Introduction
Hippocrates and Aristotle recognized that blood clots outside the body but it was not until 1720 that John Louis Petit recognized that control of hemorrhage following amputation was associated with the blood clotting process.1 138 years later Rudolph Virchow formulated his postulates regarding clots in venous thrombosis.2 The association of blood clots with acute arterial occlusion was advanced by James B. Herrick3 in 1912 but the significance of blood clotting in acute arterial events was only resolved by studies in the 1980s.4–6 As a consequence, our knowledge base has largely been driven by studies of the hemostatic process which have been extrapolated to describe the pathologic occlusive clotting events in venous and arterial thrombosis.
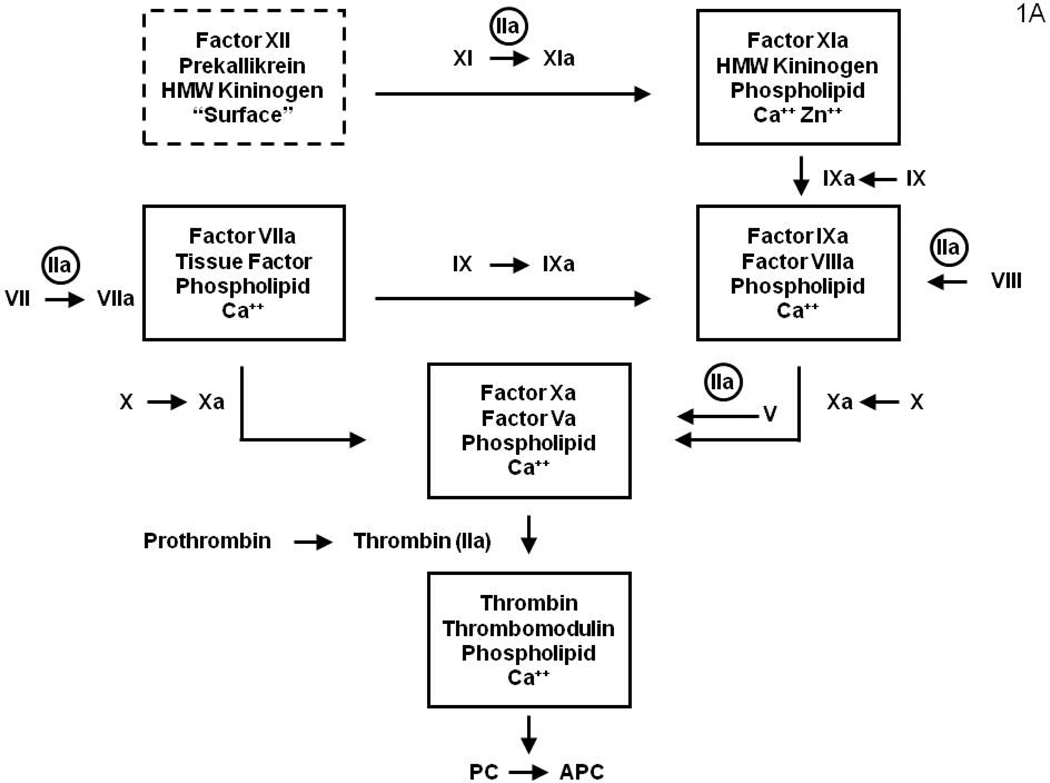
Vascular integrity and blood fluidity are maintained by complex interplay between pro and anticoagulant properties provided by the blood, the vasculature and subvascular elements. Our knowledge of the inventory and connectivity between the blood supplied components of the hemostatic and anticoagulant matrix was initially provided by genetic accidents which produced hemostatic and thrombotic defects; most of which have been subsequently ratified by experiments conducted with transgenic mice. Additional blood and vascular elements were discovered utilizing in vitro assays which identified new entities and required additions to the coagulation/anticoagulation circuits. Pathways for the clotting process were described dependent upon the plasma coming in contact with a foreign surface (the intrinsic pathway)7–9 or associated with the introduction of tissue components (now identified as membrane displayed tissue factor (Tf)) into the plasma (the extrinsic pathway) (Figure 1A).10 However, the absence of bleeding pathology with individuals or transgenic mice displaying molecular abnormalities in the factor (f) XII11 and high molecular weight kininogen12 components of the contact enzyme (dotted box, Figure 1) led to the conclusion that this catalyst is not in the primary pathway maintaining hemostasis. Thus while the contact activation catalyst most likely does not have a role in hemostasis, recent data, with fXII knock out animals11 and in human inflammatory syndromes,13 suggest this pathway may be relevant in some thrombotic disorders.
Figure 1.
Figure 1A: A diagram illustrating the surface bound complex enzymes of the “intrinsic” and “extrinsic” coagulation pathways. The surface bound complex of the intrinsic pathway is outlined by a dashed line. The amplification by thrombin feedback activations of fV, fVIII, fVII and fXI are also illustrated.
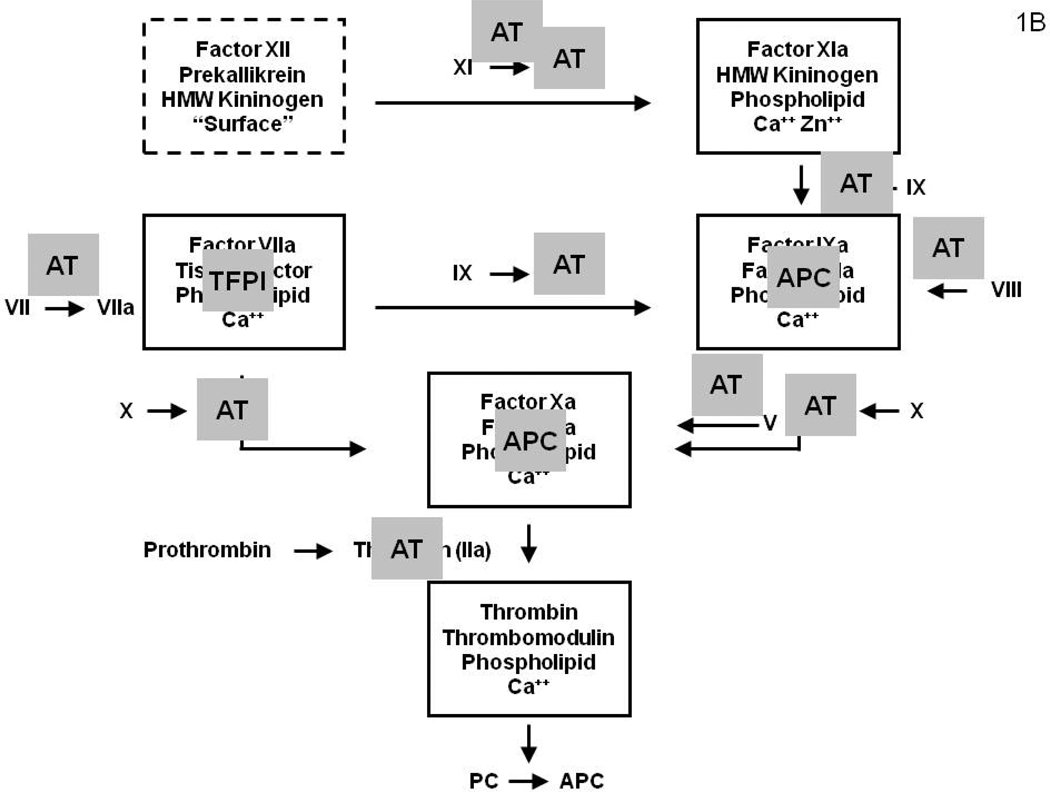
Figure 1B: Coagulation system regulation by the stoichiometric (AT and TFPI) inhibitors which down-regulate the system by binding the serine proteases and the dynamic APC system, which causes proteolytic inactivation the cofactors (fVa and fVIIIa). (Figure 1 from Mann KG. Thrombin Formation CHEST 2003; 124:4S–10S with permission)
The coagulation process is strictly regulated by stoichiometric and dynamic inhibitory processes; the former are primarily a consequence of antithrombin (AT) and tissue factor pathway inhibitor (TFPI); the latter a consequence of the dynamic protein C (PC) system whose function is directly linked to the generation of thrombin (Figure 1B). AT inhibits all the serine proteases of the coagulation system with its function accelerated by heparin and by heparan sulfate14 proteoglycans expressed on the vascular endothelium. TFPI is a kunitz type inhibitor15 which will inhibit both fXa and fVIIa-Tf independently however its primary target is the fXa-fVIIa-Tf product complex.16 TFPI is a very high affinity inhibitor but present in blood at very low concentration and on the vascular endothelium, from which it is secreted by heparin.17
The dynamic PC system integrates endothelial cell thrombomodulin (Tm)18, 19 and its cofactor, the endothelial protein C receptor (EPCR), with thrombin generation.20, 21 The Tm-EPCR-thrombin vascular membrane bound catalyst activates plasma PC to the enzyme activated protein C (APC).20 The anticoagulant APC cleaves and inactivates fVa and fVIIIa.22, 23 AT in conjunction with TFPI and the PC system act in synergy to provide a more potent anticoagulant effect.24, 25 The combination of the stochiometric and dynamic inhibitors produce “go-no go” thresholds for the clotting process, ensuring that the triggering insult is of sufficient magnitude to warrant initiation of a potentially thrombotic response.
