Abstract
Previous results have suggested that cGMP is involved in hippocampal long-term potentiation (LTP), perhaps as the presynaptic effector of a retrograde messenger. However, other studies have failed to replicate some of those results, making the role of cGMP uncertain. We therefore reexamined this question and identified several variables that can affect the contribution of cGMP. First, brief perfusion with 8-Br–cGMP before weak tetanic stimulation produced long-lasting potentiation in the CA1 region of hippocampal slices, but more prolonged perfusion with 8-Br–cGMP before the tetanus did not produce long-lasting potentiation. Second, the activity-dependent long-lasting potentiation by cGMP analogs was reduced when NMDA receptors were completely blocked, indicating that NMDA receptor activation contributes to, but is not required for, the potentiation. The amount of reduction of the potentiation differed with different protocols, and in some cases could be complete. Third, LTP produced by strong tetanic stimulation in the stratum radiatum of CA1 (which expresses eNOS) was blocked by inhibitors of soluble guanylyl cyclase or cGMP-dependent protein kinase, but LTP in the stratum oriens (which does not express eNOS) was not. The results of these experiments should help to explain some of the discrepant findings from previous studies, and, in addition, may provide insights into the mechanisms and functional role of the cGMP-dependent component of LTP.
Several lines of evidence suggest that cGMP is involved in long-term potentiation (LTP) in the hippocampus, perhaps as the presynaptic effector of a retrograde messenger. Three major candidate retrograde messengers for LTP, arachidonic acid, nitric oxide, and carbon monoxide (Williams et al. 1989; Stevens and Wang 1993; Zhuo et al. 1993), all stimulate soluble guanylyl cyclase to produce cGMP (Snider et al. 1984; Garthwaite et al. 1988; Verma et al. 1993). Moreover, several laboratories have now found that inhibitors of guanylyl cyclase or cGMP-dependent protein kinase can block the induction of LTP (Zhuo et al. 1994a; Blitzer et al. 1995; Boulton et al. 1995) and that membrane-permeable analogs of cGMP can produce long-lasting potentiation if they are applied at the same time as spike activity in the presynaptic fibers (Haley et al. 1992; Zhuo et al. 1994a). The activity is thought to make the presynaptic terminals responsive to a diffusible retrograde messenger, thus preserving the pathway specificity of LTP (Hawkins et al. 1993). Consistent with that idea, cGMP analogs can still produce activity-dependent long-lasting potentiation in the presence of AP5, an antagonist of N-methyl-d-aspartate (NMDA) receptors (Zhuo et al. 1994a), or L-NAME, an inhibitor of nitric oxide synthase (Haley et al. 1992), suggesting that exogenous cGMP can bypass postsynaptic events in the induction of LTP. Additional support for this hypothesis has come from experiments on hippocampal neurons in dissociated cell culture, where intracellular injection of cGMP into the presynaptic neuron can produce activity-dependent long-lasting potentiation in the presence of AP5 (Arancio et al. 1995).
However, the role of cGMP in long-lasting potentiation remains uncertain, in part because some studies have failed to replicate either activity-dependent long-lasting potentiation by cGMP analogs or block of LTP by inhibitors of cGMP-dependent protein kinase (Schuman et al. 1994; Selig et al. 1996). We therefore have reexamined this question in several ways: First, we have replicated the original findings of Zhuo et al. (1994a); second, we have attempted to find experimental variables that might account for the different results in different studies; and third, we have used additional, independent methods to test the role of cGMP in LTP. These studies have revealed that cGMP plays an important role in LTP under some circumstances but not others and thus may provide insights into the mechanisms and functional role of the cGMP-dependent component of LTP.
Materials and Methods
Male guinea pigs 3–5 weeks of age and male C57 mice 3–4 months of age were housed and sacrificed in accordance with the guidelines of the Health Sciences Division of Columbia University. Transverse slices of hippocampus (400 μm) were maintained in an interface chamber at 29°C, where they were subfused with saline (ACSF) consisting of 124 mm NaCl, 4.4 mm KCl, 1.0 mm Na2HPO4, 25 mm NaHCO3, 2.0 mm or 2.5 mm CaCl2, 2.0 mm or 1.3 mm MgSO4, 10 mm glucose, bubbled with 95% O2 and 5% CO2. A bipolar tungsten stimulating electrode was placed in the middle of the stratum radiatum in the CA1 region, and extracellular field potentials were recorded using a glass micropipette (5–10 MΩ, filled with ACSF) also in the s. radiatum in CA1. In some experiments both electrodes were placed in the stratum oriens. For two-pathway experiments, a second stimulating electrode was placed on the opposite side of the recording electrode, and the two pathways were stimulated alternately. The pulse duration was 10 or 50 μsec, and test responses were elicited at 0.016 or 0.02 Hz. The perfusion rate of ACSF was ∼1.5–2.0 ml/min. To increase the effectiveness of drugs that were applied through the perfusion system, the ACSF level in the recording chamber was sufficiently high to cover the slice but not to float it. 8-Br–cGMP (Biolog) was directly dissolved in ACSF at the desired concentration immediately before use. The other drugs were made as stock solutions and then diluted to the desired concentration in ACSF. DL-AP5 (RBI), MK801 (RBI), 8-pCPT–cGMP (Biolog), and Rp-8-Br–cGMPS (Biolog) were dissolved in distilled water, and CNQX (RBI), 7-chlorokynurenic acid (Tocris), ODQ (Alexis), and KT5823 (Calbiochem) were dissolved in DMSO. The final concentration of DMSO was <0.1%. The time of perfusion with the drugs is described with respect to the time of their arrival in the recording chamber, which was estimated by perfusion with dye in preliminary experiments.
For the culture experiments, dissociated cell cultures of hippocampal neurons from 1- to 2-day-old Sprague–Dawley rats were prepared as described previously (O’Dell et al. 1991). The electrophysiological methods were also as described previously (Arancio et al. 1995). cGMP was applied intracellularly by means of a fast internal perfusion method that allowed control of the timing of the perfusion. A perfluoroethylenepropylene (FEP) tube was pulled to a very fine diameter, filled with the electrode solution and cGMP (50 μm), and inserted to within 300 μm of the tip of the electrode. At the time of injection, a motorized micrometer spindle began pushing a Hamilton microliter syringe filled with Nujol mineral oil and connected to the FEP tubing. Two minutes after the beginning of injection at a rate of 2 μl/min, weak tetanic stimulation was delivered to the presynaptic neuron. The injection was stopped immediately after the end of the tetanic stimulation, allowing the drug to be diluted in the much larger volume of the electrode (∼80 μl).
Results
cGMP ANALOGS PRODUCE ACTIVITY-DEPENDENT LONG-LASTING POTENTIATION
Replicating the results of Zhuo et al. (1994a), we found that perfusion of slices of guinea pig hippocampus with the membrane-permeable analog 8-Br–cGMP (100 μm) for 5 min before weak tetanic stimulation (50 Hz, 0.5 sec) resulted in rapid potentiation of the excitatory postsynaptic potential (EPSP) that lasted for at least 1 hr [183.6 ± 12.8%, n = 4, t(3) = 6.53, P < 0.01 comparing the mean EPSP 55–60 min post-tetanus to the average for 15 min before the start of 8-Br–cGMP perfusion; Fig. 1A]. In contrast, perfusion with 8-Br–cGMP alone did not produce either a significant change in synaptic transmission during the perfusion or significant long-lasting potentiation after the perfusion (109.7 ± 11.4%, n = 3; Fig. 1B). Similarly, weak tetanic stimulation alone produced post-tetanic potentiation (PTP) and short-term potentiation (STP) but no LTP (102.6 ± 1.9%, n = 5; Fig. 1C). Thus, 8-Br–cGMP and weak tetanus act synergistically and not simply additively to produce long-lasting potentiation.
Figure 1.
