Abstract
Serotonin (5HT)-induced facilitation of synaptic transmission from tail sensory neurons (SNs) to motor neurons (MNs) in the marine mollusc Aplysia provides a cellular model of short- and long-term memory for behavioral sensitization of the tail withdrawal reflex. Synaptic facilitation at these synapses occurs in three temporal phases: short-term (STF, lasting minutes), intermediate-term (ITF, lasting more than an hour), and long-term (LTF, lasting >24 hr). STF, ITF, and LTF differ in their induction requirements: A single brief exposure of 5HT induces STF, whereas five applications are required for ITF and LTF. Moreover, STF and LTF can be induced independently.
Different forms of memory often show differential sensitivity to the pattern of training trials. To begin to explore this effect at a cellular level, we examined ITF and LTF induced by one of two patterns of 5HT application: a spaced pattern (five 5-min exposures with an interval of 15 min) or a massed pattern (one continuous 25-min application). The spaced and massed patterns both induced ITF; however, spaced 5HT application was significantly more reliable at inducing LTF than was massed application. Thus, whereas induction of ITF and LTF require similar amounts of 5HT, the cellular mechanisms underlying the induction of LTF are more sensitive to the pattern of the induction trials. In the massed group, further analysis revealed a relationship between the expression of ITF and the subsequent expression of LTF, suggesting that these two processes may be mechanistically related.
A general property of many forms of memory is that retention typically increases as a function of the amount of training. Thus, multiple training trials usually produce better performance or longer-lasting memory than a single training trial (Bugelski 1962; Cooper and Pantle 1967; Zacks 1969). However, the amount of training is not the only factor determining retention. Since early experimental studies of human memory (Ebbinghaus 1885), it has been appreciated that the temporal distribution of training trials can have a dramatic effect on later performance. This important feature of memory formation has received extensive experimental and theoretical attention (Woodworth 1938; Hull 1943; Davis 1970; Hintzman 1974; Ewing et al. 1985; Rescorla 1988). The general importance of the temporal patterning of training is highlighted by the emergence of several multiphasic models of long-term memory formation, across invertebrate and vertebrate species, which emphasize that the duration of different forms of memory is largely dependent on the nature, intensity, and pattern of training stimuli (McGaugh 1966; Carew et al. 1972; Gibbs and Ng 1979; Rosenzweig and Bennett 1984; deZazzo and Tully 1995; Hammer and Menzel 1995). For example, in a recent study using an olfactory-conditioning paradigm in Drosophila, temporally spaced training (several training sessions separated by intervals of no training) and massed training (delivered with no rest intervals) were found to produce two apparently independent forms of long-lasting memory that differed in their duration and in their dependence upon protein synthesis (Tully et al. 1994; Yin et al. 1994). Using different species and different experimental approaches, considerable progress has been made in identifying some of the critical cellular and molecular steps involved in different forms of memory processing (for review, see Byrne 1987; Hawkins et al. 1987; Squire 1987; Dudai 1989; deZazzo and Tully 1995; Carew 1996).
The marine mollusc Aplysia exhibits several types of both associative and nonassociative memory that can exist in both short-term (lasting minutes to hours) and long-term (lasting days to weeks) forms (Carew and Sahley 1986; Byrne 1987; Hawkins et al. 1987). Memory for some of these forms of learning is modulated in strength or duration by the pattern of training stimuli. For example, long-term habituation of the siphon withdrawal reflex is greater in magnitude after temporally spaced training trials than after massed exposure to the habituating stimulus (Carew et al. 1972). Behavioral sensitization, an increase in reflex amplitude following strong stimulation (such as tail shock), can also exist in both short-term and long-term forms (Carew et al. 1971; Pinsker et al. 1973). This type of nonassociative memory has proven especially useful for studying the cellular mechanisms of short-term and long-term memory. Behavioral training that produces long-term sensitization also induces long-term facilitation (LTF) of the monosynaptic connections between identified sensory neurons (SNs) and motor neurons (MNs) in Aplysia (Frost et al. 1985). Repeated application of serotonin (5HT), a transmitter released by tail shock (Glanzman 1989; Mercer et al. 1991), mimics the effects of tail shock in isolated ganglia and in cell cultures (Montarolo et al. 1986; Mercer et al. 1991; Emptage and Carew 1993; Clark and Kandel 1993; Ghirardi et al. 1995; Mauelshagen et al. 1996). Using the SN–MN synapse as a model system, considerable progress has been made recently in elucidating important synaptic, biophysical, and molecular steps involved in both short-term and long-term memory in Aplysia (Montarolo et al. 1986; Bacskai 1993; Emptage and Carew 1993; Bartsch et al. 1995; Homayouni et al. 1997; Zhang et al. 1997; for review, see Byrne and Kandel 1996; Carew 1996).
Three phases of facilitation have been described at Aplysia sensory–motor connections, both in the pleural–pedal ganglia (Mauelshagen et al. 1996) and in cell cultures (Ghirardi et al. 1995): short-term facilitation (STF), intermediate-term facilitation (ITF), and LTF. These three phases are defined by their temporal dynamics: STF lasts seconds to minutes (Walters et al. 1983b; Mauelshagen et al. 1996); ITF lasts 1–2 hr (Ghirardi et al. 1995; Mauelshagen et al. 1996); and LTF lasts >24 hr (Montarolo et al. 1986; Emptage et al. 1993; Clark and Kandel 1993; Zhang et al. 1997). They can also be distinguished by their different induction requirements and cellular mechanisms. In the pleural–pedal ganglion preparation, between one and four applications of 5HT (5 min each) induces STF, which is expressed immediately after 5HT exposure and lasts ∼15 min (Mauelshagen et al. 1996). This transient form of facilitation is thought to be caused by covalent modifications mediated by protein kinases A and C (PKA and PKC, respectively) (for review, see Byrne and Kandel 1996). Five applications of 5HT induce two additional phases: (1) a more prolonged form of facilitation, ITF, which is expressed immediately after 5HT exposure and lasts ∼90 min, and (2) LTF, which is not expressed until ∼10 hr after 5HT administration and lasts at least 1 day (Mauelshagen et al. 1996). In cultured SNs and MNs, ITF and LTF expression both require synthesis of new protein, but each relies on a different stage of protein synthesis; ITF is sensitive only to translational blockers (Ghirardi et al. 1995), whereas LTF is blocked by inhibitors of both transcription and translation (Montarolo et al. 1986). The cellular mechanisms underlying STF and LTF induction are likely independent because LTF can be induced under conditions that do not induce STF (Emptage and Carew 1993; see Clark and Kandel 1993 for a related observation in SNs and MNs in the abdominal ganglion).