Hemostasis and Vascular Occlusion
From the biochemical perspective, common molecular and cellular events are associated with thrombin generation whether involved in protective or pathologic clotting processes. However, it is also clear that the biomechanics of flow, and the vascular architecture and composition are not equivalent in the venous and arterial circuits. Further, in hemorrhage control the source of tissue factor is extravascular while it is intravascular in thrombosis. Nonetheless, for all coagulation processes, the essential enzyme, produced by the choreography of the blood and vascular components, is thrombin; produced from prothrombin as a consequence of three surface bound catalysts (Figure 1A).
In this review, the initial focus is on the process of hemorrhage control with more speculative extensions to clotting in venous and arterial circuits.
Good Clots: Hemorrhage Control
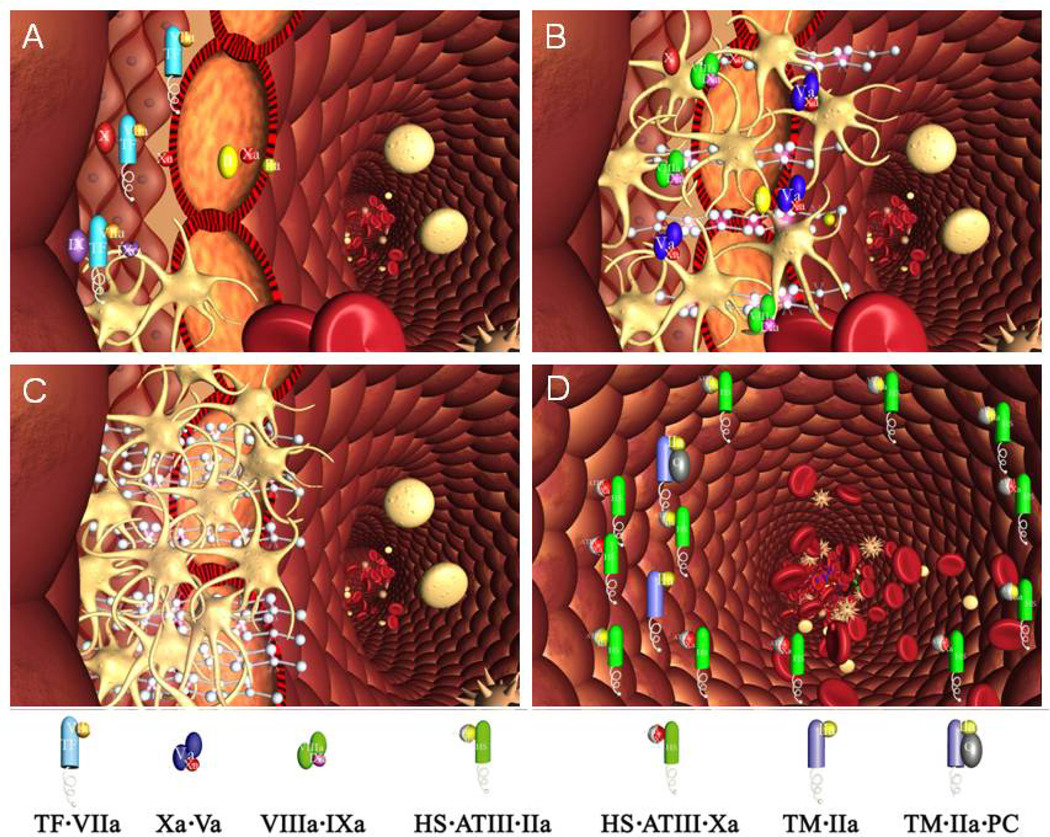
Hemorrhage control involves extravascular thrombin generation leading to a stable fibrin-platelet “dam” which stops blood flow. The environment of the clot is primarily extravascular tissue which elicits thrombin formation following a vascular perforation by platelet adhesion, secretion and aggregation by interactions with connective tissue collagen and blood derived von Willebrand factor.26, 27 Thrombin production is initiated with the presentation of plasma fVIIa, a biologically inactive serine protease,28 to extraluminal subendothelial Tf as a consequence of the vascular perforation (Figure 2A). The fVIIa-Tf complex which generates the initial burst of fXa is primarily regulated by TFPI. The fVIIa-Tf complex activates two substrates fX and fIX to their respective products fXa and fIXa29–31 (Figure 1A). Small amounts of fXa in combination with platelet and tissue membrane surfaces activates a small amount of prothrombin to thrombin.32 Thrombin amplifies its own generation in the adverse environment of overwhelming inhibitor concentrations by activating additional platelets33, 34 and the procofactors (fV and fVIII)35, 36 to produce the cofactors (fVa and fVIIIa) essential to the formation of these membrane bound catalysts (Figure 2B). The membrane binding sites for the intrinsic factor Xase (fVIIIa-fIXa),37 which becomes the major fX activator and prothrombinase (fVa-fXa) include those expressed on the activated platelets, damaged cells at the site of injury and other peripheral blood cells.38, 39 The complex catalysts are 105–107 fold more active than their constituent serine proteases and their membrane binding localizes their reactivity to the site of vascular injury.38, 40 The formation of these surface bound catalysts leads to local “explosive” generation of thrombin in the blood leaked into extravascular space. These catalysts anchored at the wound site are protected from inactivation by AT. The catalysts continue to build up in the wound site as more platelets and plasma substrates are delivered by blood leaking through the site. The α-thrombin end product of the coagulation reaction system cleaves fibrinopeptides A and B from fibrinogen leading to expanding fibrin clot formation which is stabilized by the concomitant thrombin activation of fXIII to fXIIIa which produce cross links between the aggregated fibrin monomers. The dynamics of fibrin formation aggregation and cross-linking are tightly integrated leading to a stable clot of sufficient mechanical strength to prevent blood loss.41–43 The reaction continues to proceed as long as more reactants are delivered to the wound site. The intra-extra vascular two compartment reaction system will produce thrombin, fibrin and aggregated platelets until the extravascular compartment becomes starved of reactants and by closure of the wound site (Figure 2C) by the thrombus. Coagulant enzymes escaping from the wound sites by complex dissociation are inactivated by AT. Thrombin down regulates its intravascular production through activation of the PC system. Thrombin binds to endothelial cell Tm and this thrombin-Tm complex aided by EPCR activates plasma PC to APC. APC cleaves fVa and fVIIIa inactivating the cofactors23, 44 (Figure 2D). Systemic thrombotic occlusion does not occur because of the potent negative regulatory elements provided by the vascular wall and plasma. Thrombin also initiates secretion of plasminogen activators which initiate clot dissolution concurrent with vascular repair.45
Figure 2. Schema of a two compartment model of the regulation of TF-initiated blood coagulation.
A cross section of a blood vessel showing the luminal space, endothelial cell layer and extravascular region is presented at the site of a perforation. The blood coagulation process in response is depicted in four stages. Tissue factor-factor VIIa complex, TF•VIIa; prothrombinase complex, Xa•Va; intrinsic factor Xase, VIIIa•IXa; ATIII-endothelial cell heparan sulfate proteoglycan complex bound to thrombin or factor Xa, HS•ATIII• (IIa or Xa); protein C bound to thrombomodulin-thrombin,TM•IIa•PC. Panel A. Perforation results in delivery of blood, and with it circulating factor VIIa and platelets, to an extravascular space rich in membrane bound TF. Platelets adhere to collagen and von Willebrand factor associated with the extravascular tissue, and TF binds factor VIIa, initiating the process of factor IX and factor X activation. Factor Xa activates small amounts of prothrombin to thrombin that activates more platelets and converts factor V and factor VIII to factor Va and factor VIIIa. Panel B. The reaction is propagated by platelet-bound intrinsic factor Xase and prothrombinase with the former being the principle factor Xa generator. Initial clotting occurs and fibrin begins to fill in the void in cooperation with activated platelets. Panel C. A barrier composed of activated platelets ladened with procoagualant complexes and enmeshed in fibrin scaffolding is formed. The reaction in the now filled perforation is terminated by reagent consumption attenuating further thrombin generation but functional procoagulant enzyme complexes persist because they are protected from the dynamic inhibitory processes found on the intravascular face. Panel D. View downstream of the perforation. Enzymes escaping from the plugged perforation are captured by antithrombin-heparan complexes and the protein C system is activated by residual thrombin binding to endothelial cell thrombomodulin, initiating the dynamic anticoagulant system. These intravascular processes work against occlusion of the vessel despite the continuous resupply of reactants across the intravascular face of the thrombus. From Orfeo, J. Biol. Chem. 280:42887–42896, 2005. With permission.
