing 2 wt% CNTs [98]. Evaluation of the TEM images revealed that the HA nanoparticles were closely deposited on individually dispersed CNT molecules. Moreover, they found that the compressive strength of the CNT composite sintered at 1200°C was improved by about 61% from 63 to 102 MPa when compared with that of the single-phase HA hot-pressed at 1100°C. A similar procedure for mineralization using SDS-functionalized CNTs under different conditions was recently reported by Tan et al. [103]. They carried out the mineralization by dispersing the SDS-CNTs into an aqueous solution of CaCl2 and Na2HPO4 at 37.8°C and pH = 7.4. TEM micrographs provided in the report showed that a needle-like HA mineral formed on the SDS decorated CNTs in relatively small quantity. Tasis et al. attempted CNT modification by citrate coating and mineralization in an aqueous solution of CaCl2 and Na2HPO4 at 37°C and pH = 7.4 [104]. The mineralization was conducted on CNT buckypapers (CNT thin sheets) obtained after filtration of citrate-coated CNTs. They observed grain- and plate-like crystal images that were not evenly dispersed on the citrate-treated CNT material.
4.2.2. Use of Carboxylated CNTs
Before combining CNTs with HA, many studies of HA-CNT hybrids focused on the oxidative functionalization of CNTs to create hydrophilic functional groups such as –COOH, –OH, and –C=O. The number of the negatively charged functional groups created on the surface of CNTs during oxidative modifications is dependent on the reaction conditions such as oxidant type, reaction time, and temperature and also has a great effect on the formation rate of the HA crystals, because, as previously mentioned, the functional groups can serve as calcium chelating sites to accelerate the growth of HA crystals. Studies of HA mineralization using carboxylated CNTs are briefly reviewed in Table 6.
Table 6.
HA mineralization on carboxylated CNTs.
Aryal et al. used carboxylated CNTs made in 60% nitric acid by reflux for 18 h at 110°C to prepare HA-CNTs with simulated body fluid (SBF-3 in Table 3) [106]. They found that the carboxylated CNTs effectively nucleated the HA crystals to form assemblies of HA and CNTs within 7 days. When they treated the CNT derivative with aqueous solutions of calcium chloride (0.01 M) and disodium hydrogen phosphate (0.01 M), multilayered plate-like assemblies with HA crystals were obtained [107, 108]. Recently, Pan et al. produced HA-CNT nanohybrids using carboxylated CNTs and Ca(NO3)2 and (NH4)2HPO4 solutions by a one-step sonication process [105]. They dispersed the CNT derivatives into 0.1 M Ca(NO3)2 solution at pH = 10–12, and then 0.1 M (NH4)2HPO4 solution was added dropwise at a rate of 2–4 ml/min with continuous sonication. They confirmed the formation of spindle-like HA nanoparticles with a width of 20–25 nm and a length of 50–100 nm bound to the functional groups of CNTs based on TEM images.
4.2.3. Covalently Further Treatment of the Carboxylated CNTs
The carboxylated CNTs were sometimes further functionalized with other chemical groups. The formation of amide and ester groups via the treatment of the carboxylated CNTs with amines and alcohols is one case (see Table 7) [94, 98].
Table 7.
Subsequent functionalization of the covalently derivatized CNTs for the HA mineralization [109].