In this study we compare the effects of massed and spaced patterns of 5HT application on the induction of ITF and LTF. We find that both massed and spaced 5HT applications induce ITF. However, massed applications produce LTF significantly less reliably than do spaced applications. A second analysis, exploring the relation between ITF and LTF expression, revealed that expression of ITF may be predictive of later LTF expression.
Some of the data presented in this paper have been reported previously in preliminary form (J. Mauelshagen, C.M. Sherff, and T.J. Carew, unpubl.).
Materials and Methods
PREPARATION
Wild-caught adult Aplysia californica (supplied by Marinus, Inc., Long Beach, CA, or Marine Specimens Unlimited, Pacific Palisades, CA) were anesthetized by injection of isotonic MgCl2 (100 ml/100 grams body weight). Single pleural–pedal ganglion pairs were dissected and bathed for 45–60 sec in 0.4% glutaraldehyde in artificial sea water (ASW, 460 mm NaCl, 55 mm MgCl2, 11 mm CaCl2, 10 mm KCl, 10 mm Tris at pH 7.5) to reduce contraction of connective tissue during application of 5HT (Sigma). The ganglia were pinned in a Sylgard (Dow-Corning)-coated recording dish, and desheathed to expose the somata clusters of tail SNs in the pleural ganglion and MNs in the pedal ganglion (Walters et al. 1983a; Mercer et al. 1991). Ganglia were bathed in a 1:1 mixture of ASW and isotonic MgCl2 to block synaptic transmission during dissection.
INTRACELLULAR RECORDINGS
During the experiment, the preparation was perfused with ASW (or 50 μm 5HT in ASW) at room temperature (20–22°C). The perfusion rate was adjusted to ∼5 ml/min. The preparation was transilluminated with a dark-field condenser. Tail SNs are located in a cluster of neurons in the pleural ganglion (Walters et al. 1983a). Tail MNs are found in a cluster at the base of the pleural–pedal connective in the pedal ganglion. Electrodes for intracellular recordings had resistances of between 6 and 12 MΩ when filled with 3 m KCl. Intracellular signals were amplified by an Axon2 intracellular amplifier (Axon Instruments) and a Getting intracellular amplifier (model 5A). Data were recorded and analyzed using the Spike acquisition and analysis program (Hilal Associates, Englewood Cliffs, NJ; sampling rate 16 kHz).
Throughout the experiments, resting potentials of the SNs were not manipulated experimentally, but the MNs were prevented from spiking by continuously hyperpolarizing them to −70 mV. The input resistance and resting potential of each neuron was checked frequently to judge the healthiness of the preparation. If any SN or MN had a resting potential <−30 mV or an input resistance <10 MΩ, the experiment was terminated (∼30% of all preparations). Average resting potentials and input resistances (±s.d.) for SNs were −44 ± 4 mV and 27 ± 8 MΩ, and for MNs were −49 ± 8 mV and 2 ± 6 MΩ.
Monosynaptic EPSPs in the MN were evoked by 30-msec depolarizing-current pulses in the SN. At the beginning of the experiment the amount of stimulating current in the SN was adjusted to evoke a single action potential; usually 1–2 nA. This same current pulse was used to evoke action potentials for the remainder of the experiment. EPSPs examined in this study ranged in amplitude from 3 to 10 mV.
EXPERIMENTAL PROTOCOL
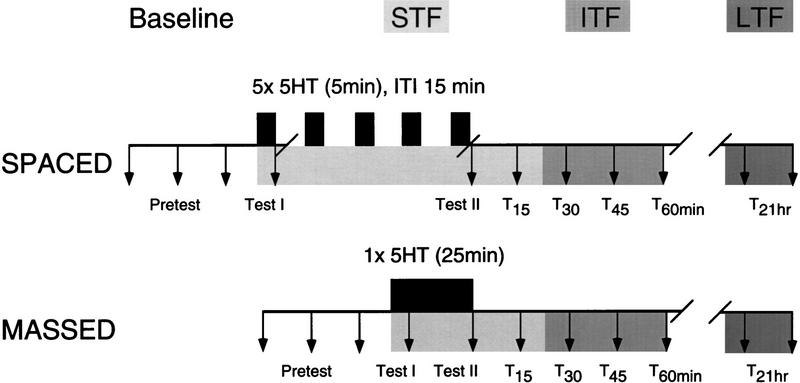
Synaptic facilitation of the tail SN–MN synapse was induced by either massed or spaced applications of 5HT to examine the effect of these different patterns of 5HT on (1) the time course and expression of ITF and (2) the expression of LTF. The experimental protocol is outlined in Figure 1. A nondepressed baseline EPSP amplitude was established by activating the SN three times at an interstimulus interval (ISI) of 15 min. If depression of >20% was observed, the preparation was discarded (17% of all preparations). There was no significant depression of the baseline EPSP amplitude in the remaining experiments; the amplitude of the third baseline test was not significantly different from the first (mean ± s.d. = 4 ± 16%, N.S., paired t-test). In one experimental group, ITF and LTF were induced by five applications of 50 μm 5HT (5 min each) at an intertrial interval (ITI) of 15 min (spaced induction). The other group received a single continuous application of 50 μm 5HT for 25 min (massed induction). Thus the patterning of 5HT application, but not the total time of 5HT exposure, differed between experimental groups.
Figure 1.