The procoagulant reaction is multi-phasic and can be divided operationally into three segments: initiation, propagation and termination.a The latter is concurrent throughout the entire process. The events occurring during the initiation phase include the activation of platelets, cofactors and serine proteases which form the membrane assembled complexes which drive most thrombin generation during the propagation phase.
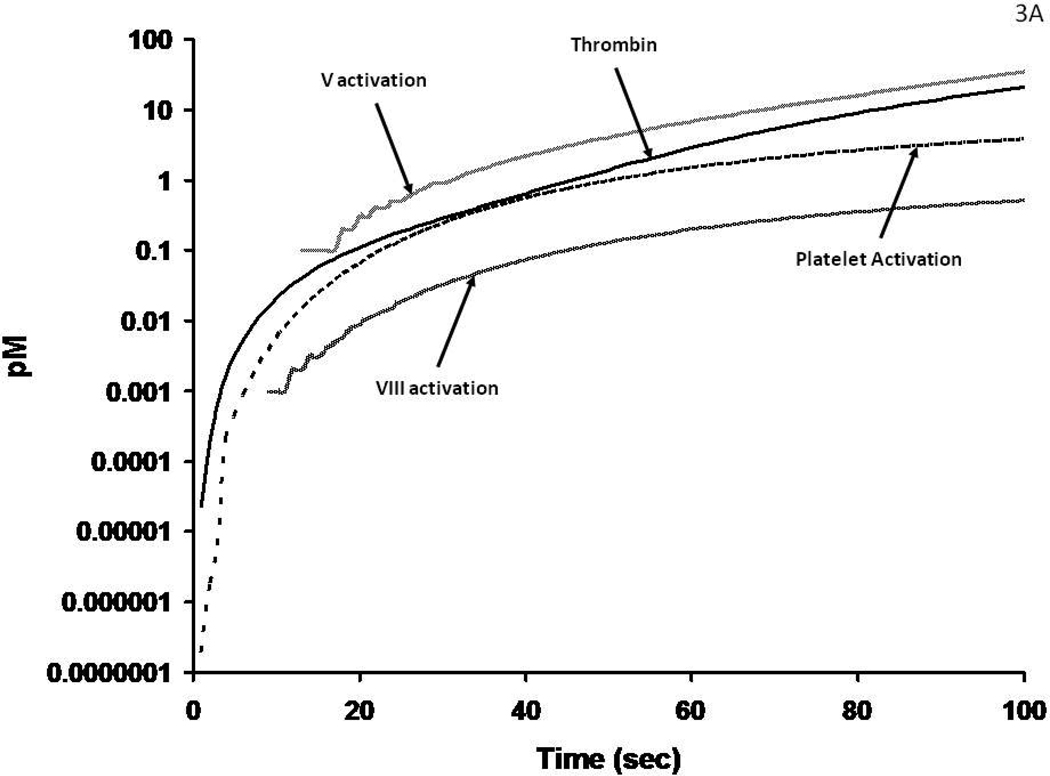
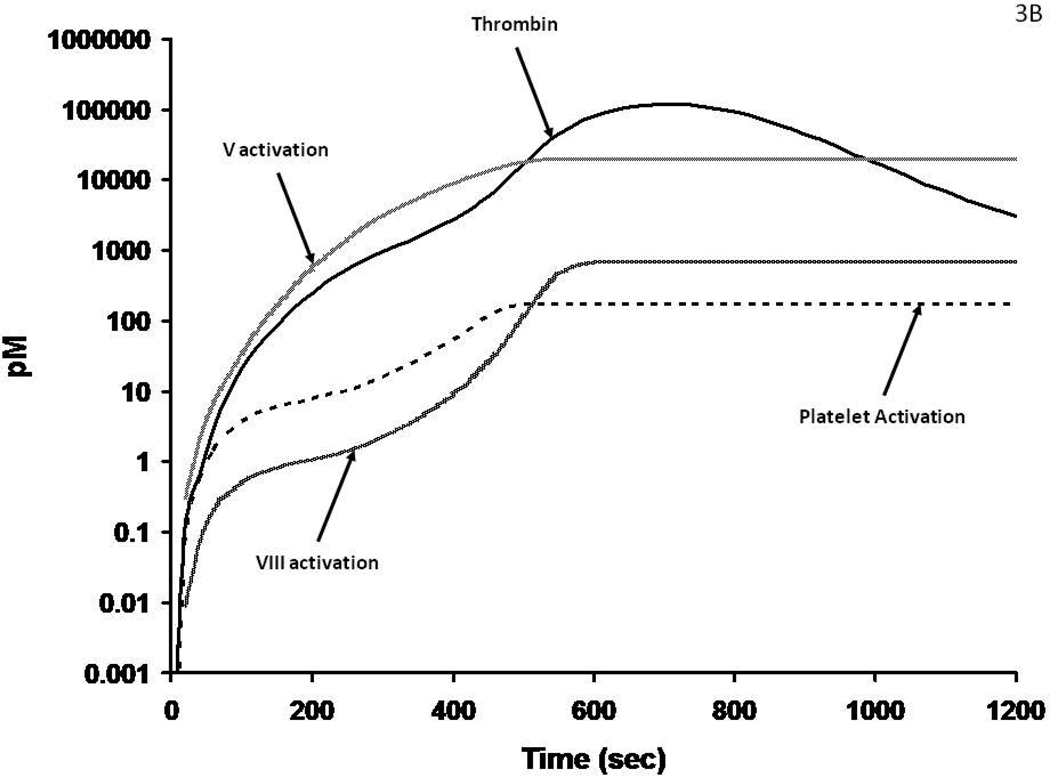
The initiation phase activation events are illustrated by a numerically modeled time course46 (Figure 3A) illustrating the dynamics of initial thrombin formation, the activation of platelets, fV and fVIII. These processes involve thrombin cleavages of PAR-1 and PAR-434, 47 which activate platelets and cleavages of the procofactors fV and fVIII that release activation peptides and provide the cofactors viz: fVa and fVIIIa. This interval of the reaction is driven by the tiny amounts (10−17 – 10−14 molar) thrombin (Figure 3A) produced by fXa during the initiation phase. The activated platelets provide specific (and presently uncharacterized) receptor sites for the fVIIIa-fIXa and fVa-fXa complexes. The overall time course for presentation of the procoagulant catalysts is presented in Figure 3B. Enhanced activation occurs because of accelerated thrombin formation by prothrombinase which is >105 times more active than unbound fXa, with amplification of fXa generation via fVIIIa. FIXa resulting in accelerated thrombin formation. Further when bound in complex with fVIIIa and fVa, fIXa and fXa are not accessible to AT.
Figure 3.
Figure 3A: Numerical simulations of1 the earliest events leading to catalyst formation with a Tf insult. The scale is logarithmic illustrating the initial activation over time of fV, fVIII and platelets by the thrombin initially produced (by fXa) in the reaction.
Figure 3B: The generation of thrombin, fVa and fVIIIa and platelet activation over the entire course of the reaction. The thrombin catalyzed feedback amplifications resulting in enhanced platelets and fVIII activation associated with the generation of increased amounts of thrombin by the prothrombinase complex are shown in this numerical simulation.
In both whole blood and plasma, fibrinogen clotting41, 48 occurs at the intersection between the initiation and propagation phases when ≤ 5% of the prothrombin is converted to thrombin. Thus clot base assays, i.e., the activated clotting time (ACT), the prothrombin time (PT) and the partial thromboplastin time (PTT) exclude 95% of the reaction.
