Hughes et al.1 suggested that a common deletion of the CFHR1 and CFHR3 genes (CFHR1–3Δ) is associated with lower risk of age related macular degeneration (AMD) and that the effect is independent from that of the previously described Y402H allele (rs1061170) in the adjacent CFH gene2. Others have replicated the CFHR1–3Δ association3,4, and this has spurred further research on the function of the CFHR gene family5. In addition to the Y402H coding variant, we and others have described a second independent CFH allele, marked by the rs1410996 intronic SNP6,7.
Since the CFH–CFHR1–CFHR3 genomic region containing both of these risk SNPs and CFHR1–3Δ has strong linkage disequilibrium (see Supplementary Fig. 1) with common haplotypes extending across the entire region4, we sought to understand the relationship between these AMD associations in a large sample collection. This issue is potentially relevant to atypical hemolytic uremic syndrome (MIM235400), which has also been linked separately to CFH alleles and to CFHR1–3Δ (ref. 8).
We genotyped CFHR1–3Δ and 20 common SNPs within the CFH and CFHR1–CFHR3 region in 711 individuals with visually impairing advanced AMD of AMD and 1041 controls (see Supplementary Methods) with the Affymetrix 6.0 chip9. This genotyping included the rs10801555 SNP, a close proxy for Y402H (r2 = 0.99 in a subset of 288 geno-typed controls), located 1 kb away, and also the rs10737680 SNP, a perfect proxy for the rs1410996 allele (r2 = 1 in Centre d'Etude du Polymorphisme Humain (CEU) HapMap) located 17.5 kb away in the ninth CFH intron. CFHR1–3Δ frequencies in affected and unaffected individuals were similar to those of Hughes et al.1 and correlated closely with the rs7542235 SNP (r2 = 0.98).
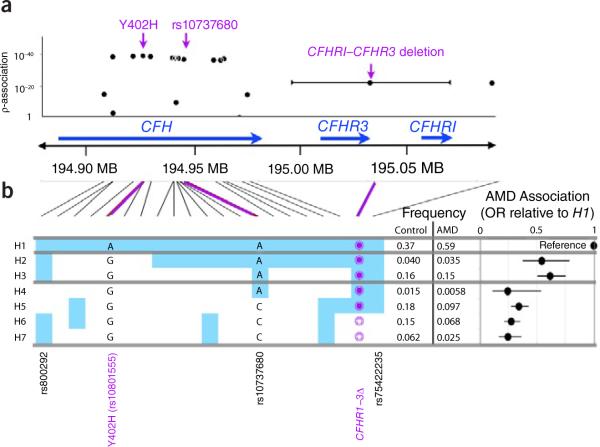
First, we tested each of the 21 markers individually (Fig. 1a and Supplementary Table 1). We reproduced associations at the CFH Y402H allele (P = 1.5 × 10−39 at rs10801555) and the CFH rs10737680 allele (P = 1.8 − 10−37). We observed more modest evidence of association of CFHR1–3Δ (P = 7.0 × 10−23), with 22% frequency in affected individuals compared to 10% in controls.
Figure 1.
Genetics of the CFH–CFHR1–CFHR3 region. Statistical results of 20 SNP markers and a CFHR1–CFHR3 common copy number polymorphism. (a) Single marker tests. For each individual marker we plot the statistical strength of association as a function of its genomic position within the region. Violet, previously described SNP associations. (b) The seven haplotypes with frequencies >1%. H1 is presented as the reference haplotype. If genotypes for SNPs in other haplotypes are the same as in H1, then they are shaded blue; if genotypes for SNPs differ from H1, they are shaded white. For each haplotype we list the nucleotide for the CFH Y402H proxy rs10801555 and for CFH rs10737680, and also the deletion status of the CFHR1–CFHR3 region: empty circle, deleted; filled circle, not deleted. There are two other SNPs of interest: rs7542235, a SNP that tags the CFHR1–CFHR3 deletion; and rs800292, a CFH nonsynonymous (I62V) allele. To the right of each haplotype is the observed frequency in controls and affected individuals. To the far right of each haplotype is the relative ratio of the odds of disease for each haplotype relative to that of the most common haplotype, H1.