Diagram of experimental protocol for spaced and massed application of 5HT. Three pretests were recorded to establish the baseline EPSP amplitude. 5HT was then applied (solid bars) in one of two patterns: five 5-min pulses (spaced), or one continuous pulse of 25 min (massed). Synapses were tested for the expression of STF (light shading), ITF (medium shading), and LTF (dark shading) at the time points indicated by arrows.
Adhering to the 15-min ISI, we tested for STF (test I) either within 15 sec after the first (5-min) 5HT application in the spaced group or, correspondingly, after the first 5 min of 5HT exposure in the massed group. A second test for facilitation (test II) was taken immediately following the termination of 5HT exposure. To minimize damage to the cells caused by the longer recording time in the spaced group as compared to the massed group, the microelectrodes were removed after test I, and the same pair of cells was reimpaled after completion of the fifth 5HT application, at which time test II was performed immediately. If during reimpalement any spikes in the SN were elicited, test II was excluded from the analysis. In the massed group, recording was continued throughout the 5HT application, and test II was then taken within 15 sec of its completion. In both groups, the EPSP was tested subsequently every 15 min for 1 hr to monitor the time course of ITF expression (for review, see Mauelshagen et al. 1996). The microelectrodes were then removed, the perfusion was turned off, and the cells surrounding the SN and MN were marked by placing small crystals of DiI (Molecular Probes, Inc.) onto the somata using a microelectrode (Emptage and Carew 1993). After allowing the dye crystals to dissolve into the membrane for 10–15 min, the recording dish was immersed in a 50-ml Pyrex dish containing ASW and stored at 15°C until the long-term test. At 20–22 hr after the completion of the 5HT application the same pairs of neurons were reimpaled for two long-term tests (ISI = 15 min) measuring the expression of LTF.
DATA ANALYSIS AND STATISTICS
EPSP amplitudes were measured as the peak voltage of the EPSP. In cases of two summating EPSPs, if the first EPSP was still rising when the second started, the transition point between the first and second EPSP was taken as a conservative estimate of EPSP amplitude. For all experiments, the mean of the first three EPSP amplitudes (pretest) was used as a baseline measure. Only synapses with a stable baseline EPSP amplitude (all three pretests within 20% of the mean) were used. All EPSP amplitudes were expressed as a percent of this pretest baseline. The mean of the two long-term tests was used as the long-term test score. For statistical analysis, the mean ± s.e.m. was calculated for each group of EPSPs. Overall differences within each group (for example, all test points within the massed group) were first evaluated by an ANOVA for repeated measurements. Between-group comparisons (such as massed vs. spaced) were determined by an ANOVA and subsequent t-tests for independent means. Differences in the probability of LTF in spaced and massed conditions were examined by a χ2 test. All probability values described are two-tailed. Differences were considered significant if P < 0.05.
Results
At Aplysia tail SN–MN synapses, one to four spaced applications of 5HT (5 min/application) produce STF that decays back to baseline within ∼15 min after the last 5HT treatment, whereas five applications induce ITF that lasts at least 90 min (Mauelshagen et al. 1996). Five spaced applications of 5HT are also known to induce LTF at these synapses (e.g., Emptage and Carew 1993; Mauelshagen et al. 1996). We examined the importance of the temporal patterning of 5HT application by comparing the effects of two different patterns that provide the same total 5HT exposure: either (1) five spaced applications of 5HT (5 × 5 min), or (2) a single massed application (1 × 25 min) on the expression and time course of ITF, and the expression of LTF.
BOTH SPACED AND MASSED 5HT APPLICATIONS ARE CAPABLE OF INDUCING ITF AND LTF
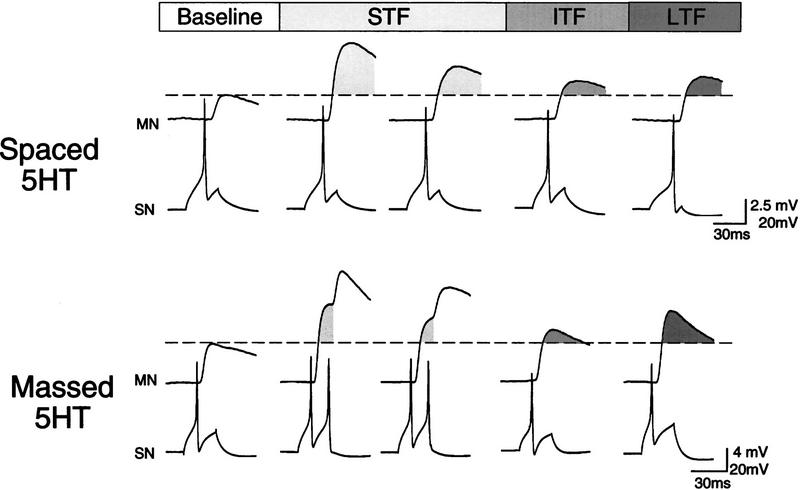
We compared the expression and time course of ITF over the first hour after spaced or massed 5HT treatment and the expression of LTF 21 hr later. Representative recordings showing STF (tests I and II), ITF (T60 min), and LTF (T21 hr) for both groups are shown in Figure 2. All three forms of facilitation were expressed in these two preparations. The increased SN excitability during the short-term tests (e.g., the massed example) is a 5HT-induced effect that is often seen after either spaced or massed 5HT exposure (Emptage and Carew 1993; Mauelshagen et al. 1996).
Figure 2.
Sample recordings showing EPSP amplitude at representative short-term, intermediate-term, and long-term time points from two preparations that received either spaced (5 × 15 min) or massed (1 × 25 min) application of 5HT. STF, ITF, and LTF were all expressed with both the spaced and massed protocols. In each set of traces, the dashed line represents the baseline EPSP amplitude and the shaded regions (light shading for STF, medium shading for ITF, and dark shading for LTF) indicate change from baseline.