| Modified CNTs | Ca-P solution | Reaction condition |
|---|---|---|
| CNT-C(=O)OCH2P(=O) (OEt)2 |
CaCl2, Na2HPO4 | 118°C, pH > 10 |
| CNT-C(=O)NHC6H4CH2 P(=O)(OEt)2 | ||
| CNT-C(=O)NH(C6H3SO3HNH)n | ||
| CNT-CONHC6H4CH2P(=O)(OH)2 | ||
Zhao et al. covalently transformed the carboxylated CNTs into a series of CNT derivatives bearing several functionalities including phosphonate groups such as diethyl methylene phosphonate in ester form {CNT-C(=O)OCH2P(=O)(OEt)2}, diethyl benzyl phosphonate in amide form {CNT-C(=O)NHC6H4CH2P(=O)(OEt)2}, and the sulfonic acid group, poly(aminobenzene sulfonic acid) {CNT-C(=O)NH(C6H3SO3HNH)n} [109]. They investigated the efficacy of various functionalities on CNTs to attract calcium cation which leads to self-assembly of HA. To accomplish this, mineralization was conducted on a hydrophobic Teflon membrane using 10 mM CaCl2 and 5 mM Na2HPO4 as mineral sources. High-resolution SEM images indicated that the phosphonate and sulfonate groups generally formed a large crystal-like HA bulk layer after only 1 day. To investigate the effect of the negative charges, they compared CNT-CONHC6H4CH2P(=O)(OEt)2 and the hydrolyzed form, CNT-CONHC6H4CH2P(=O)(OH)2; however, little difference in the effects of these compounds was observed. In the case of mineralization of CNT-C(=O)NH(C6H3SO3HNH)n, they claimed the formation of aligned plate-shaped HA crystals with a thickness of 3 μm after 14 days.
5. Conclusions
Because of the excellent properties of CNTs, such as mechanical strength and toughness, they are considered a promising reinforcing material for HA to find potential bone replacements. However, the major problem in the production of well-organized HA-CNTs nanocomposites and hybrids is the difficulty in homogenous distribution of pristine CNTs within the HA matrix. CNTs aggregation via the π-π interactions of the CNT surface and the lack of nucleation sites limit the dispersion of CNTs in HA nanopowders or the mineralization of HA crystals within ionic solutions. Therefore, functionalization of the CNTs surface with various methodologies has been popularly employed, which mainly include wrapping with surfactants or polymers and oxidation in harsh acidic conditions. Recent studies have shown some progress by activating the CNTs surface to have surface charges by an ionic-modification method. Using the modified CNTs, studies have shown a level of improvements in the dispersion status and/or the properties of the final composites. However, more studies need to be conducted in terms of the new modification techniques of the CNTs and addressing more well-controlled processes in composites or hybrids through the mineralization. This is because the exceptional properties of CNTs are still expected to provide a great opportunity to produce nanocomposites and hybrids with HA in the development of bone replacements.
Supplementary Material
Zeta (ζ) potential values of hydroxyapatite, SWCNT, and MWCNT powders were recorded using a Zetasizer nano ZS90 (Malvern) at room temperature and pH = 7.0.
1. The Zeta (ζ) potential value of hydroxyapatite after calcinations at 900°C for 3h.
2. The Zeta (ζ) potential value of SWCNTs before functionalization.
3. The Zeta (ζ) potential value of MWCNTs before functionalization.
Acknowledgments
This work was supported by the Priority Research Centers Program (Grant no. 2009-0093829) and WCU (World Class University) program (Grant no. R31-10069) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology.
References