Second, because Y402H (rs10801555), rs10737680, and CFHR1–3Δ, are in linkage disequilibrium (LD) (D′ ≥ 0.99), we used conditional logistic regression to assess whether they independently conferred risk (Table 1). A univariate analysis demonstrated significant association to disease for each marker. When we conditioned on Y402H alone, the CFHR1–3Δ effect was present (odds ratio 0.58, 95% confidence interval 0.46–0.72, P = 2 × 10−6), as previously reported1. However, when we conditioned on rs10737680, the statistical strength of the protective effect of CFHR1–3Δ was substantially mitigated (0.72, 0.55–0.95, P = 0.02), though not entirely eliminated. At the same time, conditioning on CFHR1–3Δ did not mitigate the effect of the Y402H and rs10737680 associations (P < 1 × 10−13). On the basis of these results, we concluded that the previously reported associations at CFHR1–3Δ and rs10737680 were not entirely independent.
Table 1.
Conditional logistic regression of CFH Y402H, CFH rs10737680 and CFHR1–3Δ
| Logistic regression model | Y402H (rs10801555) | rs10737680 | CFHR1–CFHR3 deletion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% c.i. | P | OR | 95% c.i. | P | OR | 95% c.i. | P | |
| Single marker model | 0.39 | 0.34–0.46 | 1.2 × 10−35 | 0.38 | 0.33–0.45 | 1.6 × 10−32 | 0.37 | 0.30–0.45 | 6.5 × 10−21 |
| Conditional on Y402H (rs10801555) | – | – | – | 0.58 | .47–0.71 | 1.1 × 10−7 | 0.58 | 0.46–0.72 | 2.3 × 10−6 |
| Conditional on rs10737680 | 3.55 | 0.46–0.66 | 4.5 × 10−10 | – | – | – | 0.72 | 0.55–0.95 | 0.02 |
| Conditional on CFHR1–3Δ | 0.47 | 0.40–0.55 | 7.7 × 10−21 | 0.45 | 0.37–0.55 | 1.8 × 10−14 | – | – | – |
Measurement of whether each of the three biallelic markers has a significant additive effect on AMD risk. For each marker we present the additive odds ratio (OR), the 95% c.i and the statistical significance of that OR. The rs10737680 SNP is a perfect proxy for the previously associated rs1410996 intronic CFH SNP. The first row presents an unconditional univariate analysis for each marker. The next three rows present the effect sizes of each marker after conditioning on each of the markers.
To better understand the disease association within that locus, we identified common haplotypes of 21 biallelic markers (Fig. 1b and Supplementary Table 2). A total of seven haplotypes with frequencies >1% accounted for 95.7% of 3,354 chromosomes. The most frequent H1 haplotype, containing the Y402H risk allele, was present in 59% of chromosomes from affected individuals but only 37% of control chromosomes. For other haplotypes, we calculated the odds ratio of disease association relative to that of H1. As previously observed6, the haplotype risk profiles can be most parsimoniously divided into three groups: high risk (H1, odds ratio = 1; reference), intermediate risk (H2 and H3, odds ratio = 0.60, 95% confidence interval (c.i.) 0.50–0.73) and low risk (H4, H5, H6 and H7, odds ratio = 0.32, 95% c.i. 0.27–0.38). The haplotypes within each group had effect sizes that were indistinguishable from each other (P = 0.71 for H2 and H3; P = 0.30 for H4, H5, H6 and H7). The three haplotype groups had distinct effects on AMD risk (P = 6.8 × 10−43), with nonoverlapping confidence intervals; breaking groups to assign independent risk to each of the seven haplotypes did not better define risk (P = 0.43).