SPACED APPLICATION OF 5HT IS MORE EFFECTIVE IN INDUCING BOTH ITF AND LTF THAN IS MASSED APPLICATION
The composite results of all test points for preparations that received spaced (n = 10) or massed (n = 9) 5HT applications are summarized in Figure 3. Because STF decays in 15 min or less after 5HT, the facilitation observed at T30, T45, and T60 can be attributed to the expression of ITF (Mauelshagen et al. 1996). In the spaced group, the EPSP amplitude was facilitated significantly at all short-term, intermediate-term, and long-term test points [ANOVA: F(7) = 2.88, P < 0.03, paired comparisons: P < 0.04 in all cases]. Moreover, as in previous results with this preparation (Emptage and Carew 1993; Mauelshagen et al. 1996), facilitation at the short-term test (TI) was not significantly different from facilitation at the long-term test.
Figure 3.
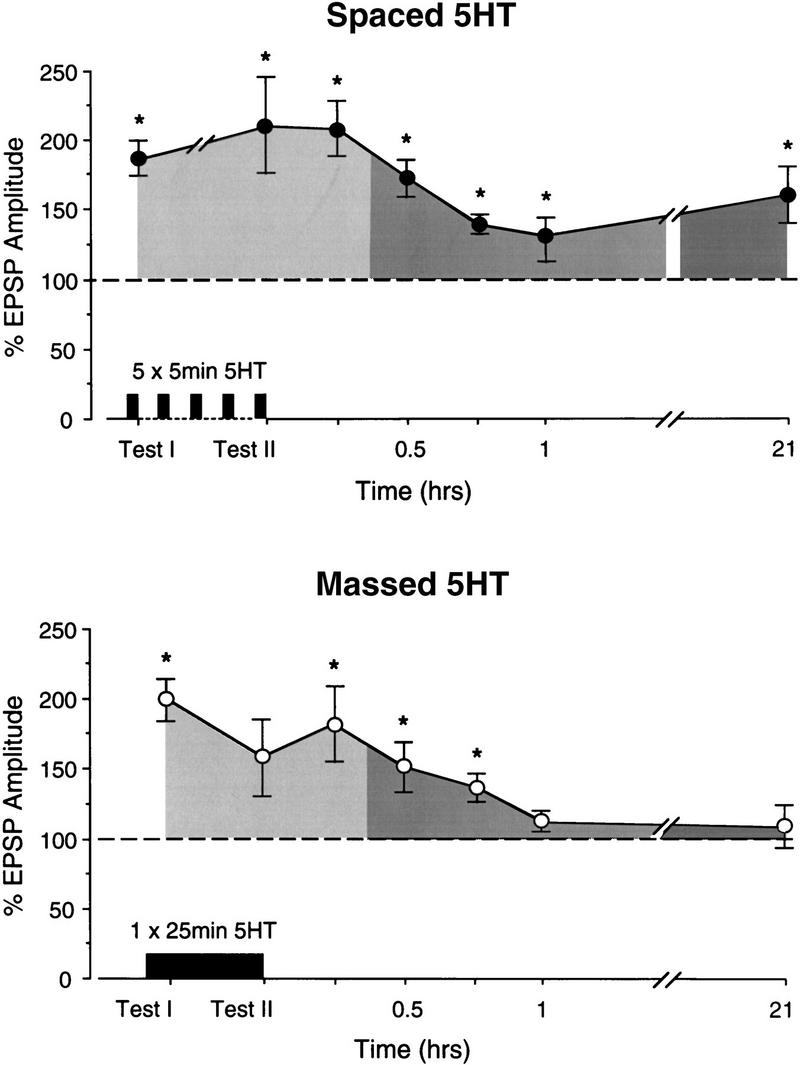
Summary of results. Significant STF and ITF were expressed with either spaced or massed application of 5HT. Significant LTF was expressed in the spaced group but not in the massed group. (*) Significant facilitation from baseline (P < 0.05). For the spaced group (top), from left to right, n = 10, 6, 8, 7, 8, 9, and 10. For the massed group (bottom), n = 9 for all tests except T45, where n = 7.
There was also significant facilitation exhibited by the massed group [ANOVA: F(7) = 3.81, P < 0.004; Fig. 3]. Paired comparisons revealed significant STF [t(8) = 6.65, P < 0.001] and ITF at T30 and T45 [t(7) = 3.05 and t(6) = 3.69, P < 0.02], but the ITF was less pronounced and decayed more quickly than in the spaced group. For example, whereas the 1-hr time point was still elevated significantly in the spaced group, it was not significantly different from baseline in the massed group [T60: t(8) = 1.68, N.S.]. In addition, although massed 5HT application sometimes induced LTF similar to that induced by spaced application (as shown in Fig. 2) there was on average no significant LTF expressed in the massed group [t(8) = 0.6, N.S.; Fig. 3]. Consistent with this observation, the EPSP amplitude at the short-term test (TI) in the massed group was significantly greater than the EPSP amplitude at the long-term test [t(8) = 5.06, P < 0.002]. These data show that spaced applications of 5HT are significantly better at inducing LTF at the sensorimotor synapse than are massed applications.