Most clotting analyses are conducted in a “closed” system for which the reactants present at the beginning of the reaction are largely consumed in the resulting pro and anticoagulant processes. In contrast, during hemorrhage, flow would continue until a coagulant platelet-fibrin “dam” is established. Insights into the continuity of the process with flow are provided by experiments in which new supplies of blood are made available to an apparently quiescent Tf initiated reaction after no additional thrombin-AT (TAT) is accumulating i.e. in “resupply” experiments.49 The addition of a new blood supply-reactants to a quiescent thrombus results in rapid and more extensive thrombin generation. This over-response is a consequence of the platelet bound procoagulant complex enzymes resident in the original thrombus generating more products from the substrates present in the newly added blood. In hemophilia the absence of intrinsic factor Xase (fVIIIa-fIXa) prevents this accelerated fXa generation and lesser amounts of thrombin are produced at a slower rate. As a consequence an imperfect cross-linked fibrin clot is formed with inadequate strength to block blood flow.50, 51
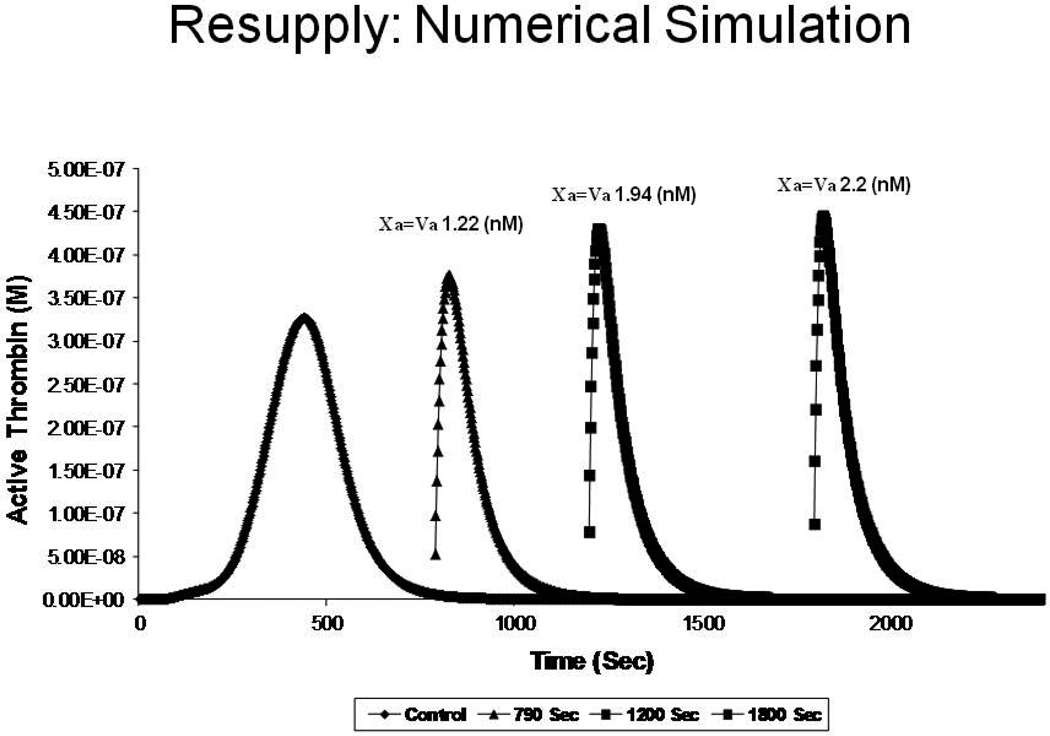
A numerical simulation of consecutive resupplies of “electronic blood” is illustrated in Figure 4. In this case, total thrombin is plotted vs. time for an experiment in which fresh aliquots of “plasma” are added after the initial Tf reaction becomes quiescent with respect to active thrombin. Each new resupply produces a faster rise of thrombin activity to a higher level as a consequence of the increased catalyst concentrations generated. The data show that the catalysts present in the quiescent thrombus when provided with more substrate will generate more catalysts which provide more intense thrombin generation, a process that will continue to expand until the leak is sealed. It should be noted here that no additional Tf is required and the reaction is no longer dependent on the initial Tf trigger which began the thrombin generating process.49
Figure 4.
A numerical simulation of consecutive resupply of “electronic” plasma reactants to a quiescent thrombus; at 790, 1200 and 1800 seconds resulting in a more intense thrombin generation and providing higher concentrations of prothrombinase (Xa = Va). From Orfeo, J. Biol. Chem. 280:42887–42896, 2005. With permission.
Thus, hemorrhage control can be described as a two compartment model for Tf initiated blood coagulation. Vascular perforation and blood extravascular flow leads to platelet adhesion, secretion and aggregation. Plasma fVIIa binds extravascular Tf leading to the generation of the procoagulant complexes and thrombin. In an open system, flow will continue to supply substrates to the reactive and growing thrombus until the vascular leak is plugged or the blood supply is exhausted. The reaction system is then deprived of additional substrates and the proteases are ultimately neutralized by AT. Any reactive enzyme components escaping downstream are inactivated by the excess of stoichiometric inhibitors and vascular Tm which combines with surplus thrombin to activate PC to APC which inactivates fVa and fVIIIa.
Bad Thrombin Generation: Venous Thrombosis
Virchow postulated elements that are associated with the formation of occlusive venous clots including hypercoagulable elements within the blood, endothelial injury or dysfunction and alterations of blood flow.2 Clinical experience with venous thrombosis endorses the use of the anticoagulants heparin and Warfarin which serve to enhance the AT neutralization process14 or impair the biosynthesis of the procoagulant zymogens.52 In general, platelet inhibitors are ineffective.
Congenitally acquired plasma risk factors for venous thrombosis are fVLeiden and PC and protein S (PS) deficiencies.53–55 These deficiency states are not ordinarily associated with arterial events. Thus, the venous occlusive pathologies associated with these three congenital deficiencies clearly suggest that the impaired inhibition of thrombin production by elements associated with the Tm-EPCR-PC system are contributors to venous thrombosis at the level of the vascular wall.18, 19, 21
The endothelial cells which line the vascular wall are heterogeneous with respect to their display of anticoagulant and fibrinolytic properties56–59. Unfortunately insufficient quantitative data are available to define the concentrations of the vascular wall anticoagulants Tm, EPCR, heparan-sulfate proteoglycan (HSP) and TFPI; all of which are major regulators of the blood clotting pathway.
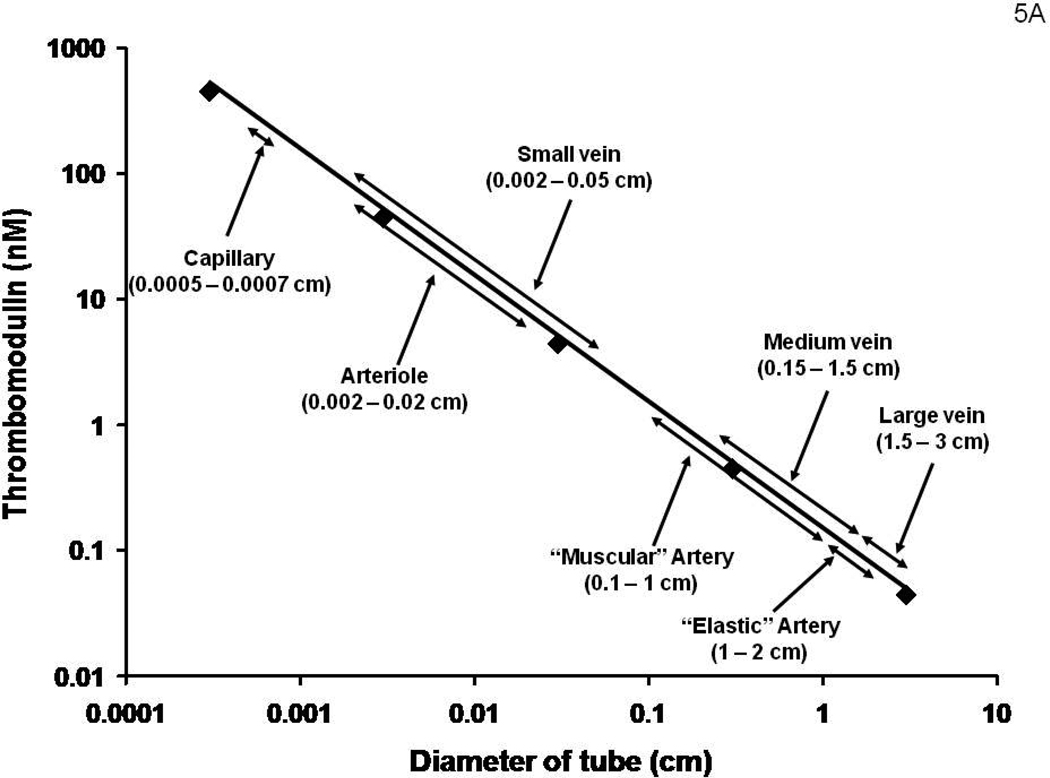
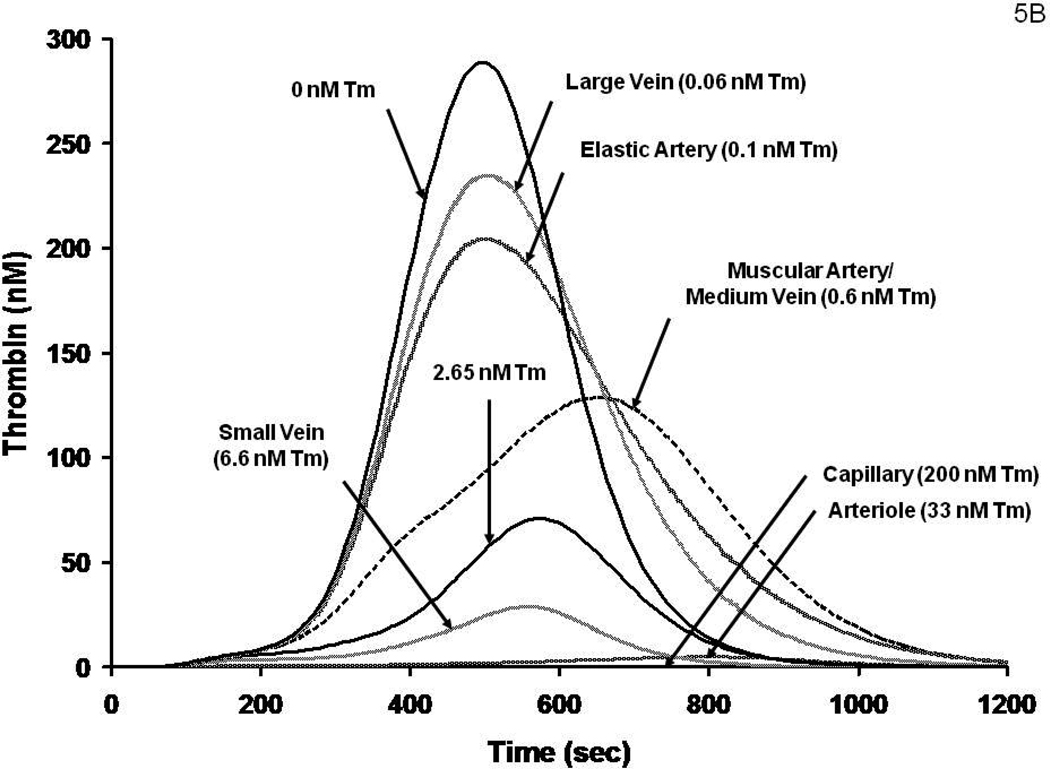
It has been suggested that the endothelial cell surface area per unit blood volume would permit a “first pass” estimate of the anticoagulant concentrations provided by the vasculature.60 We have used this approach to estimate the effective TM concentrations in different vessels. These data are shown in Figure 5A. Numerical simulations for the thrombin generation which would occur in a closed system (no flow) with these vessels with identical Tf insults are presented in Figure 5B. The high concentrations of Tm present in arterioles and capillaries suggest that these vessels are protected from a Tf insult even under relatively static conditions of blood flow unless impaired with respect to Tm presentation or other defects in the PC regulatory pathway. PC and PS congenital deficiencies represent the extremes of such pathologies (lethality). 54, 55 The result is purpura fulminans; a consequence of systemic microvascular thrombosis. FVLeiden represents a better tolerated and more common venous thrombosis risk factor that is also an impairment in this system (but at the substrate level). Therefore it is reasonable to speculate that among the constellation of candidate events likely occurring in venous thrombosis are impairments of the thrombin-PC-EPCR-Tm system. A reduction in anticoagulation potency coupled with low flow or stasis and a blood-borne or vascular cell source of Tf would result in an intra-vascular triggering of the coagulation mechanism.