Long-lasting potentiation by 8-Br–cGMP plus weak tetanus. (A) Perfusion with 8-Br–cGMP (horizontal bar) for 5 min before weak tetanic stimulation (arrow) resulted in rapid and long-lasting potentiation. The initial slope of the EPSP has been normalized to the average baseline value during the 15 min before perfusion with 8-Br–cGMP in each experiment (broken line). The points represent the means, and the error bars represent the s.e.s of the means. (Inset) Representative records of the EPSP before and 60 min after the weak tetanus. Scale bars, 2 mV, 5 msec. (B) Perfusion with 8-Br–cGMP alone had no effect on the EPSP. (C) Weak tetanic stimulation alone produced PTP and STP but no LTP. Average baseline values: −0.27 mV/msec (A); −0.24 (B); −0.36 (C); not significantly different by a one-way ANOVA.
Some of the long-lasting potentiation in these experiments could be attributable to an upward drift in the baseline following perfusion with 8-Br–cGMP. The fact that 8-Br–cGMP alone had no effect argues against that possibility. As another control, we performed two-pathway experiments in which weak tetanic stimulation was delivered to only one pathway. Perfusion with 8-Br–cGMP for 5 min before the weak tetanic stimulation resulted in rapid and long-lasting potentiation only in the stimulated pathway [157.4 ± 15.3% vs. 110.9 ± 7.0%, n = 6, t(5) = 4.61, P < 0.01; Fig. 2A]. This result indicates that the potentiation is not owing to baseline drift and is consistent with the hypothesis that activity dependence of the effects of cGMP preserves the pathway specificity of LTP.
Figure 2.
Potentiation by 8-Br–cGMP plus weak tetanus is restricted to the stimulated pathway in both guinea pigs and mice. (A) In two-pathway experiments in guinea pigs, perfusion with 8-Br–cGMP for 5 min before weak tetanic stimulation resulted in rapid and long-lasting potentiation in the tetanized pathway (•) but not in the control pathway (○). The slices were also perfused wih MK801 (10 μm) for 20 min before the tetanus. (B) Similar two-pathway experiments in mice. Average baseline values: −0.29 mV/msec (tetanized, A); −0.27 (control, A); −0.34 (tetanized, B); −0.32 (control, B); not significantly different.
Previous studies that failed to replicate activity-dependent potentiation by cGMP analogs (Schuman et al. 1994; Selig et al. 1996) used experimental methods that differed in many ways from the methods used in these studies and previous studies that demonstrated potentiation (Zhuo et al. 1994a). We therefore attempted to find experimental variables that might account for the different results in the different studies. We obtained qualitatively similar results when we used different Ca2+ and Mg2+ concentrations in the ACSF (either 2.0 mm Ca2+ and 2.0 mm Mg2+ or 2.5 mm Ca2+ and 1.3 mm Mg2+) and also when we used different methods for setting the test stimulation intensity (producing baseline EPSPs with either an amplitude of 1.0 mV or a slope of 30%–35% maximum), suggesting that those are not important variables (data not shown). The different studies also used different species. To determine whether our results are peculiar to guinea pigs, we performed similar experiments on hippocampal slices from mice. As in guinea pigs, in two-pathway experiments, perfusion with 8-Br–cGMP for 5 min before weak tetanic stimulation of one pathway resulted in rapid and long-lasting potentiation only in the stimulated pathway [142.6 ± 19.6% vs. 96.5 ± 8.8%, n = 7, t(6) = 2.64, P < 0.05; Fig. 2B]. This result suggests that cGMP may play a role in LTP in mice and other rodents as well as guinea pigs.
To further test the generality of our results, we also tried a different analog, 8-pCPT–cGMP, that is more membrane permeable and more selective for activation of cGMP-dependent protein kinase (Geiger et al. 1992). Perfusion with 8-pCPT–cGMP (10 μm) for 10 min before weak tetanic stimulation resulted in rapid and long-lasting potentiation that was somewhat larger than the potentiation by 8-Br–cGMP plus weak tetanus [261.2 ± 42.8%, n = 4, t(3) = 3.77, P < 0.05; Fig. 3A), whereas perfusion with 8-pCPT–cGMP alone had no effect (101.0 ± 12.5%, n = 4; Fig. 3B), again replicating Zhuo et al. (1994a). These results suggest that cGMP may act through cGMP-dependent protein kinase to produce activity-dependent potentiation.
Figure 3.
Potentiation by 8-pCPT–cGMP plus weak tetanus is reduced but not blocked by AP5. (A) Perfusion with 8-pCPT–cGMP for 10 min before weak tetanic stimulation resulted in rapid and long-lasting potentiation. (Inset) Representative records of the EPSP before and 60 min after the weak tetanus. Scale bars, 1 mV, 5 msec. (B) Perfusion with 8-pCPT–cGMP alone had no effect on the EPSP. (C) Perfusion with AP5 for 25 min and with 8-pCPT–cGMP for 10 min before weak tetanic stimulation resulted in slow onset, long-lasting potentiation. Average baseline values: −1.12 mV/msec (A); −1.04 (B); −1.20 (C); not significantly different.
NMDA RECEPTORS CONTRIBUTE TO, BUT ARE NOT REQUIRED FOR, ACTIVITY-DEPENDENT POTENTIATION BY cGMP ANALOGS
According to the retrograde messenger hypothesis, cGMP acts downstream of NMDA receptors, so that potentiation by cGMP analogs should not be blocked by the NMDA receptor antagonist AP5. Perfusion with DL-AP5 (50 μm) for 25 min and with 8-pCPT–cGMP for 10 min before weak tetanic stimulation resulted in long-lasting potentiation [157.0 ± 16.7%, n = 5, t(4) = 3.42, P < 0.05; Fig. 3C) that was ∼35% of the potentiation without AP5 (Fig. 3A). As a control, perfusion with AP5 for 25 min before strong tetanic stimulation (two trains of 100-Hz, 1-sec stimulation separated by 20 sec) completely blocked normal LTP [206.2 ± 21.5%, n = 4 in normal saline (Fig. 4A) vs. 102.8 ± 3.4%, n = 4 in AP5 (Fig. 4B)], suggesting that the AP5 completely blocked NMDA receptors. These results demonstrate that NMDA receptor activation is not required for activity-dependent potentiation by 8-pCPT–cGMP, consistent with the retrograde messenger hypothesis. However, the finding that the potentiation was reduced by AP5 indicates that NMDA receptors contribute to the potentiation, which was not predicted by that hypothesis. In preliminary experiments we have also obtained similar results using hippocampal slices from mice, although the potentiation by 8-pCPT–cGMP plus tetanus in the presence of AP5 was somewhat smaller [119.5 ± 5.2%, n = 4, t(3) =T3.78, P < 0.05]. These results suggest that there may be species differences in this aspect of potentiation by cGMP analogs.
Figure 4.
Potentiation by 8-Br–cGMP plus strong tetanic stimulation is reduced but not blocked by AP5. (A) Strong tetanic stimulation (double arrows) produced LTP. (B) Perfusion with AP5 for 25 min before strong tetanic stimulation blocked LTP. (C) Perfusion with AP5 for 25 min and with 8-Br–cGMP for 5 min before strong tetanic stimulation resulted in rapid and long-lasting potentiation. (Inset) Representative records of the EPSP before and 60 min after strong tetanic stimulation. Scale bars, 1 mV, 5 msec. Average baseline values: −1.09 mV/msec (A); −1.10 (B); −1.06 (C); not significantly different.
We also examined the effect of AP5 on activity-dependent potentiation by 8-Br–cGMP. Perfusion with AP5 for 25 min and with 8-Br–cGMP for 5 min before strong tetanic stimulation resulted in long-lasting potentiation that was ∼40% of normal LTP [140 ± 11.8%, n = 5, t(4) = 3.41, P < 0.05; Fig. 4C], demonstrating that activity-dependent potentiation by 8-Br–cGMP, like 8-pCPT–cGMP, does not require activation of NMDA receptors. The fact that 8-Br–cGMP did not completely reverse the block of LTP by AP5 is consistent with the idea that NMDA receptors contribute to activity-dependent potentiation by cGMP, so that the potentiation in the presence of AP5 may underestimate the contribution of cGMP in the absence of AP5. In addition, cGMP probably contributes to only part of normal LTP.