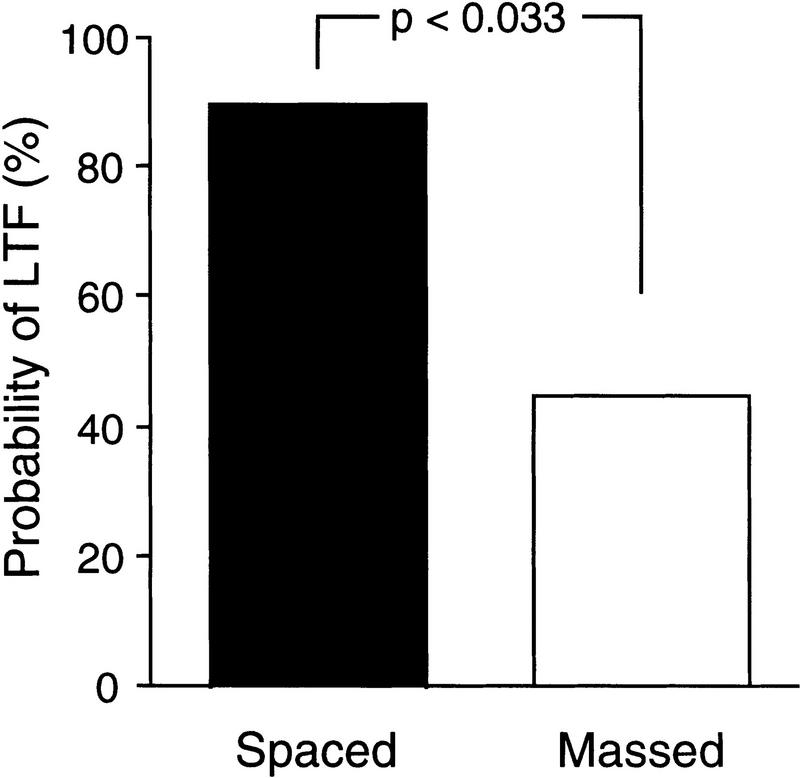
THE PROBABILITY OF INDUCING LTF IS LOWER AFTER MASSED THAN AFTER SPACED 5HT APPLICATIONS
Taken alone, the results for the massed group shown in Figure 3 could reflect the fact that this pattern of 5HT exposure is simply incapable of inducing LTF. Since we did observe expression of LTF in some preparations in the massed group (as shown in the example in Fig. 2), a more likely possibility is that massed training is capable of inducing LTF but with a lower probability than spaced training. To evaluate this possibility, we determined the number of preparations in which the EPSP amplitude in the long-term test was above (LT+) or below (LT−) baseline. In the spaced group, 9 of 10 preparations were LT+ (Fig. 4), confirming previous results (Emptage and Carew 1993; Mauelshagen et al. 1996). However, after massed 5HT applications, only four of nine preparations had EPSP amplitudes above baseline at the long-term test. A χ2 analysis revealed that the probability of inducing LTF after massed 5HT applications was significantly lower than after spaced 5HT applications [χ2(1) = 4.55, P < 0.033; see Fig. 4]. These results suggest that, although both massed and spaced application of 5HT can induce ITF and LTF, under our experimental conditions massed applications induce LTF significantly less reliably.
Figure 4.
The probability of inducing LTF is higher with spaced application (n = 10) of 5HT than with massed application (n = 9). Each bar represents the percentage of experiments in which the EPSP amplitude, measured 21 hr after 5HT application, was significantly higher than baseline.
WITH MASSED 5HT APPLICATIONS, EXPRESSION OF ITF PREDICTS THE EXPRESSION OF LTF
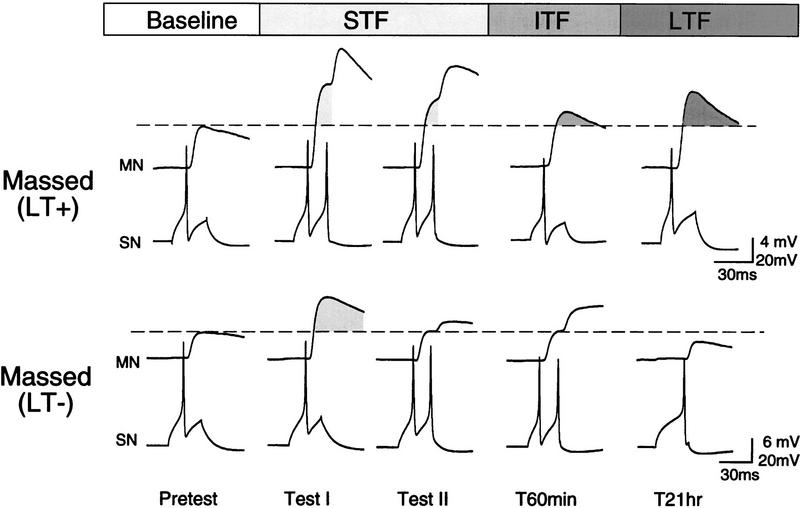
To explore the relationship between ITF and LTF, we took advantage of the fact that massed 5HT applications resulted in similar numbers of preparations that did (n = 4) and did not (n = 5) produce LTF. Figures 5 and 6 show examples and summary graphs for the subsets of preparations in which massed 5HT applications either induced LTF (LT+) or did not (LT−). As illustrated by the sample recording for the LT+ group (Fig. 5, top), the EPSP amplitude was, as in the spaced group (see Fig. 2), above baseline at test I and test II, T60 min, and the 21-hr test. The summary graph in Figure 6 confirms that, despite the small number of preparations, the EPSP was significantly facilitated at all test points [ANOVA: F(7) = 2.88, P < 0.03; P < 0.05 for TI through T60; because the data were selected on the basis of a LT+ score, the 21-hr test was not included in the statistical analysis]. There was no significant difference in the amplitude of LTF expressed in the spaced group and the LT+ group [F(1) = 0.31, N.S.], indicating that, in cases in which LTF is induced during massed application of 5HT, ITF and LTF expression appear to be indistinguishable from that induced by spaced application (cf. Figs. 6, top, with 3, top).
Figure 5.
Examples of two preparations that received a massed application of 5HT, one of which expressed LTF (LT+), the other of which did not (LT−). ITF was expressed only in preparations that later expressed LTF. Shading indicates change from baseline (broken line).
Figure 6.