- 1.Melde BJ, Stein A. Periodic macroporous hydroxyapatite-containing calcium phosphates. Chemistry of Materials. 2002;14(8):3326–3331. [Google Scholar]
- 2.Takechi M, Miyamoto Y, Ishikawa K, et al. Effects of added antibiotics on the basic properties of anti-washout- type fast-setting calcium phosphate cement. Journal of Biomedical Materials Research. 1998;39(2):308–316. doi: 10.1002/(sici)1097-4636(199802)39:2<308::aid-jbm19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Watson A, Latchman D. Gene delivery into neuronal cells by calcium phosphate-mediated transfection. Methods. 1996;10(3):289–291. doi: 10.1006/meth.1996.0105. [DOI] [PubMed] [Google Scholar]
- 4.Stigter M, De Groot K, Layrolle P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials. 2002;23(20):4143–4153. doi: 10.1016/s0142-9612(02)00157-6. [DOI] [PubMed] [Google Scholar]
- 5.Choi D, Marra KG, Kumta PN. Chemical synthesis of hydroxyapatite/poly(ε-caprolactone) composites. Materials Research Bulletin. 2004;39(3):417–432. [Google Scholar]
- 6.Tsui YC, Doyle C, Clyne TW. Plasma sprayed hydroxyapatite coatings on titanium substrates—part 1: mechanical properties and residual stress levels. Biomaterials. 1998;19(22):2015–2029. doi: 10.1016/s0142-9612(98)00103-3. [DOI] [PubMed] [Google Scholar]
- 7.Hukovic M, Tkalacec E, Kwokal A, Piljac J. An in vitro study of Ti and Ti-alloys coated with sol-gel derived hydroxyapatite coatings. Surface and Coatings Technology. 2003;165(1):40–50. [Google Scholar]
- 8.Tadic D, Peters F, Epple M. Continuous synthesis of amorphous carbonated apatites. Biomaterials. 2002;23(12):2553–2559. doi: 10.1016/s0142-9612(01)00390-8. [DOI] [PubMed] [Google Scholar]
- 9.Banks E, Nakajima S, Shapiro LC. Fibrous apatite grown on modified collagen. Science. 1977;198(4322):1164–1166. doi: 10.1126/science.929194. [DOI] [PubMed] [Google Scholar]
- 10.Nam S, Won JE, Kim CH, Kim HW. Odontogenic differentiation of human dental pulp stem cells stimulated by the calcium phosphate porous granules. Journal of Tissue Engineering. 2011;2011:10 pages. doi: 10.4061/2011/812547. Article ID 812547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Shi B, Fairchild A, Cale T. Applications of plasma coatings in artificial joints: an overview. Vacuum. 2004;73(3-4):317–326. [Google Scholar]
- 12.Ruys AJ, Wei M, Sorrell CC, Dickson MR, Brandwood A, Milthorpe BK. Sintering effects on the strength of hydroxyapatite. Biomaterials. 1995;16(5):409–415. doi: 10.1016/0142-9612(95)98859-c. [DOI] [PubMed] [Google Scholar]
- 13.Bonfield W, Grynpas MD, Tully AE, Bowman J, Abram J. Hydroxyapatite reinforced polyethylene-a mechanically composite implant material for bone replacement. Biomaterials. 1981;2(3):185–186. doi: 10.1016/0142-9612(81)90050-8. [DOI] [PubMed] [Google Scholar]
- 14.Bradt JH, Mertig M, Teresiak A, Pompe W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chemistry of Materials. 1999;11(10):2694–2701. [Google Scholar]
- 15.Miyaji F, Kim HM, Handa S, Kokubo T, Nakamura T. Bonelike apatite coating on organic polymers: novel nucleation process using sodium silicate solution. Biomaterials. 1999;20(10):913–919. doi: 10.1016/s0142-9612(98)00235-x. [DOI] [PubMed] [Google Scholar]
- 16.Ignjatović N, Tomić S, Dakić M, Miljković M, Plavšić M, Uskoković D. Synthesis and properties of hydroxyapatite/poly-L-lactide composite biomaterials. Biomaterials. 1999;20(9):809–816. doi: 10.1016/s0142-9612(98)00234-8. [DOI] [PubMed] [Google Scholar]
- 17.Bigi A, Boanini E, Panzavolta S, Roveri N. Biomimetic growth of hydroxyapatite on gelatin films doped with sodium polyacrylate. Biomacromolecules. 2000;1(4):752–756. doi: 10.1021/bm0055854. [DOI] [PubMed] [Google Scholar]