The potentiation by 8-pCPT–cGMP paired with weak tetanus in the presence of AP5 (Fig. 3C) had a slow onset, similar to potentiation by 8-pCPT–cGMP paired with low-frequency stimulation in the absence of AP5 (Zhuo et al. 1994a), or nitric oxide (NO) or carbon monoxide (CO) paired with weak tetanus in the presence of AP5 (Zhuo et al. 1993). In contrast, the potentiation by 8-Br–cGMP plus strong tetanic stimulation in the presence of AP5 (Fig. 4C) had a rapid onset. Similarly, the potentiation by either 8-pCPT–cGMP (Fig. 3A) or 8-Br–cGMP (Fig. 1A) plus weak tetanus in the absence of AP5 had a rapid onset and early phase that was larger than the STP by weak tetanus alone (Fig. 1C). These results suggest that cGMP analogs may be able to produce two temporally different phases of activity-dependent potentiation (one with a rapid onset and early decline and one with a slow onset) that normally overlap, resulting in a characteristic saddle-shaped time course of potentiation (Figs. 1A, 2, 3A, and 5A).
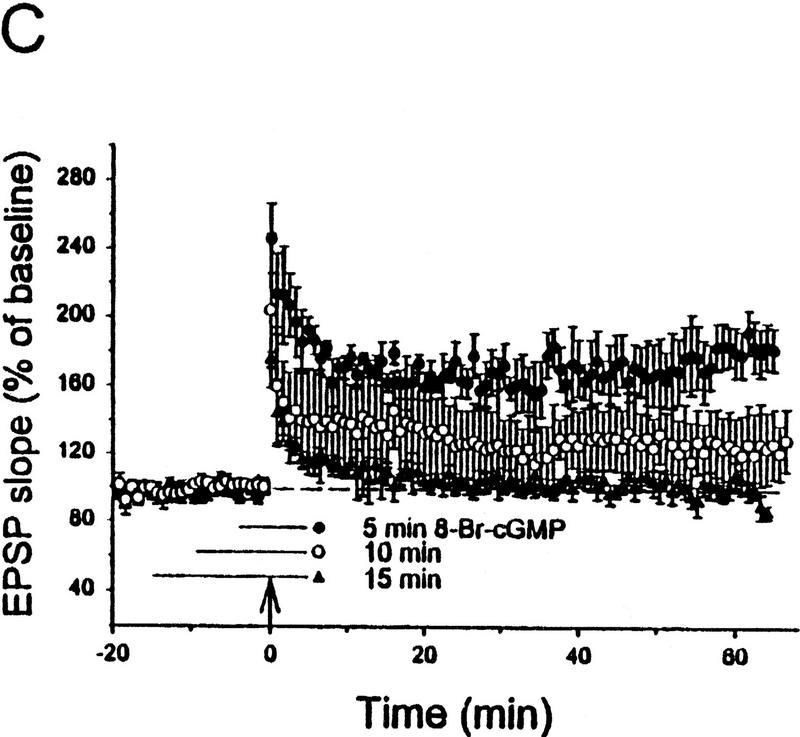
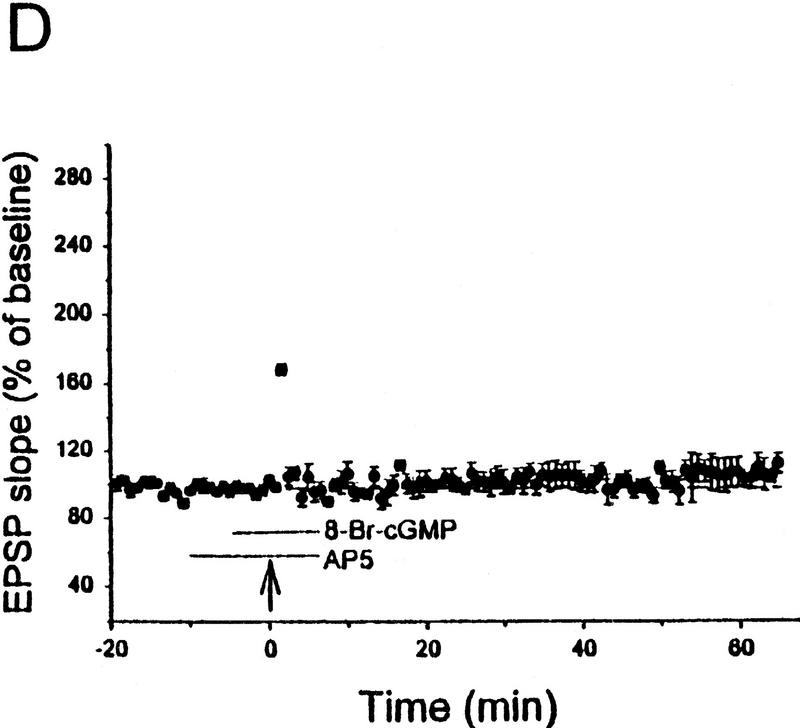
Figure 5.
Potentiation by 8-Br–cGMP plus weak tetanus depends on the duration of perfusion with either 8-Br–cGMP or AP5. (A) Perfusion with 8-Br–cGMP and AP5 for 5 min before weak tetanic stimulation resulted in rapid and long-lasting potentiation. (B) Perfusion with 8-Br–cGMP and AP5 for 10 min before weak tetanic stimulation resulted in no long-lasting potentiation. (C) Perfusion with 8-Br–cGMP alone for 5 min (•) before weak tetanic stimulation resulted in rapid and long-lasting potentiation, but perfusion for 10 min (○) resulted in less potentiation, and perfusion for 15 min (▴) resulted in no long-lasting potentiation. (D) Perfusion with AP5 for 10 min and with 8-Br–cGMP for 5 min before weak tetanic stimulation resulted in no long-lasting potentiation. Average baseline values: −0.23 mV/msec (A); −0.25 (B); −0.27 (5 min, C); −0.30 (10 min, C); −0.27 (15 min, C); −0.26 (D); not significantly different.
POTENTIATION BY 8-BR–cGMP PLUS WEAK TETANIC STIMULATION DEPENDS ON THE EXPERIMENTAL PROTOCOL
We then examined the effect of AP5 on potentiation by 8-Br–cGMP plus weak tetanus, which might be expected to be less robust than potentiation by either the more potent analog 8-pCPT–cGMP plus weak tetanus (Fig. 3C) or 8-Br–cGMP plus strong tetanus (Fig. 4C). Using methods similar to Zhuo et al. (1994a), we first replicated their finding that simultaneous perfusion with AP5 and 8-Br–cGMP for 5 min before weak tetanic stimulation resulted in rapid and long-lasting potentiation [151.4 ± 12.1%, n = 6, t(5) = 4.24, P < 0.01; Fig. 5A]. We then tried longer perfusion with AP5 and 8-Br–cGMP, which is more similar to the methods of Schuman et al. (1994) and Selig et al. (1996). Unlike 5-min perfusion, simultaneous perfusion with these two drugs for 10 min before weak tetanic stimulation resulted in PTP but no STP or LTP (98.4 ± 4.5%, n = 5; Fig. 5B), replicating the results of Schuman et al. (1994) and Selig et al. (1996). These results therefore suggest that some of the discrepancies between the previous reports might be attributable to differences in the duration of perfusion with either AP5 or 8-Br–cGMP.
DURATION OF PERFUSION WITH 8-BR–cGMP IS AN IMPORTANT VARIABLE
To distinguish between these possibilities, we first tried longer perfusion with 8-Br–cGMP in the absence of AP5. Surprisingly, perfusion with 8-Br–cGMP for 10 min before weak tetanic stimulation resulted in less long-lasting potentiation than perfusion for 5 min (124.9 ± 17.2%, n = 3), and perfusion with 8-Br–cGMP for 15 min before weak tetanic stimulation resulted in no long-lasting potentiation at all (98.8 ± 6.8%, n = 6; Fig. 5C). Thus, three different durations of perfusion with 8-Br–cGMP resulted in significantly different amounts of potentiation [F(2,10) = 19.24, P < 0.001]. These results differed from results with 8-pCPT–cGMP, which still produced LTP when the duration of perfusion was increased to 30 min before the weak tetanic stimulation (214.5 ± 17.5%, n = 2). Duration of perfusion with 8-Br–cGMP (but not 8-pCPT–cGMP) before tetanic stimulation is therefore an important variable that might account for some failures to observe potentiation in similar experiments (Schuman et al. 1994; Selig et al. 1996).