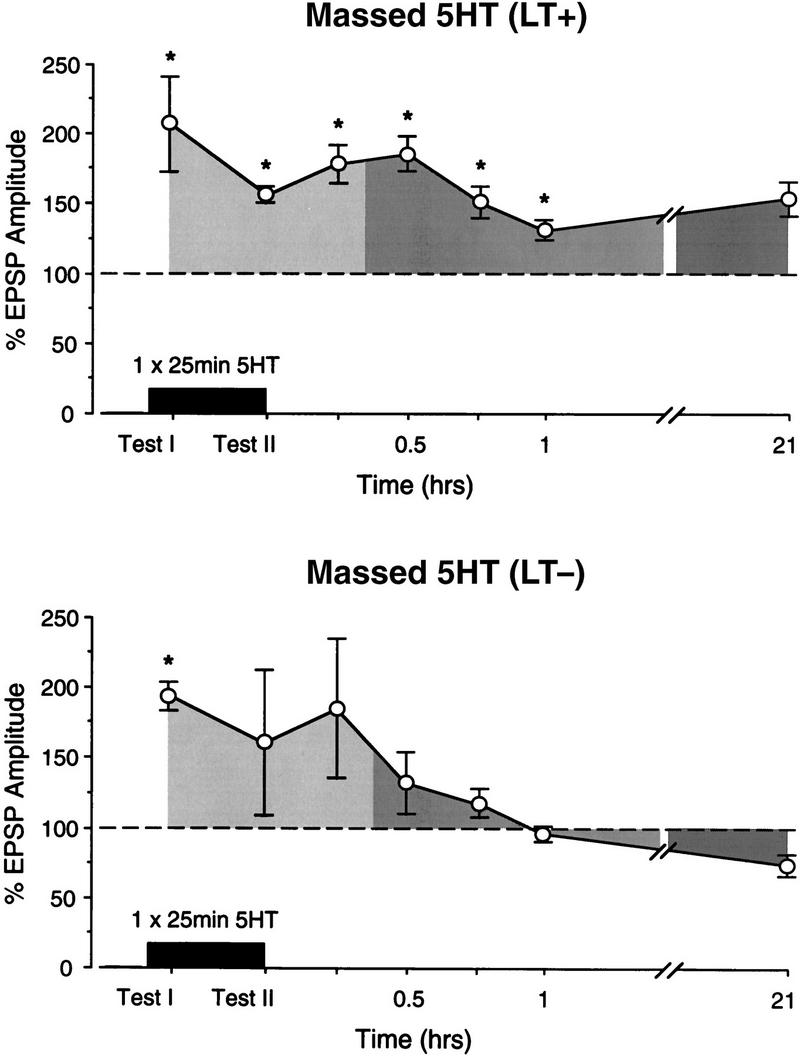
LTF expression is associated with ITF expression with massed application of 5HT. Summary graphs of the preparations that received massed application of 5HT, divided into those that expressed LTF (LT+, top) and those that did not (LT−, bottom). Significant ITF was seen only in the group that also expressed LTF. Shading indicates change from baseline at STF (light), ITF (medium), and LTF (dark) time points. Because the two groups were selected according to their T21-hr test score, this point was not included in the statistical analysis. n = 4 (LT+); n = 5 (LT−).
In contrast, the example and summary graph of the LT− massed group in Figures 5 and 6 indicate that, in those preparations in which massed 5HT applications did not induce LTF, there was also no significant ITF [F(7) = 2.18, N.S.]. A between-group ANOVA revealed an overall difference in ITF between the LT+ and LT− groups [F(1) = 4.53, P < 0.005]. Moreover, the EPSP amplitude at the T60 time point in group LT+ was significantly larger than that in the group LT− [t(7) = 4.25, P < 0.004], indicating that the time course of ITF in the preparations that later expressed LTF were significantly longer than in those in which LTF was not induced.
Discussion
In this paper we examined the effects of massed and spaced applications of 5HT on the induction and expression of ITF and LTF at Aplysia tail SN–MN synapses. The most pronounced effects were observed in the differential expression of LTF, in which the probability of LTF induction was significantly lower after massed than after spaced 5HT application. ITF expression was also sensitive to the pattern of stimulation, decaying to baseline significantly more rapidly after massed than after spaced patterns of 5HT application.
ITF
In previous experiments examining this synapse, we showed that one to four applications of 5HT (5 min each) spaced by 15 min, typically induces only STF that decays back to baseline levels after ∼15 min, whereas five spaced applications induce ITF that lasts at least 90 min after the last 5HT pulse (Mauelshagen et al. 1996). Consistent with this previous work, in this paper we have identified ITF by its prolonged time course and its requirement of five pulses of 5HT. Thus any significant facilitation at 30, 45, and 60 min after the completion of 5HT treatment was considered to reflect ITF. We found that the expression of ITF is only moderately sensitive to the pattern of the five pulses of 5HT. Either a single continuous application lasting 25 min or five spaced applications of 5 min each induced significant ITF (at time points T30 and T45); however, the ITF induced by massed application of 5HT is not as robust, decaying to baseline significantly sooner. The difference in the profiles of ITF expression seen after massed and spaced stimuli may reflect a lower probability of ITF induction with massed application, as was observed in the induction of LTF (see below).
The cellular machinery necessary for the induction and expression of ITF can be activated in a relatively short time period (compared to LTF), within 25 min, and appears to be less sensitive to the pattern of 5HT exposure than are the mechanisms responsible for the induction of LTF. Because the SN synapses onto tail MNs are quite far from the soma (several mm), the rapidity of ITF induction and its expression at the synapse suggest that these processes do not require activation of transcriptional machinery in the nucleus of the SN. This, in turn, suggests that induction of ITF can be independent of LTF induction, which (using spaced 5HT applications) is known to require transcription and translation of new proteins up to half an hour after five pulses of 5HT (Montarolo et al. 1986; Ghirardi et al. 1995). However, the ITF that we observed may still be dependent on the translation of new proteins. The first demonstration of ITF in Aplysia SNs was provided in cultured SNs by Ghirardi et al. (1995), who showed that a defining feature of ITF was its susceptibility to translational, but not transcriptional inhibitors. Given the distance from SN soma to MN synapses in the pleural–pedal ganglia that we used in our experiments, the time required to transport newly synthesized proteins from the SN soma to the terminals does not fit easily with the rapid expression of ITF following massed or spaced application of 5HT. Thus, the molecular changes responsible for ITF induction are likely restricted to the vicinity of the SN terminals, or alternatively, may be located in the postsynaptic region of the MN (Steward and Banker 1992; Lin and Glanzman 1994; Murphy and Glanzman 1996; Bao et al. 1998); these of course are not mutually exclusive possibilities.