Figure 5.
Figure 5A: A hypothetical construct displaying the potential molar concentrations of Tm in various regions of the vasculature. An underlying assumption is that the Tm concentration by each endothelial cell is independent of the vascular source.
Figure 5B: A numerical simulation of thrombin generation versus time in vessels displaying the Tm concentrations estimated in Figure 5A following a Tf insult.
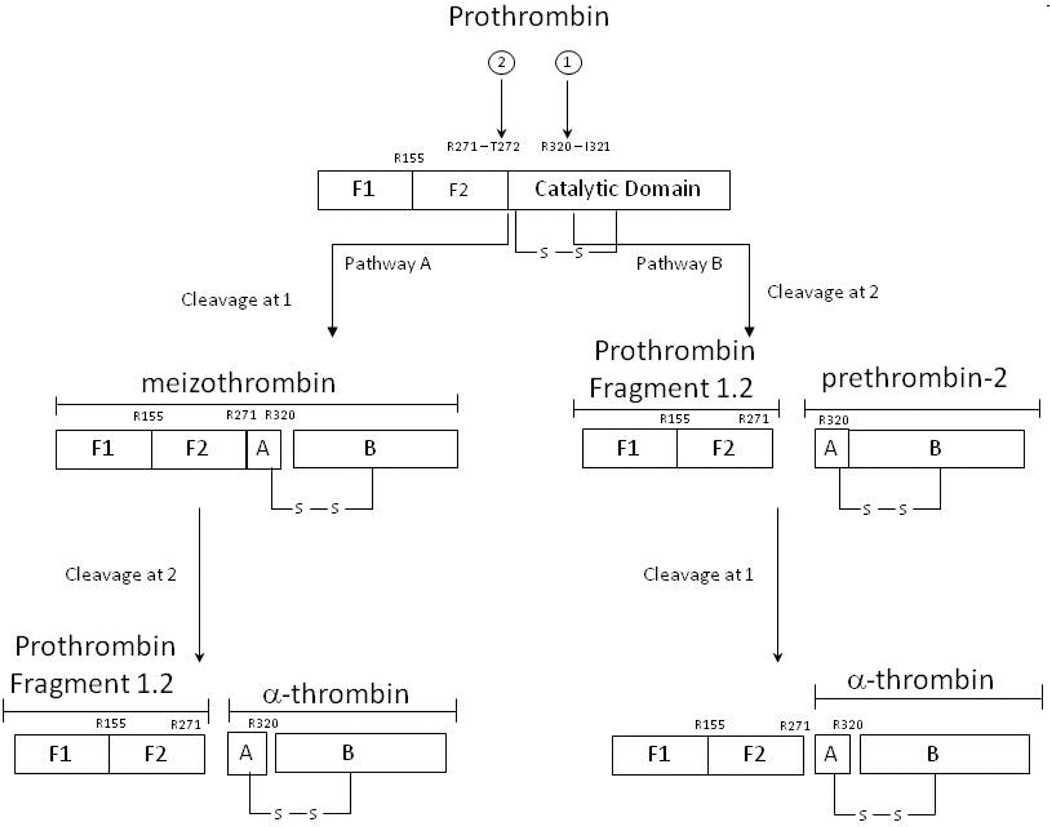
Paradoxically thrombin is not only a procoagulant but also an anticoagulant. Prothrombin activation (Figure 6) involves two peptides bond cleavages with the predominant reaction pathway proceeding through meizothrombin which is subsequently cleaved to give α-thrombin. The activity of meizothrombin is preferentially anticoagulant in character (Table 1). In closed systems, meizothrombin is short lived and rapidly converted to α-thrombin, however under conditions of flow significant amounts of meizothrombin accumulate. This significant fraction (~50%) of thrombin formed at the vessel wall under conditions of flow is primarily anticoagulant in nature.61
Figure 6.
Pathways of Prothrombin Activation. The order of the two bond cleavages yield either the thrombin precursor prethrombin-2 or the enzyme, meizothrombin, as intermediates From Krishnaswamy J. Biol. Chem. 262:3291-99, 1987. With permission.
Table 1.
| Meizothrombin Activity | |
|---|---|
| Substrate | Relative Activity (% α-IIa)* |
| Fibrinogen | 7%A |
| Platelets | 2%A |
| Factor V | 41%B |
| Tm + Protein C | 93%A |
| Tm + TAFI | 10%A |
| Antithrombin | 25%A |
Based on comparison between r-wt-α-IIa and r(R155A, R271A, R284A)mIIa
Côté HCF, Bajzar L, Stevens WK, Samis JA, Morser J, MacGillivray RT, Nesheim ME J. Biol. Chem. 272:6194–6200, 1997
Orfeo T, Brufatto N, Nesheim ME, Xu H, Butenas S, Mann KG. J. Biol. Chem. 279: 19580–19591, 2004.
Virchow postulated a circulating source of hypercoagulability which may be attributed to a source of Tf within the blood. Potential mobile targets include inflammatory blood cells expressing Tf on their surfaces or cell-derived microparticles bearing Tf.62–65 In addition to blood borne procoagulants, the endothelium itself may contribute. The unperturbed endothelial cell in vitro does not appear to express Tf at concentration levels which would likely provoke a coagulant response.62, 66, 67 However, with inflammatory cytokine (TNF-α) stimulation a variety of observations suggest that relevant levels of Tf may be expressed by endothelial cells in vitro.68 Conversely, there is no question that the leukocyte populations in blood, in particular the monocyte, when subjected to inflammatory cytokines, translocates, internally stored, highly procoagulant Tf to their surface. These cells are likely candidates for Virchow’s mobile source of systemic hypercoagulability. Similarly, cell-derived “microparticles” in circulation may provide a mobile source of Tf which under conditions of low flow and impaired endothelium would produce a venous clot.69–72 A Tf trigger with low flow and impaired local regulation would produce explosive thrombin generation and occlusive clot formation within the vessel.
Ugly Clots: Arterial Occlusion
In contrast to the three centuries of experience with clotting in hemorrhage control and two centuries of experience with venous thrombosis, clotting in the arterial circuit has been investigated intensely only in more recent times. Arterial clots differ from venous clots in a number of respects. They are highly platelet dependent (“white clots”), occur in vessels of high shear and occur in response to a local intravascular presentation of Tf associated with rupture of an atherosclerotic lesion.6, 73 Clinical anticoagulation in venous and arterial environments is routinely provided by Warfarin anticoagulation. While systemic antiplatelet agents are largely ineffective in venous thrombosis they are, in contrast, highly effective for arterial thrombosis. Prophylaxis with systemic antiplatelet therapy has proven quite effective. 74, 75 The basis for the higher quantities of accumulated platelets in arterial thrombi is most likely a consequence of the superposition of a high shear environment with other aspects governing thrombin generation.