POTENTIATION BY 8-BR–cGMP PLUS WEAK TETANUS REQUIRES SOME NMDA CURRENT
The lack of potentiation by perfusion with both 8-Br–cGMP and AP5 for 10 min before weak tetanic stimulation (Fig. 5B) might also be attributable to longer perfusion with AP5, which could allow AP5 to reach a higher concentration in the slice. Consistent with that possibility, perfusion with AP5 for 10 min and with 8-Br–cGMP for 5 min before weak tetanic stimulation resulted in PTP but no STP or LTP (106.8 ± 2.8%, n = 7; Fig. 5D), demonstrating that duration of perfusion with AP5 is an important variable. Five-minute perfusion with AP5 also did not completely block LTP by strong tetanic stimulation (123.3 ± 12.1%, n = 4), suggesting that it may not completely block NMDA receptors. To check that possibility more directly, we examined the NMDA component of the EPSP during perfusion with CNQX (10 μm), which blocks the non-NMDA component (see Fig. 6A). On average, the NMDA component of the EPSP was significantly but not completely blocked by perfusion with AP5 for 5 min and was completely blocked by perfusion with AP5 for 10 min (Table 1A). These results suggest that there is a relationship between the degree of NMDA receptor activation and the degree of potentiation produced by 8-Br–cGMP plus weak tetanus (Table 1B).
Figure 6.
8-Br–cGMP does not affect NMDA current during the weak tetanus. (A) Following block of the non-NMDA component of the EPSP by CNQX, 8-Br–cGMP had no effect on the remaining NMDA component. The stimulation intensity was increased at 110 min (arrow). (Inset) Representative records of the NMDA component of the EPSP before (a) and during (b) perfusion with 8-Br–cGMP. Scale bars, 1 mV, 5 msec. Subsequent perfusion with AP5 completely blocked the EPSP. (B) Examples of the EPSPs at the beginning of the weak tetanus before and during perfusion with 8-Br–cGMP in normal saline. Scale bars, 3 mV, 30 msec.
Table 1.
Effect of NMDA blockers on the NMDA component of the EPSP and activity-dependent potentiation by 8-Br–cGMP
| No drug | AP5 (50 μm 5 min) | AP5 (50 μm 10 min) | 7-CLKA (20 μm 10 min) | MK801 (40 μm 10 min) + weak tetanus | |
|---|---|---|---|---|---|
| A. NMDA-dependent | 100 | 33.4** | 0.2** | 75.6** | 71.6* |
| component of EPSP | — | ±7.6 | ±0.2 | ±7.7 | ±14.8 |
| (15) | (7) | (9) | (9) | (3) | |
| B. Potentiation by | 183.6 | 151.4* | 106.8** | 122.0** | 129.3** |
| 8-Br–cGMP plus | ±12.8 | ±12.1 | ±2.8 | ±5.5 | ±7.8 |
| weak tetanus | (4) | (6) | (7) | (5) | (4) |
Effect of different NMDA blockers and protocols on the NMDA component of the EPSP (A) and potentiation by 8-Br–cGMP plus weak tetanus (B). The numbers represent the mean percent of baseline ± the s.e. of the mean, and the numbers in parentheses indicate the n. ** = p < 0.01, * = p < 0.05 compared with no drug by planned comparisons (A) or Duncan’s multiple range tests (B) following one-way ANOVAs. (A) F(3,16) = 19.12; (B) F(4,21) = 11.49, P < 0.01 in each case.
To explore that relationship further, we performed similar experiments with two different types of NMDA blockers, 7-chlorokynurenic acid (7-CLKA), which acts at the glycine site on the NMDA receptor (Kemp et al. 1988), and MK801, which produces an activity-dependent block of ion channels associated with NMDA receptors (Huettner and Bean 1988). Both drugs partially blocked the NMDA-dependent component of the EPSP and also partially blocked potentiation by perfusion with 8-Br–cGMP for 5 min before weak tetanic stimulation (Table 1). These results demonstrate a rough correlation between potentiation by 8-Br–cGMP plus weak tetanus and the magnitude of the NMDA component of the EPSP. However, the rank order effectiveness of the three drugs was different for the two phenomena. In particular, 5-min perfusion with AP5 was more effective than 7-CLKA or MK801 in blocking the NMDA component of the EPSP (P < 0.01 in each case by Duncan’s multiple range test) but less effective in blocking potentiation by 8-Br–cGMP plus weak tetanus (Table 1).
8-BR–cGMP DOES NOT ENHANCE NMDA CURRENT
The results of these experiments indicate that potentiation by 8-Br–cGMP plus weak (but not strong) tetanus requires some NMDA current. One hypothesis that might be consistent with these results is that 8-Br–cGMP produces activity-dependent potentiation in part by enhancing postsynaptic NMDA current during the weak tetanus, making it functionally equivalent to a stronger tetanus. We tested this possibility in two ways. First, following block of the non-NMDA component of the EPSP with CNQX, we perfused the slices with 8-Br–cGMP (100 μm) for 30 min (Fig. 6A). 8-Br–cGMP had no effect on the area of the remaining NMDA component (100.7 ± 6.6%, n = 6, 30–35 min after the start of perfusion). Second, we examined whether 8-Br–cGMP increased facilitation of the EPSP during the weak tetanus, which might enhance NMDA current indirectly by enhancing depolarization (Figurov et al. 1996). However, 8-Br–cGMP had no effect on facilitation of the amplitude of the EPSP during the tetanus (157 ± 12% vs. 154 ± 4% in control saline for the second EPSP in the train and 64 ± 4% vs. 57 ± 6% in control saline for the last EPSP in the train, n = 3; Fig. 6B). These results indicate that 8-Br–cGMP does not produce potentiation by enhancing postsynaptic NMDA current during the weak tetanus. An alternative possibility that could account for our results is that NMDA current contributes to the activity-dependent enhancement of potentiation by cGMP, so that when NMDA receptors are blocked, training with 8-Br–cGMP plus weak (but not strong) tetanus is below threshold for producing long-lasting potentiation.