The haplotype analysis demonstrates the relationship between the CFH rs10737680 association and the CFHR1–3Δ association: both markers tag a collection of low-risk haplotypes. The rs10737680 SNP is closely linked to the low-risk haplotypes but misses the rare (1.2%) H4 haplotype, whereas CFHR1–3Δ misses both H4 and H5. Neither tags all of the low-risk haplotypes perfectly, suggesting that there could be one or more not-yet-identified variants that better explain disease risk.
One parsimonious explanation is a single protective functional variant present on low-risk haplotypes H4–H7, in addition to the Y402H risk allele present on H1; such a variant would have very high LD to rs10737680 (r2 > 0.9). Alternatively, a risk variant on intermediate risk haplotypes H2 and H3 could also explain the data. We searched for such markers by (i) imputing 171 ungenotyped SNPs with 205 HapMap CEU and Toscani in Italia (TSI) samples as a reference and (ii) imputing 72 ungenotyped CFH SNPs with 812 published cases and controls as a reference7 (Supplementary Methods). No geno-typed or imputed SNP fulfilled these criteria. Potentially, dense resequencing of this region to ascertain all common variants within this region could identify a functional mutation that fulfills the above criteria.
An alternative but less parsimonious explanation would be the presence of multiple protective functional mutations on the H4–H7 haplotypes that confer approximately equal effect on risk. For example, CFHR1–3Δ or a CFH variant in LD on H6 and H7 haplotypes and the rs800292 CFH coding variant (I62V) on H4 and H5 haplotypes might each confer equivalent protection from disease, and this would explain the observed data.
We and others have published examples in which common genomic copy number variation might alter disease risk. For example, the IRGM association to Crohn's disease maps to an upstream deletion in the regulatory region, that affects the expression of the gene itself10. However, these results suggest the possibility that CFHR1–3Δ may not confer any independent risk of AMD, but may simply be associated with protective CFH haplotypes.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the support of an anonymous donor to the research of J.M.S., without whom this research would not have been possible. We thank the participants and many ophthalmologists throughout the country who contributed to this study, and D. Mirel and the US National Center for Research Resources (NCRR) Broad Institute Center for Genotyping and Analysis for help with design and execution of the genotyping. This research was supported in part by grants K08AR055688-01A1 (S. Raychaudhuri), RO1-EY11309 (J.M.S.), K12-EY16335 (L.S.), U01 MH085520-01 (S. Ripke) and R01-HG004517 (M.L.) from the US National Institutes of Health (NIH); NIH National Eye Institute intramural program; Massachusetts Lions Eye Research Fund, Inc.; a Challenge Grant from Research to Prevent Blindness to the New England Eye Center, Department of Ophthalmology, Tufts University School of Medicine; a Career Development Award from Research to Prevent Blindness (L.S.); a Harvard Catalyst Faculty Fellowship (L.S.); and the Macular Degeneration Research Fund of the Ophthalmic Epidemiology and Genetics Service, New England Eye Center, Tufts Medical Center, Tufts University School of Medicine. We thank the Myocardial Infarction Genetics Consortium (MIGen) study for the use of their genotype data as control data in our study. The MIGen study was funded by grants from the NIH-National Heart, Lung and Blood Institute (R01HL087676) and the NIH-NCRR.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Note: Supplementary information is available on the Nature Genetics website.
References
- 1.Hughes AE, et al. Nat. Genet. 2006;38:1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 2.Klein RJ, et al. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hageman GS, et al. Ann. Med. 2006;38:592–604. [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer KL, et al. Hum. Mol. Genet. 2008;17:971–977. doi: 10.1093/hmg/ddm369. [DOI] [PubMed] [Google Scholar]
- 5.Jozsi M, Zipfel PF. Trends Immunol. 2008;29:380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Maller J, et al. Nat. Genet. 2006;38:1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 7.Li M, et al. Nat. Genet. 2006;38:1049–1054. doi: 10.1038/ng1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zipfel PF, et al. PLoS Genet. 2007;3:e41. doi: 10.1371/journal.pgen.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neale BM, et al. Proc. Natl. Acad. Sci. USA. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarroll SA, et al. Nat. Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.