Under conditions of flow, thrombin generation from prothrombinase is highly shear dependent. 76–78 When prothrombin is activated under conditions of flow with a vessel wall-bound prothrombinase catalyst, the substrate, prothrombin, arrives to the wall-bound catalyst prothrombinase primarily by diffusion only from the solvent in close proximity to the vessel wall and the reaction product is limited to the fluid volume immediately adjacent to the vessel wall. As the flow rate is increased, new solvent entering the vessel segment dilutes the thrombin which is produced at the vessel wall. In blood, that thrombin would be surrounded by high concentrations of AT and blocked from function. As a consequence, as flow rates increase decreasing concentrations of active thrombin are found in regions proceeding from the vessel wall into the vessel lumen downstream from the point of its generation.
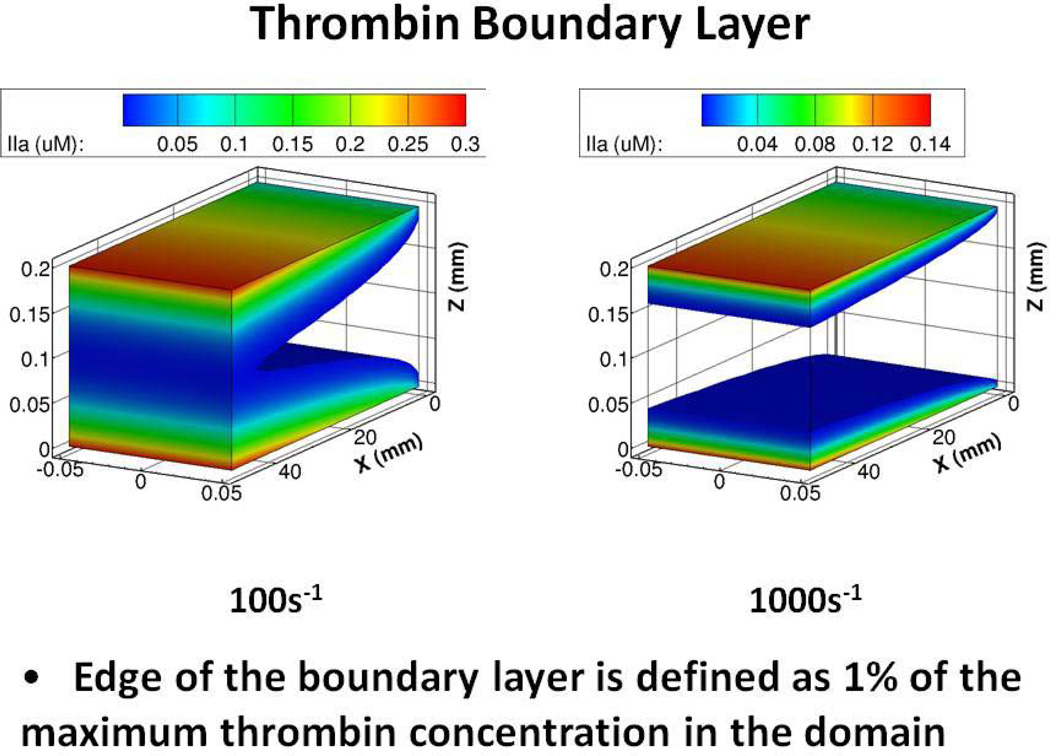
From a biomechanical standpoint, thrombin generation by immobilized prothrombinase under conditions of flow is largely under dilutional control. As flow rates increase thrombin produced in a local region of a vessel is largely “washed out” by the incoming blood supply leading to greatly reduced thrombin concentrations in regions extending from the vessel wall site of its production. These boundary layer concentrations can be predicted by computational fluid dynamics model data for concentrations downstream from the point of formation (Figure 7). At venous flow rates (100 per sec) significant thrombin concentrations extend throughout the vessel. In contrast, at arterial flow rates, because of dilution effects by the incoming stream fluid over the reaction site, thrombin is rapidly diluted and high concentrations are only generated in very close proximity to the vessel wall. As a consequence, abnormalities which reduce flow serve to effectively reduce vessel aperture to dimensions essential to generate clot-effective thrombin concentrations in larger vessels at arterial shear rates. Flow restrictions by the vascular architecture alterations associated with plaque and platelet accumulation are thus essential components altering flow parameters to permit a concentration of thrombin to be produced throughout the vessel to achieve a stable platelet-fibrin clot.
Figure 7.
Computational flow dynamic model of thrombin concentrations extending from the wall site of formation at venous (100s−1) and arterial (1000s−1) shear rates. (from the data of Haynes et al (with permission) . Figure provided by Yves Dubief.
Platelets are essential components for hemorrhage control as evidenced by the sensitivity of the bleeding time to their function and number.79 In vitro in static blood, clotting becomes sensitive to platelets at concentrations below 10,000/µl while effective thrombin generation during the propagation phase requires platelet counts approaching 200,000/µl.80 Impairment of platelet function with the platelet IIbIIIa antagonists, Integrilin and Abcixamab, and with aspirin have a significant influence on the rate of thrombin generation during the propagation phase of the reaction in blood and are recognized antiocclusive pharmaceuticals81–85 in the arterial circuit.
Platelet function is required to provide the binding sites upon which coagulation complexes are formed,39, 86 but in arterial flow, platelets also provide the anchor for clot location and probably contribute to flow impairment which permits extension of the clot into the lumen to produce an occlusion of the vessel.
In whole blood activated with Tf, the initial platelet-fibrin clot forms at thrombin concentrations of approximately 2 nM in a closed (no flow) system. Thus 2 nM thrombin seems to be a reasonable concentration which must be locally developed in a flowing system to produce a fibrin clot.
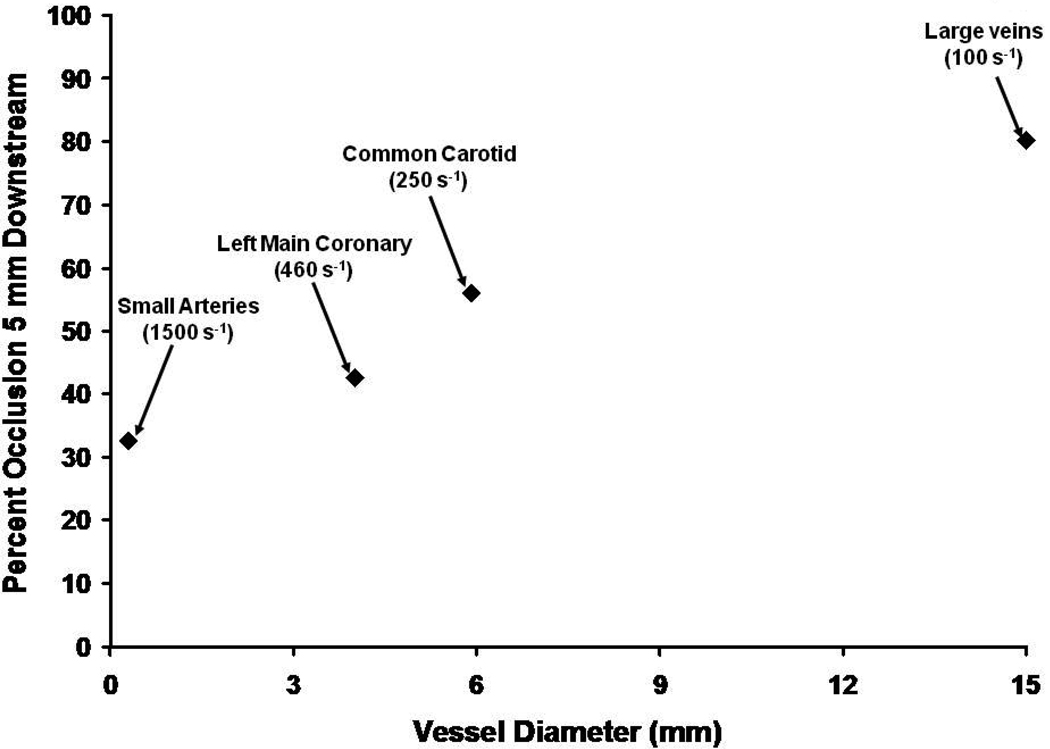
If we use 2 nM thrombin as the platelet-fibrin clot threshold,87we can estimate the vertical height intruding into the vessel lumen that a clot might extend from the vessel wall as a function of shear (Figure 8). This analysis suggests that at (healthy) arterial flow rates clot penetration into the vessel lumen would be significantly reduced by flow dilution and inhibition as the shear rate increases. Vessel diameter thus would also play a role. Therefore from fluid mechanics-and geometry, at high shear rates, a vascular obstruction or irregularity must be present to permit development of an occlusive clot in a vessel of moderate diameter. It is likely this phenomenon and the deviation in vascular architecture associated with an atherosclerotic lesion requires the accumulation of platelets to product a “white” platelet rich clot which is also dependent on thrombin generation and fibrin accumulation. Furthermore, it is likely that architectural features which influence the shear rate in local thrombin concentrations will also influence the biophysical properties and stability of the fibrin-platelet thrombus. 43
Figure 8.