Considering that both spaced and massed 5HT applications can induce ITF, one possible model consistent with our results is that some intracellular signal accumulates during 5HT exposure (and is not degraded during the rest interval between spaced 5HT applications) and, after a certain exposure time, reaches a critical threshold level required to induce ITF. One mechanism that could contribute to the relatively reduced efficacy of massed 5HT exposure is desensitization of 5HT receptors from the prolonged exposure to 5HT during massed application. If receptor desensitization is involved, it is unlikely to be the only mechanism because in other studies examining LTF, even longer continuous 5HT applications have produced significant long-lasting facilitation (Emptage and Carew 1993; Ghirardi et al. 1995; Zhang et al. 1997). Finally, given that LTF in this system can be induced by 5HT restricted to the soma (Emptage and Carew 1993), and given the suggestion above that ITF induction is restricted to the synaptic region, it is plausible that the induction of ITF and LTF can occur in spatially separate cellular compartments within the SN. Even if this hypothesis is correct, we cannot rule out the possibility that ITF expression may play a facilitatory role in LTF induction. This possibility takes on greater significance in light of recent findings by C.M. Sherff and T.J. Carew (unpubl.), who showed that local, brief (5-min) exposure of the SN synapse to 5HT can significantly facilitate the expression of LTF induced by 5HT at the soma.
LTF
Whereas there is only a modest difference in ITF expression induced by spaced and massed 5HT applications, LTF was induced with a significantly lower probability after massed 5HT applications than after spaced applications. The sensitivity of LTF induction to the temporal pattern of 5HT exposure is interesting at both the cellular and behavioral levels. At the cellular level, it will be of considerable interest to determine the mechanisms responsible for enhanced LTF when there are rest intervals between 5HT exposures. Important clues can be obtained from recent studies in Drosophila and Aplysia examining the molecular mechanisms contributing to long-term memory. These studies have provided evidence that cAMP response element-binding proteins (CREBs), a class of transcription factors, are critically involved in inducing long-term memory (LTM) in Drosophila (Yin et al. 1994) and LTF in Aplysia (Kaang et al. 1993; Alberini et al. 1994) via gene activation and that activator and repressor isoforms of CREB can induce or inhibit LTM/LTF induction, respectively (Dash et al. 1990; Alberini et al. 1994; Bartsch et al. 1995; Yin et al. 1995).
Of particular relevance to this paper, massed and spaced training sessions in an olfactory avoidance learning task in Drosophila induce two parallel forms of long-lasting memory that differ in their duration and dependence on protein synthesis (Tully et al. 1994; Yin et al. 1994). To account for these results Yin et al. (1995) developed a conceptual model of the underlying molecular mechanisms. The model proposes equal activation of activator and repressor isoforms of CREB during training, but differential breakdown kinetics during the rest interval accompanying spaced training sessions, which allows the (posited) more slowly degrading CREB activator to build up during multiple spaced training sessions. Such an accrual of CREB activator, which is hypothesized to be responsible for long-term memory induction, does not occur during massed training because the rest intervals required to allow CREB activator levels to exceed CREB repressor levels do not occur.
The interplay between activator and repressor isoforms of CREB have also been directly implicated in the induction of LTF in Aplysia. For example, interference with CREB activation or downstream effects of CREB in the SNs blocks the induction of LTF yet leaves STF intact (Dash et al. 1990; Alberini et al. 1994). Moreover, blocking an inhibitory isoform of CREB enables procedures that normally induce only STF (e.g., a single pulse of 5HT) to produce long-term functional and structural changes in SNs (Bartsch et al. 1995). Using these and related observations it will be of considerable interest to explore the cellular and molecular mechanisms contributing to the differential effectiveness of spaced and massed 5HT exposures in the induction of LTF. In considering such mechanisms it is important to stress that prolonged and continuous exposure of the SNs to 5HT is certainly capable of inducing LTF, not only in the present studies, but under several other experimental conditions (Emptage and Carew 1993; Ghirardi et al. 1995; Zhang et al. 1997). Thus any complete mechanistic explanation will have to account not only for cases in which continuous 5HT exposure is inferior to distributed exposure in the induction of LTF (as in the present paper), but also for cases in which continuous prolonged exposure does in fact induce significant LTF. Finally, although STF, ITF, and LTF can have clearly different induction requirements, our results suggest that there are not sharp boundaries between these different phases of facilitation, which give rise, in all-or-none fashion, to the induction of one phase over another. Rather, there appear to be temporal domains in which the probability of inducing one or another form of facilitation depends on the nature and pattern of the modulatory events that initiate facilitation. From this perspective it will be of considerable interest to identify the cellular and molecular mechanisms that can shift the probability of induction of specific phases of facilitation.
A final important question concerns the degree to which these different, presumably functionally independent forms of synaptic plasticity induced by 5HT are relevant for the behavioral expression of memory in Aplysia. Assuming that 5HT-induced synaptic facilitation is an important component of behavioral sensitization (Brunelli et al. 1976; Walters et al. 1983b; Abrams et al. 1984; Glanzman et al. 1989), our data are in agreement with the well-known behavioral phenomenon that spaced training sessions are typically superior to massed training sessions in producing LTM (Davis et al. 1970; Carew et al. 1972; Tully et al. 1994; Yin et al. 1994). Thus the preparation we have developed now permits the study of possible cellular analogs of different memory phases and their dependence on different training schedules, such as spaced and massed training, and provides unique access to examining the molecular mechanisms underlying multiphasic forms of memory processing. Multiphasic memory processing has been suggested for many other vertebrate and invertebrate systems (McGaugh 1966; Gibbs and Ng 1979; Rosenzweig and Bennett 1984; Ewing et al. 1985; deZazzo and Tully 1995; Hammer and Menzel 1995). Thus it is possible that an overall behavioral retention profile for sensitization of Aplysia is actually composed of multiple phases that can be dissociated by their time course, and by their dependence on intensity, duration, or pattern of training stimuli. Though these phases have not yet been investigated for behavioral sensitization in Aplysia, a multiphasic retention profile in Aplysia feeding behavior has been described recently (D. Botzer, A. Weller, A.J. Susswein, unpubl.). Such a composite retention profile may reflect the dynamics of multiple memory systems, common to most animals, that may have evolved across very different species to provide optimal adaptation to changing environmental conditions.