PRESYNAPTIC cGMP PRODUCES ACTIVITY-DEPENDENT LONG-LASTING POTENTIATION IN CULTURE
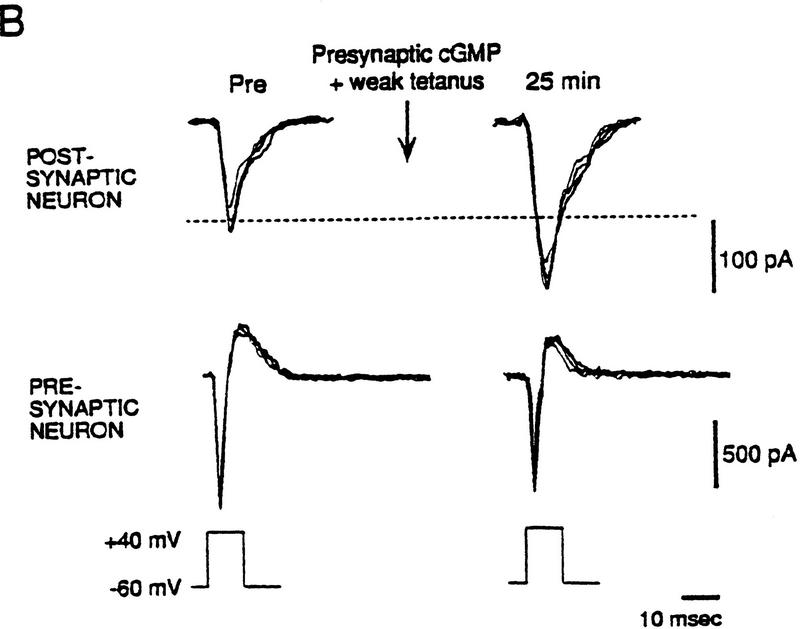
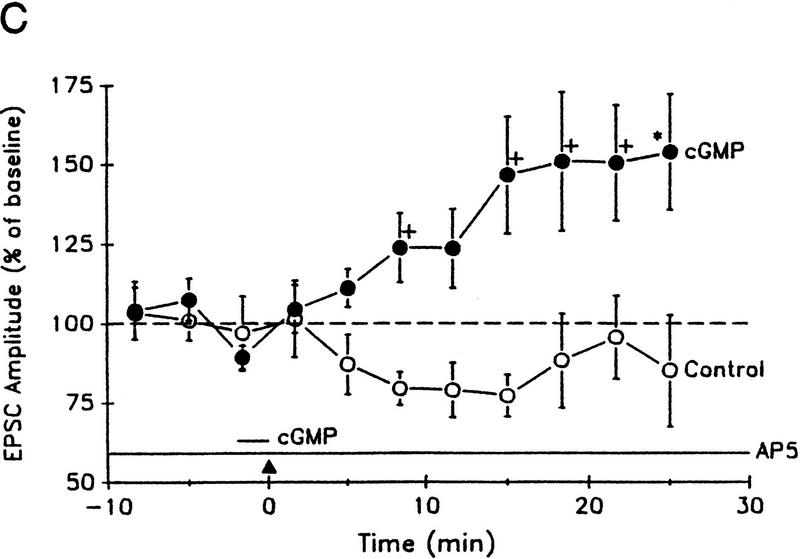
The experiments described so far indicate that cGMP analogs can produce long-lasting potentiation but do not address the question of whether cGMP acts in the pre- or postsynaptic cell. To examine that question, we performed experiments on hippocampal neurons in dissociated cell culture, where both the pre- and postsynaptic sides of the synapse are accessible (Arancio et al. 1995, 1996). A synaptically connected pair of neurons was held under ruptured whole-cell voltage clamp, and excitatory postsynaptic currents (EPSCs) were produced in the postsynaptic neuron by step depolarization of the presynaptic neuron every 10 sec (Fig. 7A). Injection of cGMP (50 μm) into the presynaptic neuron for 2 min before weak tetanic stimulation of that cell resulted in a long-lasting increase in the amplitude of the EPSC [151.4 ± 16.1%, n = 5, t(4) = 3.20, P < 0.05, comparing the mean EPSC 20–25 min post-tetanus to the average for 10 min pre-tetanus; Fig. 7B,C]. In contrast, test-alone control neurons exhibited slight run-down of the EPSC [92.3 ± 12.9%, n = 5, t(8) = 2.89, P < 0.05 compared with presynaptic cGMP]. The cultures were perfused with AP5 (50 μm) throughout the experiments to ensure that the weak tetanus by itself did not produce potentiation. Furthermore, injection of cGMP into the postsynaptic neuron before weak tetanic stimulation resulted in no potentiation (88.0 ± 16.8%, n = 5). These results indicate that cGMP acts directly in the presynaptic neuron to produce activity-dependent long-lasting potentiation.
Figure 7.
Potentiation by injection of cGMP into the presynaptic neuron plus weak tetanus in the presence of AP5 in cultured hippocampal neurons. (A) Experimental arrangement. (B) Example of potentiation by injection of cGMP into the presynaptic neuron plus weak tetanus. EPSCs were produced in the postsynaptic neuron by step depolarization that elicited an inward current in the presynaptic neuron once every 10 sec. The current in the presynaptic neuron has had leakage subtracted. Both recordings are a.c. coupled. Sample traces are shown before (Pre) and 25 min after injection of cGMP into the presynaptic neuron for 2 min before weak tetanic stimulation of that neuron during continuous perfusion with AP5. Four successive traces are superimposed at each time period. The broken line shows the average Pre value. (C) Average potentiation by presynaptic injection of cGMP for 2 min before weak tetanus (•) compared with test-alone control (○). EPSC amplitude has been normalized to the average baseline value during the 10 min before training in each experiment (broken line). Each point represents the average of 30 successive trials. Weak tetanic stimulation (▴) occurred at time zero. The short horizontal bar indicates the time of cGMP injection. AP5 was present throughout the experiments. The points indicate the means, and the error bars indicate the s.e.m.s. (Adapted from Arancio et al. 1995.)
LTP IN S. RADIATUM, BUT NOT S. ORIENS, IS BLOCKED BY INHIBITORS OF SOLUBLE GUANYLYL CYCLASE AND cGMP-DEPENDENT PROTEIN KINASE
Additional evidence suggesting that cGMP is involved in LTP comes from studies showing that potentiation by strong tetanic stimulation is blocked by inhibitors of soluble guanylyl cyclase or cGMP-dependent protein kinase (PKG) in hippocampal slices (Zhuo et al. 1994a; Blitzer et al. 1995; Boulton et al. 1995) and also in hippocampal cultures (Arancio et al. 1995; O. Arancio, J. Wood, D. Lawrence, and R.D. Hawkins, unpubl.). However, Schuman et al. (1994) failed to find any effect on LTP of a broad spectrum kinase inhibitor, H-8, that is thought to block PKG. We therefore reexamined this issue by studying the effects of specific inhibitors of soluble guanylyl cyclase and PKG on LTP produced by strong tetanic stimulation (two trains of 100-Hz, 1-sec stimulation separated by 20 sec) in the CA1 region of slices of mouse hippocampus. We examined LTP in both the s. radiatum and s. oriens because the endothelial isoform of NO synthase (eNOS) is expressed only in s. radiatum (O’Dell et al. 1994), and LTP in s. radiatum, but not s. oriens, is blocked by inhibitors or knockout of NO synthase (Haley et al. 1996; Son et al. 1996). If cGMP acts downstream of NO, one might expect a similar pattern of results for inhibitors of soluble guanylyl cyclase and PKG.
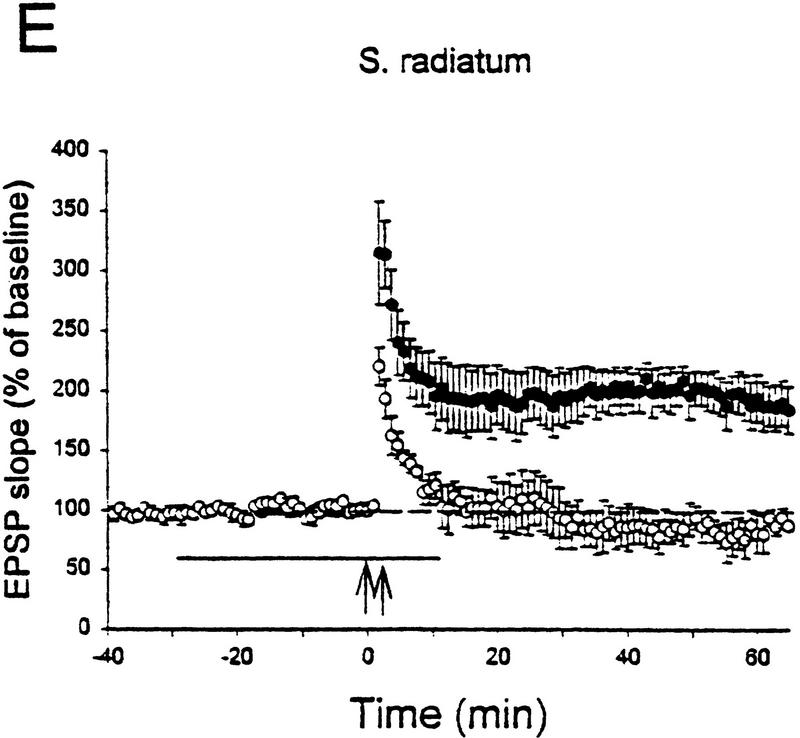
We first examined the effect of ODQ, a specific inhibitor of soluble guanylyl cyclase (Garthwaite et al. 1995). Consistent with the results of Zhuo et al. (1994a) in guinea pigs and Boulton et al. (1995) in rats, ODQ (10 μm) produced a significant reduction in LTP in the s. radiatum of mice [137.7 ± 14.9%, n = 6 vs. 213.9 ± 5.4%, n = 3 in control saline, t(7) = 3.44, P < 0.02; Fig. 8A]. Moreover, like inhibitors or knockout of NO synthase (Haley et al. 1996; Son et al. 1996), ODQ produced a smaller reduction in LTP in s. oriens that was not significant (177.7 ± 13.3%, n = 8 vs. 232.2 ± 56.2%, n = 3 in control saline; Fig. 8B).