A hypothetical estimate of the extent of vascular occlusion which would occur 5 mm downstream from an anchored thrombin generating catalyst (prothrombinase) in the absence of platelets. The illustration is based upon a combination of fluid mechanics analyses with empirically defined enzyme kinetics studied in a flowing system. The data suggest that in smaller vessels at high shear rates a Tf induced thrombin generation with plasma constituents alone would not lead to occlusion without another source of vascular obstruction (i.e. a platelet thrombus).
Summary
The outcome of a blood clotting event is either desirable or undesirable. All these events involve the generation of thrombin from plasma components, the participation of platelets and the local conditions associated with the vascular environment. “Good” hemostatic clots are largely relegated to the extravascular environment while pathologic occlusive clots are associated with the presentation of Tf within the vasculature. While reactions in both of these environments involve platelet function the high shear present in arterial environments requires the participation of platelets and both pathologies may involve systemic inflammatory-coagulation syndromes.88 The varying effectiveness of the nature and intensity of anticoagulant treatment for venous and arterial thrombosis are a consequence of these biochemical cell biological and biomechanical phenomena.
Data for the contributions of the vascular anticoagulation systems i.e. Tm, EPCR, TFPI, heparan sulfate proteoglycan are not qualitatively or quantitatively established within the vasculature. The development of data regarding the concentrations of these anticoagulants in different vascular beds is essential to the meaningful evaluation of the dynamics that influence the susceptibility of an occlusive clot forming in different sites in the vascular anatomy.
Acknowledgements
I thank Drs. Butenas, Brummel-Ziedins and Orfeo for their contributions to the science reported here; Ms. Laura Haynes and Dr. Yves Dubief for permitting inclusion of some of their unpublished data; Matt Whelihan, Matt Gissel and Laura Clayton for their assistance in preparing this manuscript; and Drs. Burt Sobel and Russell Tracy for their advice and counsel.
Funding Sources
Most of our research has been supported by the NHLBI, most recently HL46703 and HL007594.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A narrated cartoon for hemostasis is available.
Part I, Initiation: http://www.youtube.com/watch?v=RP1eLdkdJKg
Part II, Propagation: http://www.youtube.com/watch?v=oj9E33BZMsI
Conflict of Interest Disclosures
Dr. Mann is the Chairman of the Board of Haematologic Technologies, Inc. He has received research support from Johnson and Johnson. He has also received honoraria from Johnson and Johnson, Daiichi Sankyo, Boehringer Ingelheim and Merck.
References
- 1.Owen CA., Jr . A History of Blood Coagulation. Rochester, MN: Mayo Foundation for Medical Education and Research; 2001. [Google Scholar]
- 2.Virchow R. "Thrombose und Embolie. Gefässentzündung und septische Infektion". In: Matzdorff AC, Bell WR, editors. Gesammelte Abhandlungen zur wissenschaftlichen Medicin. Translation. Canton, Massachusetts: Science History Publications; 1998. p. 1856. [Google Scholar]
- 3.Herrick JB. Landmark article (JAMA 1912). Clinical features of sudden obstruction of the coronary arteries. JAMA. 1983;250:1757–1765. [PubMed] [Google Scholar]
- 4.DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, Lang HT. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N.Engl.J.Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 5.Hillis LD, Borer J, Braunwald E, Chesebro JH, Cohen LS, Dalen J, Dodge HT, Francis CK, Knatterud G, Ludbrook P, Markis JE, Mueller H, Desvigne-Nickens P, Passamani ER, Powers ER, Rao AK, Roberts R, Roberts WC, Ross A, Ryan TJ, Sobel BE, Williams DO, Zaret BL Co-Investigators. High dose intravenous streptokinase for acute myocardial infarction: preliminary results of a multicenter trial. J Am Coll Cardiol. 1985;6:957–962. doi: 10.1016/s0735-1097(85)80294-1. [DOI] [PubMed] [Google Scholar]
- 6.Davies MJ, Thomas AC. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davie EW, Ratnoff OD. Waterfall sequence for intrinstic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 8.MacFarlane RG. An enzyme in the blood clotting mechanism and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 9.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 10.Morawitz P. Die chemie der blutgerrinnung. Ergebn Physiol. 1905;4:307. [Google Scholar]
- 11.Pauer HU, Renne T, Hemmerlein B, Legler T, Fritzlar S, Adham I, Muller-Esterl W, Emons G, Sancken U, Engel W, Burfeind P. Targeted deletion of murine coagulation factor XII gene-a model for contact phase activation in vivo. Thromb Haemost. 2004;92:503–508. doi: 10.1160/TH04-04-0250. [DOI] [PubMed] [Google Scholar]
- 12.Schmaier AH. The plasma kallikrein-kinin system counterbalances the renin-angiotensin system. J Clin Invest. 2002;109:1007–1009. doi: 10.1172/JCI15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg RD, Bauer KA. The heparin-antithrombin system:A natural anticoagulant mechanism. In: Colman RW, Hirsch J, Marder VJ, Salzman EW, editors. Hemostasis and Thrombosis:Basic Principles and Clinical Practice. 3rd ed. Philadelphia: J.B. Lippincott Company; 1994. pp. 837–860. [Google Scholar]
- 15.Broze GJ., Jr Tissue factor pathway inhibitor. Thromb.Haemost. 1995;74:90–93. [PubMed] [Google Scholar]
- 16.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273:4378–4386. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 17.Maroney SA, Mast AE. Expression of tissue factor pathway inhibitor by endothelial cells and platelets. Transfus Apher Sci. 2008;38:9–14. doi: 10.1016/j.transci.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esmon NL, Owen WG, Esmon CT. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J.Biol.Chem. 1982;257:859–864. [PubMed] [Google Scholar]
- 19.Esmon CT. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J. 1995;9:946–955. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- 20.Regan LM, Stearns-Kurosawa DJ, Kurosawa S, Mollica J, Fukudome K, Esmon CT. The endothelial cell protein C receptor. Inhibition of activated protein C anticoagulant function without modulation of reaction with proteinase inhibitors. J Biol Chem. 1996;271:17499–17503. doi: 10.1074/jbc.271.29.17499. [DOI] [PubMed] [Google Scholar]
- 21.Esmon CT. Structure and functions of the endothelial cell protein C receptor. Crit Care Med. 2004;32:S298–S301. doi: 10.1097/01.ccm.0000126128.64614.81. [DOI] [PubMed] [Google Scholar]
- 22.Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994;269:31869–31880. [PubMed] [Google Scholar]
- 23.Fay PJ, Smudzin TM, Walker FJ. Activated protein C-catalyzed inactivation of human factor VIII and factor VIIIa. Identification of cleavage sites and correlation of proteolysis with cofactor activity. J Biol Chem. 1991;266:20139–20145. [PubMed] [Google Scholar]
- 24.van 't Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin- III, and heparin cofactor-II. J Biol Chem. 1997;272:4367–4377. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 25.van 't Veer C, Golden NJ, Kalafatis M, Mann KG. Inhibitory mechanism of the protein C pathway on tissue factor-induced thrombin generation. Synergistic effect in combination with tissue factor pathway inhibitor. J Biol Chem. 1997;272:7983–7994. doi: 10.1074/jbc.272.12.7983. [DOI] [PubMed] [Google Scholar]
- 26.Ruggeri ZM, De Marco L, Gatti L, Bader R, Montgomery RR. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983;72:1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brass LF. Thrombin and platelet activation. Chest. 2003;124:18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- 28.Butenas S, Mann KG. Kinetics of human factor VII activation. Biochemistry. 1996;35:1904–1910. doi: 10.1021/bi951768c. [DOI] [PubMed] [Google Scholar]
- 29.Osterud B, Rapaport SI. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc.Natl.Acad.Sci.U.S.A. 1977;74:5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bom VJ, Bertina RM. The contributions of Ca2+, phospholipids and tissue-factor apoprotein to the activation of human blood-coagulation factor X by activated factor VII. Biochem.J. 1990;265:327–336. doi: 10.1042/bj2650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komiyama Y, Pedersen AH, Kisiel W. Proteolytic activation of human factors IX and X by recombinant human factor VIIa: effects of calcium, phospholipids, and tissue factor. Biochemistry. 1990;29:9418–9425. doi: 10.1021/bi00492a016. [DOI] [PubMed] [Google Scholar]