Acknowledgments
We thank Thomas M. Fischer and Stephen A. Fisher for thoughtful criticism of an earlier draft of this paper. This research was supported by a Deutsche Forschungsgemeinschaft grant to J.M., a National Research Service Award MH12004 to C.M.S., and a National Science Foundation grant (BNS 831130) and a National Institutes of Health grant (MH41083) to T.J.C. We also acknowledge here the loss of a coauthor of this paper, Juliane Mauelshagen, a valued friend and colleague, who died in a climbing accident on October 4, 1996.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Abrams TW, Castellucci VF, Camardo JS, Kandel ER, Lloyd PE. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci. 1984;81:7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamic of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: Relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Bao J-X, Kandel ER, Hawkins RD. Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1998;18:458–466. doi: 10.1523/JNEUROSCI.18-01-00458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M, Castellucci VF, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: Possible role of serotonin and cAMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Bugelski BR. Presentation time, total time, and mediation in paired-associate learning. J Exp Psychol. 1962;63:409–412. doi: 10.1037/h0045665. [DOI] [PubMed] [Google Scholar]
- Byrne JH. Cellular analysis of associative learning. Physiol Rev. 1987;67:329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: State and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Sahley CL. Invertebrate learning and memory: From behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971;2:79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Pinsker HM, Kandel ER. Long-term habituation of a defensive withdrawal reflex in Aplysia. Science. 1972;175:451–454. doi: 10.1126/science.175.4020.451. [DOI] [PubMed] [Google Scholar]
- Carew TJ. Molecular enhancement of memory formation. Neuron. 1996;16:5–8. doi: 10.1016/s0896-6273(00)80016-1. [DOI] [PubMed] [Google Scholar]
- Clark GA, Kandel ER. Induction of long-term facilitation in Aplysia sensory neurons by local application of serotonin to remote synapses. Proc Natl Acad Sci. 1993;90:11411–11415. doi: 10.1073/pnas.90.23.11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EH, Pantle AJ. The total-time hypothesis in verbal learning. Psychol Bull. 1967;68:221–234. doi: 10.1037/h0025052. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Davis M. Effects of interstimulus interval length and variability on startle response habituation. J Comp Physiol Psychol. 1970;72:177–192. doi: 10.1037/h0029472. [DOI] [PubMed] [Google Scholar]
- deZazzo J, Tully T. Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Neurogenetic dissection of learning and short-term memory in Drosophila. Annu Rev Neurosci. 1989;11:537–563. doi: 10.1146/annurev.ne.11.030188.002541. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H. Uber das Gedachtnis. New York, NY: Dover; 1885. [Google Scholar]
- Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- Ewing MF, Larew MB, Wagner AR. Distribution-of-trials effects in Pavlovian conditioning: An apparent involvement of inhibitory backward conditioning with short intertrial intervals. J Exp Psychol. 1985;11:537–547. [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections from the sensory neurons of the gill-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of Aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Ng KT. Behavioural stages in memory formation. Neurosci Lett. 1979;13:279–283. doi: 10.1016/0304-3940(79)91507-6. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Learning and memory in the honeybee. J Neurosci. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Clark G, Kandel ER. Cell biological studies of learning in simple vertebrate and invertebrate systems. In: Mountcastle V, Plum F, editors. Handbook of physiology. Bethesda, MD: The American Physiological Society; 1987. pp. 25–83. [Google Scholar]
- Hintzman DL. Theoretical implications of the spacing effect. In: Solso RL, editor. Theories in cognitive psychology: The Loyola symposium. Hillsdale, NJ: Lawrence Erlbaum; 1974. pp. 77–99. [Google Scholar]
- Homayouni R, Nunez-Rerueiro M, Byrne JH, Eskin A. Identification of two phosphoproteins affected by serotonin in Aplysia sensory neurons. Brain Res. 1997;750:87–94. doi: 10.1016/s0006-8993(96)01335-2. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of behavior. New York, NY: Appleton-Century-Crofts; 1943. [Google Scholar]
- Kaang B-K, Kandel ER, Grant SGN. Activation of cAMP-responsive genes by stimuli that produce long-term facilitation in Aplysia sensory neurons. Neuron. 1993;10:427–435. doi: 10.1016/0896-6273(93)90331-k. [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: Regulation by postsynaptic voltage. Proc Natl Acad Sci. 1994;255:113–118. doi: 10.1098/rspb.1994.0016. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Parker GR, Carew TJ. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Mercer AR, Emptage NJ, Carew TJ. Pharmacological dissociation of modulatory effects of serotonin in Aplysia sensory neurons. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL. Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+ Proc Natl Acad Sci. 1996;93:9931–9936. doi: 10.1073/pnas.93.18.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Bennett EL. Direct processes and modulatory influences in the stages of memory formation. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of learning and memory. New York, NY: Guilford Press; 1984. pp. 263–288. [Google Scholar]
- Squire LR. Memory and brain. New York, NY: Oxford University Press; 1987. [Google Scholar]
- Steward O, Banker GA. Getting the message from the gene to the synapse: Sorting and intracellular transport of RNA in neurons. Trends Neurosci. 1992;15:180–186. doi: 10.1016/0166-2236(92)90170-d. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983a;50:1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J Neurophysiol. 1983b;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. Experimental psychology. New York, NY: Holt, Rinehart and Winston; 1938. [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quin WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yin JC, DelVecchio M, Zhou H, Tully T. CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Zacks RT. Invariance of total learning time under different conditions of practice. J Exp Psychol. 1969;82:441–447. [Google Scholar]
- Zhang F, Endo S, Cleary LJ, Eskin A, Byrne JH. Role of transforming growth factor-beta in long-term synaptic facilitation in Aplysia. Science. 1997;275:1318–1320. doi: 10.1126/science.275.5304.1318. [DOI] [PubMed] [Google Scholar]