Figure 8.
Inhibitors of guanylyl cyclase or PKG block LTP by strong tetanic stimulation in s. radiatum but not in s. oriens. (A) LTP in s. radiatum was blocked by the guanylyl cyclase inhibitor ODQ (○) compared with ACSF control (•). (B) LTP in s. oriens was not significantly blocked by ODQ. (C) LTP in s. radiatum was blocked by the PKG inhibitor Rp-8-Br–cGMPS. (D) LTP in s. oriens was not significantly blocked by Rp-8-Br–cGMPS. (E) LTP in s. radiatum was blocked by the structurally different PKG inhibitor KT5823. (F) LTP in s. oriens was not significantly blocked by KT5823. The horizontal bars indicate the time of perfusion with the drugs. Average baseline values: −0.28 mV/msec (ODQ, A); −0.35 (control, A); −0.32 (ODQ, B); −0.42 (control, B); −0.31 (Rp-8-Br–cGMPS, C); −0.35 (control, C); −0.36 (Rp-8-Br–cGMPS, D); −0.42 (control, D); −0.28 (KT5823, E); −0.31 (control, E); −0.42 (KT5823, F); −0.35 (control, F); not significantly different.
We then examined the effects of two structurally different inhibitors of PKG, Rp-8-Br–cGMPS and KT5823. Replicating the results of Zhuo et al. (1994a) in guinea pigs and Blitzer et al. (1995) in rats, Rp-8-Br–cGMPS (100 μm) significantly reduced LTP in s. radiatum in mice [105.4 ± 12.9%, n = 3 vs. 213.9 ± 5.4%, n = 3 in control saline, t(4) = 7.79, P < 0.01; Fig. 8C]. Like ODQ, Rp-8-Br–cGMPS produced a smaller reduction in LTP in s. oriens that was not significant (212.5 ± 23.2%, n = 4 vs. 232.1 ± 56.2%, n = 3 in control saline; Fig. 8D). Similarly, KT5823 (2 μm) produced a significant reduction of LTP in s. radiatum [88.9 ± 7.1%, n = 3 vs. 188.1 ± 17.1%, n = 3 in control saline, t(4) = 5.34, P < 0.01; Fig. 8E] but not in s. oriens (170.7 ± 15.7%, n = 5 vs. 219.9 ± 24.7%, n = 7 in control saline; Fig. 8F). Overall, like inhibitors or knockout of NO synthase, ODQ, Rp-8-Br–cGMPS, and KT5823 had larger effects in s. radiatum than in s. oriens [F(1,39) = 3.30, P < 0.05 one-tail]. These results indicate that the inhibition of LTP in s. radiatum is not attributable to nonspecific effects of the drugs and support the idea that LTP in s. radiatum involves guanylyl cyclase and PKG.
Discussion
Previous results suggested that cGMP is involved in hippocampal LTP, perhaps as the presynaptic effector of a retrograde messenger (Haley et al. 1992; Zhuo et al. 1994a; Arancio et al. 1995; Blitzer et al. 1995; Boulton et al. 1995). However, other studies have failed to replicate some of those results (Schuman et al. 1994; Selig et al. 1996), making the role of cGMP uncertain. We have therefore reexamined this question and have attempted to identify experimental variables that might account for the different results in different studies.
BRIEF PERFUSION WITH 8-BR–cGMP IS MORE EFFECTIVE THAN LONGER PERFUSION
We first replicated the finding of Zhuo et al. (1994a) that perfusion with either 8-Br–cGMP or 8-pCPT–cGMP plus weak tetanic stimulation produces long-lasting potentiation (Figs. 1A and 3A). However, we found that if we increased the duration of perfusion with 8-Br–cGMP from 5 min to 15 min before the weak tetanus there was no potentiation (Fig. 5C), replicating the results of Schuman et al. (1994) and Selig et al. (1996). Thus, duration of perfusion with 8-Br–cGMP is an important variable that might account for some of the conflicting results that have been reported in previous studies.
Why is duration of perfusion with 8-Br–cGMP important? One possibility is that the intracellular effects of 8-Br–cGMP undergo adaptation or feedback suppression during prolonged perfusion. Alternatively, because cGMP is thought to be involved in the induction of hippocampal long-term depression as well as potentiation (Zhuo et al. 1994b; Gage et al. 1997), prolonged perfusion with 8-Br–cGMP may produce conditions that favor depression and either cancel out or block potentiation. Unlike 8-Br–cGMP, prolonged perfusion with 8-pCPT–cGMP before weak tetanic stimulation still resulted in long-lasting potentiation. Furthermore, when paired with low-frequency stimulation (0.25 Hz, 10 sec), 8-Br–cGMP produced long-lasting depression, whereas 8-pCPT–cGMP produced long-lasting potentiation (Zhuo et al. 1994a,b). One known difference between the analogs is that 8-pCPT–cGMP has greater selectivity for stimulating PKG as opposed to cGMP-dependent phosphodiesterases (Geiger et al. 1992). Thus, the decrease in effectiveness of 8-Br–cGMP with longer perfusion times might be owing to activation of phosphodiesterases, which would produce a decrease in endogenous cGMP and cAMP levels. The long-lasting depression produced by 8-Br–cGMP paired with low-frequency stimulation (Zhuo et al. 1994b) might also involve this pathway (M. Zhuo, unpubl.).
NMDA RECEPTOR ACTIVATION CONTRIBUTES TO, BUT IS NOT REQUIRED FOR, POTENTIATION BY cGMP
We also found that potentiation by either 8-pCPT–cGMP plus weak tetanus (Fig. 3C) or 8-Br–cGMP plus strong tetanus (Fig. 4C) was reduced but not blocked by the NMDA receptor antagonist AP5. The observation that AP5 did not block the potentiation in those experiments is consistent with the idea that cGMP acts downstream of postsynaptic NMDA receptors, as might be expected if it is the presynaptic effector of a retrograde messenger. However, the finding that AP5 reduced the potentiation in those experiments and could block potentiation by 8-Br–cGMP plus weak tetanus (Fig. 5D) was not predicted by the simplest version of the retrograde messenger hypothesis.
An alternative hypothesis is that cGMP analogs act in part by enhancing the postsynaptic NMDA current during the weak tetanus, making it functionally equivalent to a strong tetanus. However, we found that 8-Br–cGMP had no detectable effect on either the NMDA component of the EPSP (Fig. 6A) or facilitation of the EPSP during the weak tetanus (Fig. 6B), which might enhance the NMDA current indirectly by enhancing depolarization (Figurov et al. 1996). These results indicate that 8-Br–cGMP does not produce potentiation by enhancing postsynaptic NMDA current during the weak tetanus. 8-Br–cGMP also probably does not act by enhancing activation of certain types of metabotropic glutamate receptors, because activity-dependent potentiation by 8-Br–cGMP is not blocked by the metabotropic glutamate receptor antagonists AP3 or MCPG (M. Zhuo, J.T. Laitinen, X-L. Li, and R.D. Hawkins, in prep.).