- 18.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials. 2001;22(13):1705–1711. doi: 10.1016/s0142-9612(00)00305-7. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Magnay JL, Cooling L, El Haj AJ. Development of a “mechano-active” scaffold for tissue engineering. Biomaterials. 2002;23(10):2119–2126. doi: 10.1016/s0142-9612(01)00342-8. [DOI] [PubMed] [Google Scholar]
- 21.Park SN, Park JC, Kim HO, Song MJ, Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials. 2002;23(4):1205–1212. doi: 10.1016/s0142-9612(01)00235-6. [DOI] [PubMed] [Google Scholar]
- 22.Dorozhkina EI, Dorozhkin SV. Surface mineralisation of hydroxyapatite in modified simulated body fluid (mSBF) with higher amounts of hydrogencarbonate ions. Colloids and Surfaces A. 2002;210(1):41–48. [Google Scholar]
- 23.Zhang W, Liao SS, Cui FZ. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chemistry of Materials. 2003;15(16):3221–3226. [Google Scholar]
- 24.Peigney A. Composite materials: tougher ceramics with nanotubes. Nature Materials. 2003;2(1):15–16. doi: 10.1038/nmat794. [DOI] [PubMed] [Google Scholar]
- 25.Lee H-H, Shin US, Jin G-Z, Kim H-W. Highly homogeneous carbon nanotube-polycaprolactone composites with various and controllable concentrations of ionically-modified-MWCNTs. Bulletin of the Korean Chemical Society. 2011;32(1):157–161. [Google Scholar]
- 26.Balázsi C, Kónya Z, Wéber F, Biró LP, Arató P. Preparation and characterization of carbon nanotube reinforced silicon nitride composites. Materials Science and Engineering C. 2003;23(6-8):1133–1137. [Google Scholar]
- 27.Xia Z, Riester L, Curtin WA, et al. Direct observation of toughening mechanisms in carbon nanotube ceramic matrix composites. Acta Materialia. 2004;52(4):931–944. [Google Scholar]
- 28.Curtin WA, Sheldon BW. CNT-reinforced ceramics and metals. Materials Today. 2004;7(11):44–49. [Google Scholar]
- 29.Peigney A, Laurent C, Flahaut E, Rousset A. Carbon nanotubes in novel ceramic matrix nanocomposites. Ceramics International. 2000;26(6):677–683. [Google Scholar]
- 30.Wang X, Padture NP, Tanaka H. Contact-damage-resistant ceramic/single-wall carbon nanotubes and ceramic/graphite composites. Nature Materials. 2004;3(8):539–544. doi: 10.1038/nmat1161. [DOI] [PubMed] [Google Scholar]
- 31.Peigney A, Flahaut E, Laurent C, Chastel F, Rousset A. Aligned carbon nanotubes in ceramic-matrix nanocomposites prepared by high-temperature extrusion. Chemical Physics Letters. 2002;352(1-2):20–25. [Google Scholar]
- 32.An JW, You DH, Lim DS. Tribological properties of hot-pressed alumina-CNT composites. Wear. 2003;255(1-6):677–681. [Google Scholar]
- 33.Ma RZ, Wu J, Wei BQ, Liang J, Wu DH. Processing and properties of carbon nanotubes-nano-SiC ceramic. Journal of Materials Science. 1998;33(21):5243–5246. [Google Scholar]
- 34.Zhan GD, Kuntz JD, Wan J, Mukherjee AK. Single-wall carbon nanotubes as attractive toughening agents in alumina-based nanocomposites. Nature Materials. 2003;2(1):38–42. doi: 10.1038/nmat793. [DOI] [PubMed] [Google Scholar]
- 35.Li GY, Wang PM, Zhao X. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon. 2005;43(6):1239–1245. [Google Scholar]
- 36.Chłopek J, Czajkowska B, Szaraniec B, Frackowiak E, Szostak K, Béguin F. In vitro studies of carbon nanotubes biocompatibility. Carbon. 2006;44(6):1106–1111. [Google Scholar]
- 37.Price RL, Waid MC, Haberstroh KM, Webster TJ. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003;24(11):1877–1887. doi: 10.1016/s0142-9612(02)00609-9. [DOI] [PubMed] [Google Scholar]