Why did prolonged perfusion with AP5 block potentiation by 8-Br–cGMP plus weak tetanus but not potentiation by either 8-pCPT–cGMP plus weak tetanus or 8-Br–cGMP plus strong tetanus? Strong tetanus may simply allow sufficient Ca2+ influx through voltage-dependent Ca2+ channels to interact with 8-Br–cGMP. The greater effectiveness of 8-pCPT–cGMP may be owing to its greater selectivity for stimulating PKG (Geiger et al. 1992). Alternatively, because 8-pCPT–cGMP is more membrane permeable, it may simply produce a more rapid rise in intracellular concentration, perhaps more closely resembling what happens during normal LTP. Consistent with that possibility, NO and CO, which are very permeable and should produce more physiological increases in cGMP levels, also produce activity-dependent potentiation that is not blocked by prolonged perfusion with AP5 (Zhuo et al. 1993).
If the different results with 8-Br–cGMP and 8-pCPT–cGMP are attributable to differences in their permeability, then one might expect more effective potentiation by cGMP analogs in cultured hippocampal neurons, which are more accessible. Consistent with that possibility, perfusion with 8-Br–cGMP alone can produce long-lasting potentiation of evoked EPSCs and a long-lasting increase in the frequency of spontaneous miniature EPSCs in dissociated cultures of hippocampal neurons (Arancio et al. 1995). The potentiation of evoked EPSCs is enhanced by weak tetanic stimulation of the presynaptic neuron, even during prolonged perfusion with AP5. Similarly, intracellular injection of cGMP into the presynaptic neuron plus weak tetanus produces long-lasting potentiation during prolonged perfusion with AP5, providing strong evidence for a presynaptic locus of action of cGMP (Arancio et al. 1995; Fig. 7). Experiments with inhibitors of PKG also indicate a presynaptic locus of action both in slices (Blitzer et al. 1995) and in culture (O. Arancio, J. Wood, D. Lawrence, and R.D. Hawkins, unpubl.).
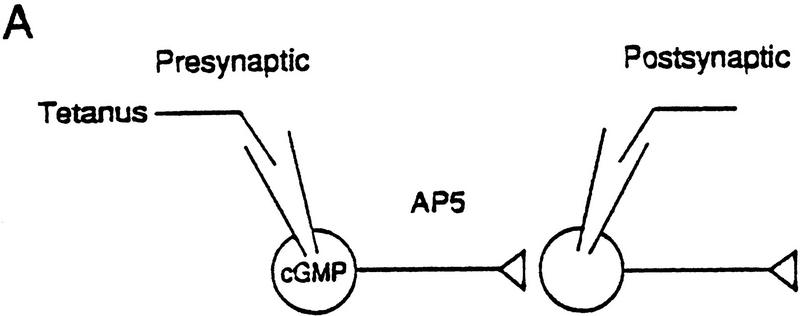
POSSIBLE ELABORATIONS OF THE RETROGRADE MESSENGER HYPOTHESIS
The finding that NMDA current contributes to activity-dependent potentiation by cGMP was not predicted by the simple retrograde messenger hypothesis. However, all of the results to date might be explained by the following elaboration of that hypothesis: (1) A sufficiently large, rapid rise in presynaptic cGMP alone (as may happen with 8-Br–cGMP in culture) can produce long-lasting potentiation of transmitter release. (2) That potentiation is enhanced by temporally paired spike activity, perhaps owing to the influx of Ca2+ that could act synergistically with cGMP. The Ca2+ influx may be partly through NMDA-receptor channels and partly through other channels (perhaps voltage-dependent Ca2+ channels). (3) If cGMP levels are low (as may happen with 8-Br–cGMP in slices), then a relatively large Ca2+ influx may be required for the cGMP to produce long-lasting potentiation. If cGMP levels are somewhat higher (as may happen with 8-pCPT–cGMP, NO, or CO in slices or with intracellular cGMP in culture), then a smaller Ca2+ influx may be sufficient.
The NMDA receptor channels that contribute to Ca2+ influx in this model might in principle be presynaptic receptors that act in series with the other channels (Fig. 9A). There is immunocytochemical evidence for presynaptic NMDA receptors in spinal cord, cortex, and the CA3 but not the CA1 region of hippocampus (Aoki et al. 1994; Liu et al. 1994; Siegel et al. 1994), and there is biochemical and electrophysiological evidence for presynaptic NMDA receptors on inputs to pyramidal neurons in both the CA3 and CA1 regions (Chernevskaya et al. 1991; Martin et al. 1991). Consistent with that possibility, the pharmacological profiles of the NMDA component of the postsynaptic potential and potentiation by 8-Br–cGMP plus weak tetanus were somewhat different (Table 1), suggesting that the two phenomena might involve somewhat different receptors. Alternatively, the NMDA receptor channels in the model might be postsynaptic. One possibility is that a tonic postsynaptic NMDA current plays some constitutive role in presynaptic potentiation by NO and cGMP (Schuman and Madison 1994; J. Noel, A. Bergamaschi, and A. Malgaroli, unpubl.). Another possibility is that potentiation by cGMP has a postsynaptic as well as a presynaptic component, perhaps involving an interaction with some step downstream from postsynaptic NMDA receptors (Fig. 9B). Such a mechanism might contribute to the role of cGMP in protein synthesis-dependent late-phase potentiation, which may involve nuclear events in the postsynaptic cell (Y.-F. Lu and R.D. Hawkins, unpubl.).
Figure 9.
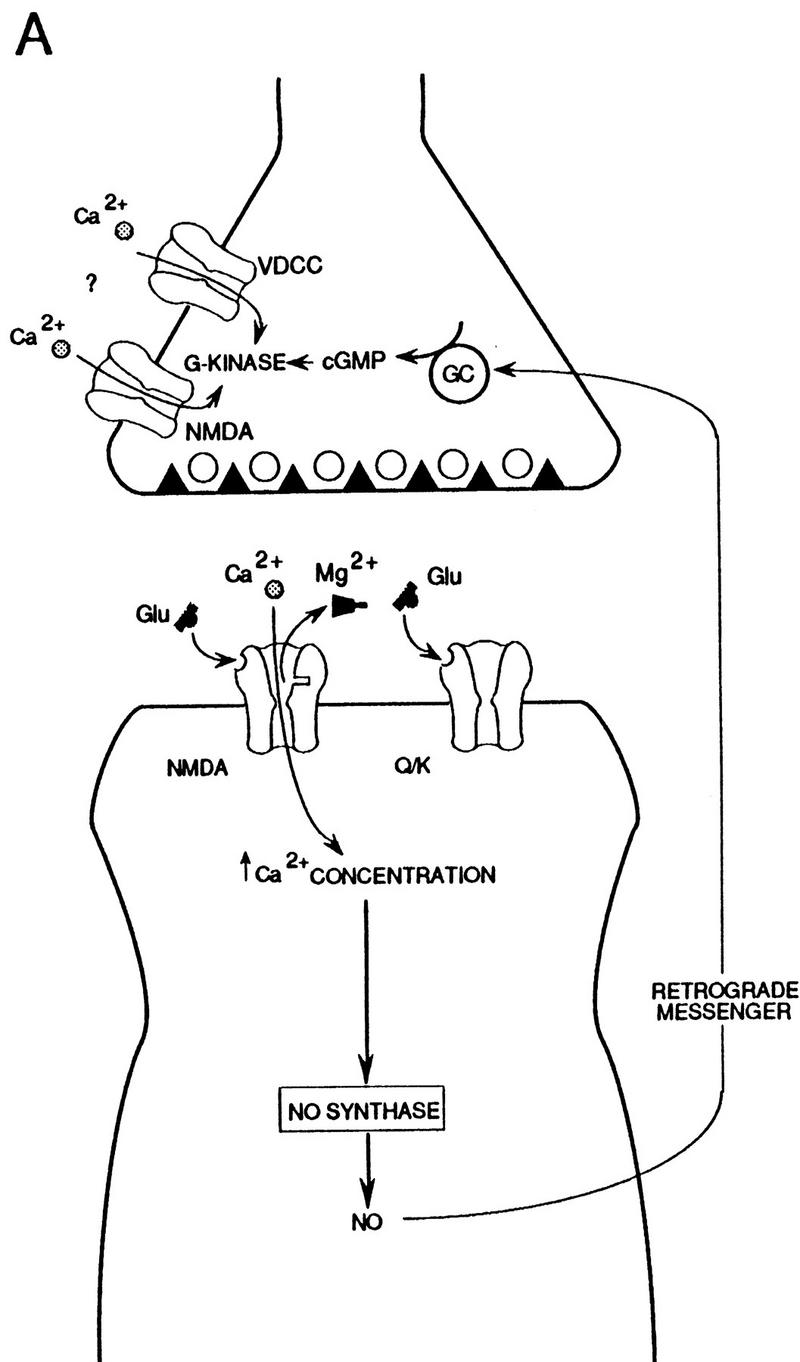

Elaborations of the simple retrograde messenger hypothesis involving NMDA receptors in the presynaptic (A) or postsynaptic (B) neuron. (See Discussion for details.)