- 38.Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Letters. 2006;6(3):562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 39.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–58. [Google Scholar]
- 40.Ajayan PM. Nanotubes from Carbon. Chemical Reviews. 1999;99(7):1787–1799. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- 41.Terrones M. Science and technology of the 21st Century: synthesis, properties, and applications of carbon nanotubes. Annual Review of Materials Research. 2003;33:419–501. [Google Scholar]
- 42.Dresselhaus MS, Dresselhaus G, Charlier JC, Hernández E. Electronic, thermal and mechanical properties of carbon nanotubes. Philosophical Transactions of the Royal Society A. 2004;362(1823):2065–2098. doi: 10.1098/rsta.2004.1430. [DOI] [PubMed] [Google Scholar]
- 43.Baughman RH, Zakhidov AA, De Heer WA. Carbon nanotubes—the route toward applications. Science. 2002;297(5582):787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Zhai Y, Zhang Y, Ong C, Guo S. Reinforcement of hydrogenated carboxylated nitrile-butadiene rubber by multi-walled carbon nanotubes. Applied Surface Science. 2008;255(5):2162–2166. [Google Scholar]
- 45.Seeger T, De la Fuente G, Maser WK, Benito AM, Callejas MA, Martínez MT. Evolution of multiwalled carbon-nanotube/SiO2 composites via laser treatment. Nanotechnology. 2003;14(2):184–187. [Google Scholar]
- 46.Gojny FH, Nastalczyk J, Roslaniec Z, Schulte K. Surface modified multi-walled carbon nanotubes in CNT/epoxy-composites. Chemical Physics Letters. 2003;370(5-6):820–824. [Google Scholar]
- 47.Zhang X, Liu T, Sreekumar TV, et al. Poly(vinyl alcohol)/SWNT composite film. Nano Letters. 2003;3(9):1285–1288. [Google Scholar]
- 48.Kumar S, Dang TD, Arnold FE, et al. Synthesis, structure, and properties of PBO/SWNT composites. Macromolecules. 2002;35(24):9039–9043. [Google Scholar]
- 49.Correa-Duarte MA, Wagner N, Rojas-Chapana J, Morsczeck C, Thie M, Giersig M. Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Letters. 2004;4(11):2233–2236. [Google Scholar]
- 50.Wang SF, Shen L, Zhang WD, Tong YJ. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules. 2005;6(6):3067–3072. doi: 10.1021/bm050378v. [DOI] [PubMed] [Google Scholar]
- 51.MacDonald RA, Laurenzi BF, Viswanathan G, Ajayan PM, Stegemann JP. Collagen-carbon nanotube composite materials as scaffolds in tissue engineering. Journal of Biomedical Materials Research - Part A. 2005;74(3):489–496. doi: 10.1002/jbm.a.30386. [DOI] [PubMed] [Google Scholar]
- 52.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Injectable nanocomposites of single-walled carbon nanotubes and biodegradable polymers for bone tissue engineering. Biomacromolecules. 2006;7(7):2237–2242. doi: 10.1021/bm060391v. [DOI] [PubMed] [Google Scholar]
- 53.Sitharaman B, Shi X, Tran LA, et al. Injectable in situ cross-linkable nanocomposites of biodegradable polymers and carbon nanostructures for bone tissue engineering. Journal of Biomaterials Science. 2007;18(6):655–671. doi: 10.1163/156856207781034133. [DOI] [PubMed] [Google Scholar]
- 54.Thostenson ET, Ren Z, Chou TW. Advances in the science and technology of carbon nanotubes and their composites: a review. Composites Science and Technology. 2001;61(13):1899–1912. [Google Scholar]
- 55.Dai H. Carbon nanotubes: opportunities and challenges. Surface Science. 2002;500(1–3):218–241. [Google Scholar]
- 56.Lupo F, Kamalakaran R, Scheu C, Grobert N, Rühle M. Microstructural investigations on zirconium oxide-carbon nanotube composites synthesized by hydrothermal crystallization. Carbon. 2004;42(10):1995–1999. [Google Scholar]