The elaborations of the simple retrograde messenger hypothesis shown in Figure 9 could account for all the data on potentiation by cGMP and cGMP analogs, but many other interpretations of those data are also possible. Furthermore, these hypotheses are not meant to be complete accounts of normal LTP, which probably has multiple components involving several different mechanisms. Thus, for example, LTP may also involve purely postsynaptic mechanisms, the presynaptic mechanisms may involve other retrograde messengers in addition to NO, and NO may have additional presynaptic effects (Williams et al. 1989; Stevens and Wang 1993; Zhuo et al. 1993; Schuman et al. 1994).
THE FUNCTIONAL ROLE OF cGMP-DEPENDENT POTENTIATION
The results of these studies indicate that cGMP analogs can produce long-lasting potentiation with some experimental protocols but not with others. These results may therefore help to explain some of the discrepant findings from previous studies and, in addition, may provide insights into the functional role of cGMP-dependent potentiation. For example, the results of all of the studies with cGMP analogs or injection of cGMP might be consistent with the idea that activity-dependent potentiation is optimal when a relatively large, rapid rise in cGMP is combined with a relatively large, rapid rise in Ca2+. Such a result might be expected if activity-dependent potentiation by cGMP serves as a temporal associative mechanism that restricts potentiation by a diffusible retrograde messenger to presynaptic fibers that are active at about the same time as the postsynaptic cells. A slower, smaller rise in cGMP or Ca2+ is hypothesized not to produce potentiation and may engage other mechanisms that lead to long-lasting depression (Zhuo et al. 1994b; Gage et al. 1997).
Although further experiments will be necessary to clarify the mechanisms of potentiation by cGMP, studies with inhibitors of guanylyl cyclase or PKG (Zhuo et al. 1994a; Blitzer et al. 1995; Boulton et al. 1995) suggest that cGMP plays a physiological role in LTP. We have found that those inhibitors were more effective in blocking LTP in s. radiatum (which synapses on the apical dendrites of the CA1 pyramidal cells) than s. oriens (which synapses on the basal dendrites) (Fig. 8). Similar results have been obtained with inhibitors or knockout of NO synthase (Haley et al. 1996; Son et al. 1996). These results are consistent with the idea that NO acts through cGMP during potentiation at synapses from s. radiatum onto apical dendrites and indicate that LTP involves different mechanisms at synapses from s. oriens onto basal dendrites of the same CA1 pyramidal cells. Both s. radiatum and s. oriens contain axons from CA3 pyramidal cells (Ishizuka et al. 1990), but the organization of interneurons is different in the two pathways, suggesting they may subserve different functions (Sik et al. 1995). Like LTP in s. radiatum, LTP in s. oriens is NMDA dependent (Haley et al. 1996; Son et al. 1996), but otherwise its mechanisms are poorly understood. Further comparison of LTP in these different pathways may therefore provide additional insights into the functional role of NO and cGMP-dependent potentiation in hippocampal information processing.
Acknowledgments
We thank A. MacDermott and S. Siegelbaum for their comments, H. Ayers and M. Pellan for typing the manuscript, and C. Lam for preparing the figures. This research was supported by grants from the National Institute of Mental Health (MH50733) and the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Aoki C, Venkatesan C, Go C-G, Mung JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′, 5′-cyclic cGMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Boulton CL, Southam E, Garthwaite J. Nitric oxide-dependent long-term potentiation is blocked by a specific inhibitor of soluble guanylyl cyclase. Neuroscience. 1995;69:699–703. doi: 10.1016/0306-4522(95)00349-n. [DOI] [PubMed] [Google Scholar]
- Chernevskaya NI, Obukhov AG, Krishtal OA. NMDA receptor agonists selectively block N-type calcium channels in hippocampal neurons. Nature. 1991;349:418–420. doi: 10.1038/349418a0. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller L-D, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Gage AT, Reyes M, Stanton PK. Nitric-oxide-guanyly-cyclase-dependent and independent components of multiple forms of long-term synaptic depression. Hippocampus. 1997;7:286–295. doi: 10.1002/(SICI)1098-1063(1997)7:3<286::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielson EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide sensitive guanylyl cyclase by 1H-[1,2,4] oxadiazolo [4,3-α] quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Geiger J, Nolte C, Butt E, Sage SO, Walter U. Role of cGMP and cGMP-dependent protein kinase in nitrovasodilator inhibition of agonist-evoked calcium elevation in human platelets. Proc Natl Acad Sci. 1992;89:1031–1035. doi: 10.1073/pnas.89.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley JE, Wilcox GL, Chapman PF. The role of nitric oxide in hippocampal long-term potentiation. Neuron. 1992;8:211–216. doi: 10.1016/0896-6273(92)90288-o. [DOI] [PubMed] [Google Scholar]
- Haley J, Schaible E, Paulidis P, Murdock A, Madison DV. Basal and apical synapses of CA1 pyramidal cells employ different LTP induction mechanisms. Learn & Mem. 1996;3:289–295. doi: 10.1101/lm.3.4.289. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Kandel ER, Siegelbaum SA. Learning to modulate transmitter release: Themes and variations in synaptic plasticity. Annu Rev Neurosci. 1993;16:625–665. doi: 10.1146/annurev.ne.16.030193.003205. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: Selective binding to open channels. Proc Natl Acad Sci. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL, Woodruff GN. 7-chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci. 1988;85:6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Bustos GA, Bowe MA, Bray SD, Nadler JV. Autoreceptor regulation of glutamate and aspartate release from slices of the hippocampal CA1 area. J Neurochem. 1991;56:1647–1655. doi: 10.1111/j.1471-4159.1991.tb02063.x. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Hawkins RD, Kandel ER, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman ML. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994;265:542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. Locally distributed synaptic potentiation in the hippocampus. Science. 1994;263:532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Meffert MK, Schulman H, Madison DV. An ADP-ribosyl transferase as a potential target for nitric oxide action in hippocampal long-term potentiation. Proc Natl Acad Sci. 1994;91:11958–11962. doi: 10.1073/pnas.91.25.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig DK, Segal MR, Liao D, Malenka RC, Malinow R, Nicoll RA, Lisman JE. Examination of the role of cGMP in long-term potentiation in the CA1 region of the hippocampus. Learn & Mem. 1996;3:42–48. doi: 10.1101/lm.3.1.42. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Brose N, Janssen WG, Gasic GP, Jahn R, Heinemann SE. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci. 1994;91:564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: An in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider RM, McKinney M, Forray C, Richelson E. Neurotransmitter receptors mediate cyclic GMP formation by involvement of arachidonic acid and lipoxygenase. Proc Natl Acad Sci. 1984;81:3905–3909. doi: 10.1073/pnas.81.12.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Hawkins RD, Martin K, Kiebler M, Huang PL, Fishman ML, Kandel ER. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Reversal of long-term potentiation by inhibitors of heme oxygenase. Nature. 1993;364:147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: A putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Williams JH, Errington ML, Lynch MA, Bliss TVP. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989;341:739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Hu Y, Schultz C, Kandel ER, Hawkins RD. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994a;368:635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Kandel ER, Hawkins RD. Nitric oxide and cGMP can produce either synaptic depression or potentiation depending on the frequency of presynaptic stimulation in hippocampus. NeuroReport. 1994b;5:1033–1036. doi: 10.1097/00001756-199405000-00004. [DOI] [PubMed] [Google Scholar]