- 57.Balázsi C, Kónya Z, Wéber F, Biró LP, Arató P. Preparation and characterization of carbon nanotube reinforced silicon nitride composites. Materials Science and Engineering C. 2003;23(6–8):1133–1137. [Google Scholar]
- 58.Xia Z, Riester L, Curtin WA, et al. Direct observation of toughening mechanisms in carbon nanotube ceramic matrix composites. Acta Materialia. 2004;52(4):931–944. [Google Scholar]
- 59.Curtin WA, Sheldon BW. CNT-reinforced ceramics and metals. Materials Today. 2004;7(11):44–49. [Google Scholar]
- 60.Peigney A, Laurent C, Flahaut E, Rousset A. Carbon nanotubes in novel ceramic matrix nanocomposites. Ceramics International. 2000;26(6):677–683. [Google Scholar]
- 61.Wang X, Padture NP, Tanaka H. Contact-damage-resistant ceramic/single-wall carbon nanotubes and ceramic/graphite composites. Nature Materials. 2004;3(8):539–544. doi: 10.1038/nmat1161. [DOI] [PubMed] [Google Scholar]
- 62.Peigney A, Flahaut E, Laurent C, Chastel F, Rousset A. Aligned carbon nanotubes in ceramic-matrix nanocomposites prepared by high-temperature extrusion. Chemical Physics Letters. 2002;352(1-2):20–25. [Google Scholar]
- 63.An JW, You DH, Lim DS. Tribological properties of hot-pressed alumina-CNT composites. Wear. 2003;255(1–6):677–681. [Google Scholar]
- 64.Ma RZ, Wu J, Wei BQ, Liang J, Wu DH. Processing and properties of carbon nanotubes-nano-SiC ceramic. Journal of Materials Science. 1998;33(21):5243–5246. [Google Scholar]
- 65.Zhan GD, Kuntz JD, Wan J, Mukherjee AK. Single-wall carbon nanotubes as attractive toughening agents in alumina-based nanocomposites. Nature Materials. 2003;2(1):38–42. doi: 10.1038/nmat793. [DOI] [PubMed] [Google Scholar]
- 66.Li GY, Wang PM, Zhao X. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon. 2005;43(6):1239–1245. [Google Scholar]
- 67.Chłopek J, Czajkowska B, Szaraniec B, Frackowiak E, Szostak K, Béguin F. In vitro studies of carbon nanotubes biocompatibility. Carbon. 2006;44(6):1106–1111. [Google Scholar]
- 68.Price RL, Waid MC, Haberstroh KM, Webster TJ. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003;24(11):1877–1887. doi: 10.1016/s0142-9612(02)00609-9. [DOI] [PubMed] [Google Scholar]
- 69.Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Letters. 2006;6(3):562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 70.Takagi A, Hirose A, Nishimura T, et al. Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. Journal of Toxicological Sciences. 2008;33(1):105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 71.Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nature Nanotechnology. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 72.Lacerda L, Soundararajan A, Singh R, et al. Dynamic imaging of functionalized multi-walled carbon nanotube systemic circulation and urinary excretion. Advanced Materials. 2008;20(2):225–230. [Google Scholar]
- 73.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28(2):344–353. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Gan C, Zhang T, Yu G, Bai P, Kaplan A. Laser-surface-alloyed carbon nanotubes reinforced hydroxyapatite composite coatings. Applied Physics Letters. 2005;86(25):1–3. Article ID 251905. [Google Scholar]
- 75.Chen Y, Zhang YQ, Zhang TH, Gan CH, Zheng CY, Yu G. Carbon nanotube reinforced hydroxyapatite composite coatings produced through laser surface alloying. Carbon. 2006;44(1):37–45. [Google Scholar]
- 76.Chen Y, Zhang TH, Gan CH, Yu G. Wear studies of hydroxyapatite composite coating reinforced by carbon nanotubes. Carbon. 2007;45(5):998–1004. [Google Scholar]
- 77.Balani K, Anderson R, Laha T, et al. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials. 2007;28(4):618–624. doi: 10.1016/j.biomaterials.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 78.Lee HY, Shin US, Won J-E, Kim H-W. Preparation of hydroxyapatite-carbon nanotube composite nanopowders. Materials Letters. 2011;65(2):208–211. [Google Scholar]
- 79.Lu X, Wang H, Xia S, Wang J, Weng J. In situ growth of carbon nanotubes in hydroxyapatite matrix by chemical vapor deposition. Advanced Materials Research. 2009;79–82:1671–1674. [Google Scholar]
- 80.Song WH, Jun YK, Han Y, Hong SH. Biomimetic apatite coatings on micro-arc oxidized titania. Biomaterials. 2004;25(17):3341–3349. doi: 10.1016/j.biomaterials.2003.09.103. [DOI] [PubMed] [Google Scholar]
- 81.Akasaka T, Watari F, Sato Y, Tohji K. Apatite formation on carbon nanotubes. Materials Science and Engineering C. 2006;26(4):675–678. [Google Scholar]
- 82.Kealley C, Elcombe M, van Riessen A, Ben-Nissan B. Development of carbon nanotube-reinforced hydroxyapatite bioceramics. Physica B. 2006;385-386:496–498. [Google Scholar]
- 83.Kealley C, Elcombe M, Van Riessen A, Ben-Nissan B. Neutron characterisation of hydroxyapatite bioceramics. Key Engineering Materials. 2006;309–311:61–64. [Google Scholar]
- 84.Abe Y, Kokubo T, Yamamuro T. Apatite coating on ceramics, metals and polymers utilizing a biological process. Journal of Materials Science. 1990;1(4):233–238. [Google Scholar]
- 85.Kim HM, Miyazaki T, Kokubo T, Nakamura T. Revised simulated body fluid. Key Engineering Materials. 2001;192–195:47–50. [Google Scholar]
- 86.Oyane A, Kawashita M, Nakanishi K, et al. Bonelike apatite formation on ethylene-vinyl alcohol copolymer modified with silane coupling agent and calcium silicate solutions. Biomaterials. 2003;24(10):1729–1735. doi: 10.1016/s0142-9612(02)00581-1. [DOI] [PubMed] [Google Scholar]
- 87.Liao S, Xu G, Wang W, et al. Self-assembly of nano-hydroxyapatite on multi-walled carbon nanotubes. Acta Biomaterialia. 2007;3(5):669–675. doi: 10.1016/j.actbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, Rinzler AG, Dai H, et al. Fullerene pipes. Science. 1998;280(5367):1253–1256. doi: 10.1126/science.280.5367.1253. [DOI] [PubMed] [Google Scholar]
- 89.Kovtyukhova NI, Mallouk TE, Pan L, Dickey EC. Individual single-walled nanotubes and hydrogels made by oxidative exfoliation of carbon nanotube ropes. Journal of the American Chemical Society. 2003;125(32):9761–9769. doi: 10.1021/ja0344516. [DOI] [PubMed] [Google Scholar]
- 90.Ramesh S, Ericson LM, Davis VA, et al. Dissolution of pristine single walled carbon nanotubes in superacids by direct protonation. Journal of Physical Chemistry B. 2004;108(26):8794–8798. [Google Scholar]
- 91.Wang Y, Iqbal Z, Mitra S. Rapidly functionalized, water-dispersed carbon nanotubes at high concentration. Journal of the American Chemical Society. 2006;128(1):95–99. doi: 10.1021/ja053003q. [DOI] [PubMed] [Google Scholar]
- 92.Liu ZJ, Xu Z, Yuan ZY, Chen W, Zhou W, Peng LM. A simple method for coating carbon nanotubes with Co-B amorphous alloy. Materials Letters. 2003;57(7):1339–1344. [Google Scholar]
- 93.Chen J, Hamon MA, Hu H, et al. Solution properties of single-walled carbon nanotubes. Science. 1998;282(5386):95–98. doi: 10.1126/science.282.5386.95. [DOI] [PubMed] [Google Scholar]
- 94.Yu B, Zhou F, Liu G, Liang Y, Huck WTS, Liu W. The electrolyte switchable solubility of multi-walled carbon nanotube/ionic liquid (MWCNT/IL) hybrids. Chemical Communications. 2006;(22):2356–2358. doi: 10.1039/b603878f. [DOI] [PubMed] [Google Scholar]
- 95.Aihara JI. Lack of superaromaticity in carbon nanotubes. Journal of Physical Chemistry. 1994;98(39):9773–9776. [Google Scholar]
- 96.Bahr JL, Yang J, Kosynkin DV, Bronikowski MJ, Smalley RE, Tour JM. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: a bucky paper electrode. Journal of the American Chemical Society. 2001;123(27):6536–6542. doi: 10.1021/ja010462s. [DOI] [PubMed] [Google Scholar]
- 97.Mickelson ET, Huffman CB, Rinzler AG, Smalley RE, Hauge RH, Margrave JL. Fluorination of single-wall carbon nanotubes. Chemical Physics Letters. 1998;296(1-2):188–194. [Google Scholar]
- 98.Zhao L, Gao L. Novel in situ synthesis of MWNTs-hydroxyapatite composites. Carbon. 2004;42(2):423–426. [Google Scholar]
- 99.Ishibashi A, Nakashima N. Indivisual dissolution of single-walled carbon nanotubes in aqueous solutions of steroid or sugar compounds and their Raman and near-IR spectral properties. Chemistry. 2006;12:7595–7602. doi: 10.1002/chem.200600326. [DOI] [PubMed] [Google Scholar]
- 100.Rao AM, Eklund PC, Bandow S, Thess A, Smalley RE. Evidence for charge transfer in doped carbon nanotube bundles from raman scattering. Nature. 1997;388(6639):257–259. [Google Scholar]
- 101.Pénicaud A, Poulin P, Derré A, Anglaret E, Petit P. Spontaneous dissolution of a single-wall carbon nanotube salt. Journal of the American Chemical Society. 2005;127(1):8–9. doi: 10.1021/ja0443373. [DOI] [PubMed] [Google Scholar]
- 102.Liu CM, Cao HB, Li YP, Xu HB, Zhang Y. The effect of electrolytic oxidation on the electrochemical properties of multi-walled carbon nanotubes. Carbon. 2006;44(14):2919–2924. [Google Scholar]
- 103.Tan Q, Zhang K, Gu S, Ren J. Mineralization of surfactant functionalized multi-walled carbon nanotubes (MWNTs) to prepare hydroxyapatite/MWNTs nanohybrid. Applied Surface Science. 2009;255(15):7036–7039. [Google Scholar]
- 104.Tasis D, Kastanis D, Galiotis C, Bouropoulos N. Growth of calcium phosphate mineral on carbon nanotube buckypapers. Physica Status Solidi B. 2006;243(13):3230–3233. [Google Scholar]
- 105.Pan D, Wang Y, Chen Z, Yin T, Qin W. Fabrication and characterization of carbon nanotube-hydroxyapatite nanocomposite: application to anodic stripping yoltammetric determination of cadmium. Electroanalysis. 2009;21(8):944–952. [Google Scholar]
- 106.Aryal S, Bhattarai SR, Remant Bahadur KC, Khil MS, Lee DR, Kim HY. Carbon nanotubes assisted biomimetic synthesis of hydroxyapatite from simulated body fluid. Materials Science and Engineering A. 2006;426(1-2):202–207. [Google Scholar]
- 107.Aryal S, Bahadur KCR, Dharmaraj N, Kim K-W, Kim HY. Synthesis and characterization of hydroxyapatite using carbon nanotubes as a nano-matrix. Scripta Materialia. 2006;54(2):131–135. [Google Scholar]
- 108.Bhattarai SR, Aryal S, Bahadur R, et al. Carbon nanotube-hydroxyapatite nanocomposite for DNA complexation. Materials Science and Engineering C. 2008;28(1):64–69. [Google Scholar]
- 109.Zhao B, Hu H, Mandal SK, Haddon RC. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chemistry of Materials. 2005;17(12):3235–3241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Zeta (ζ) potential values of hydroxyapatite, SWCNT, and MWCNT powders were recorded using a Zetasizer nano ZS90 (Malvern) at room temperature and pH = 7.0.
1. The Zeta (ζ) potential value of hydroxyapatite after calcinations at 900°C for 3h.
2. The Zeta (ζ) potential value of SWCNTs before functionalization.
3. The Zeta (ζ) potential value of MWCNTs before functionalization.