- 32.Orfeo T, Brufatto N, Nesheim ME, Xu H, Butenas S, Mann KG. The factor V activation paradox. J.Biol.Chem. 2004;279:19580–19591. doi: 10.1074/jbc.M400727200. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 34.Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 35.Nesheim ME, Mann KG. Thrombin-catalyzed activation of single chain bovine factor V. J.Biol.Chem. 1979;254:1326–1334. [PubMed] [Google Scholar]
- 36.Fay PJ. Subunit structure of thrombin-activated human factor VIIIa. Biochim.Biophys.Acta. 1988;952:181–190. doi: 10.1016/0167-4838(88)90114-8. [DOI] [PubMed] [Google Scholar]
- 37.Fay PJ, Koshibu K. The A2 subunit of factor VIIIa modulates the active site of factor IXa. J.Biol.Chem. 1998;273:19049–19054. doi: 10.1074/jbc.273.30.19049. [DOI] [PubMed] [Google Scholar]
- 38.Walsh PN, Ahmad SS, Ashby B, Walsh PN. Kinetics of coagulation factor X activation of platelet-bound factor IXa. Biochem. 1990;29:2606–2611. doi: 10.1021/bi00462a025. [DOI] [PubMed] [Google Scholar]
- 39.Tracy PB, Nesheim ME, Mann KG. Coordinate binding of factor Va and factor Xa to the unstimulated platelet. J.Biol.Chem. 1981;256:743–751. [PubMed] [Google Scholar]
- 40.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J.Biol.Chem. 1979;254:10952–10962. [PubMed] [Google Scholar]
- 41.Brummel KE, Butenas S, Mann KG. An integrated study of fibrinogen during blood coagulation. J Biol Chem. 1999;274:22862–22870. doi: 10.1074/jbc.274.32.22862. 6. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Carlisle CR, Sparks EA, Guthold M. The mechanical properties of single fibrin fibers. J Thromb Haemost. 2010;8:1030–1036. doi: 10.1111/j.1538-7836.2010.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weisel JW. Biomechanics in hemostasis and thrombosis. J Thromb Haemost. 2010;8:1027–1029. doi: 10.1111/j.1538-7836.2010.03808.x. [DOI] [PubMed] [Google Scholar]
- 44.Kalafatis M, Mann KG. Role of the membrane in the inactivation of factor Va by activated protein C. J.Biol.Chem. 1993;268:27246–27257. [PubMed] [Google Scholar]
- 45.Shatos MA, Orfeo T, Doherty JM, Penar PL, Collen D, Mann KG. Alpha-thrombin stimulates urokinase production and DNA synthesis in cultured human cerebral microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:903–911. doi: 10.1161/01.atv.15.7.903. [DOI] [PubMed] [Google Scholar]
- 46.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J.Biol.Chem. 2002;277:18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 47.Coughlin SR, Vu TK, Hung DT, Wheaton VI. Characterization of a functional thrombin receptor. Issues and opportunities. J.Clin.Invest. 1992;89:351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falvo MR, Gorkun OV, Lord ST. The molecular origins of the mechanical properties of fibrin. Biophys Chem. 2010;152:15–20. doi: 10.1016/j.bpc.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orfeo T, Brummel-Ziedins KE, Gissel M, Butenas S, Mann KG. The nature of the stable blood clot procoagulant activities. J Biol Chem. 2008;283:9776–9786. doi: 10.1074/jbc.M707435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brummel-Ziedins KE, Branda RF, Butenas S, Mann KG. Discordant fibrin formation in hemophilia. J Thromb Haemost. 2009;7:825–832. doi: 10.1111/j.1538-7836.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolberg AS, Allen GA, Monroe DM, Hedner U, Roberts HR, Hoffman M. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. Br J Haematol. 2005;131:645–655. doi: 10.1111/j.1365-2141.2005.05820.x. [DOI] [PubMed] [Google Scholar]
- 52.Suttie JW. The biochemical basis of warfarin therapy. Adv.Exp.Med.Biol. 1987;214:3–16. doi: 10.1007/978-1-4757-5985-3_2. [DOI] [PubMed] [Google Scholar]
- 53.Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature (London) 1994;369:64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 54.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. J.Clin.Invest. 1981;68:1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz HP, Fischer M, Hopmeier P, Batard MA, Griffin JH. Plasma protein S deficiency in familial thrombotic disease. Blood. 1984;64:1297–1300. [PubMed] [Google Scholar]
- 56.Campbell JE, Brummel-Ziedins KE, Butenas S, Mann KG. Cellular regulation of blood coagulation: a model for venous stasis. Blood. 2010;116:6082–6091. doi: 10.1182/blood-2010-01-266395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hockin MF, Kalafatis M, Shatos M, Mann KG. Protein C activation and factor Va inactivation on human umbilical vein endothelial cells. Arterioscler.Thromb.Vasc.Biol. 1997;17:2765–2775. doi: 10.1161/01.atv.17.11.2765. [DOI] [PubMed] [Google Scholar]
- 58.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006;4:1396–1404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 59.Shatos MA, Orfeo T, Doherty JM, Penar PL, Collen D, Mann KG. Alpha-thrombin stimulates urokinase production and DNA synthesis in cultured human cerebral microvascular endothelial cells. Arterioscler.Thromb.Vasc.Biol. 1995;15:903–911. doi: 10.1161/01.atv.15.7.903. [DOI] [PubMed] [Google Scholar]
- 60.Busch C, Cancilla PA, DeBault LE, Goldsmith JC, Owen WG. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982;47:498–504. [PubMed] [Google Scholar]
- 61.Haynes LMDYC, Orfeo T, Mann KG. Dilutional control of prothrombin activation at physiologically relevant shear rates. Biophys J. 2011;100:765–773. doi: 10.1016/j.bpj.2010.12.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sovershaev MA, Egorina EM, Gruber FX, Olsen JO, Osterud B. High tissue factor-expressing human monocytes carry low surface CD36: application to intersubject variability. J Thromb Haemost. 2007;5:2453–2460. doi: 10.1111/j.1538-7836.2007.02777.x. [DOI] [PubMed] [Google Scholar]
- 63.Eilertsen KE, Osterud B. The role of blood cells and their microparticles in blood coagulation. Biochem Soc Trans. 2005;33:418–422. doi: 10.1042/BST0330418. [DOI] [PubMed] [Google Scholar]
- 64.Tracy PB, Robinson RA, Worfolk LA, Allen DH. Procoagulant activities expressed by peripheral blood mononuclear cells. Methods Enzymol. 1993;222:281–299. doi: 10.1016/0076-6879(93)22019-c. [DOI] [PubMed] [Google Scholar]
- 65.Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breider MA, Yang Z. Tissue factor expression in bovine endothelial cells induced by Pasteurella haemolytica lipopolysaccharide and interleukin-1. Vet Pathol. 1994;31:55–60. doi: 10.1177/030098589403100107. [DOI] [PubMed] [Google Scholar]
- 67.Yang HL, Lu FJ, Wung SL, Chiu HC. Humic acid induces expression of tissue factor by cultured endothelial cells: regulation by cytosolic calcium and protein kinase C. Thromb Haemost. 1994;71:325–330. [PubMed] [Google Scholar]
- 68.Camera M, Giesen PL, Fallon J, Aufiero BM, Taubman M, Tremoli E, Nemerson Y. Cooperation between VEGF and TNF-alpha is necessary for exposure of active tissue factor on the surface of human endothelial cells. Arterioscler.Thromb.Vasc.Biol. 1999;19:531–537. doi: 10.1161/01.atv.19.3.531. [DOI] [PubMed] [Google Scholar]
- 69.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 70.Antoniak S, Boltzen U, Eisenreich A, Stellbaum C, Poller W, Schultheiss HP, Rauch U. Regulation of cardiomyocyte full-length tissue factor expression and microparticle release under inflammatory conditions in vitro. J Thromb Haemost. 2009;7:871–878. doi: 10.1111/j.1538-7836.2009.03323.x. [DOI] [PubMed] [Google Scholar]
- 71.Langer F, Spath B, Haubold K, Holstein K, Marx G, Wierecky J, Brummendorf TH, Dierlamm J, Bokemeyer C, Eifrig B. Tissue factor procoagulant activity of plasma microparticles in patients with cancer-associated disseminated intravascular coagulation. Ann Hematol. 2008;87:451–457. doi: 10.1007/s00277-008-0446-3. [DOI] [PubMed] [Google Scholar]
- 72.Morel O, Pereira B, Averous G, Faure A, Jesel L, Germain P, Grunebaum L, Ohlmann P, Freyssinet JM, Bareiss P, Toti F. Increased levels of procoagulant tissue factor-bearing microparticles within the occluded coronary artery of patients with ST-segment elevation myocaridal infarction: Role of endothelial damage and leukocyte activation. Arteriosclerosis. 2009;204:636–641. doi: 10.1016/j.atherosclerosis.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 73.Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J. 1983;50:127–134. doi: 10.1136/hrt.50.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, Fox KA, Granger CB, Jolly S, Joyner CD, Rupprecht HJ, Widimsky P, Afzal R, Pogue J, Yusuf S. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363:930–942. doi: 10.1056/NEJMoa0909475. [DOI] [PubMed] [Google Scholar]
- 75.Wiviott SD, Antman EM, Gibson CM, Montalescot G, Riesmeyer J, Weerakkody G, Winters KJ, Warmke JW, McCabe CH, Braunwald E. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) Am Heart J. 2006;152:627–635. doi: 10.1016/j.ahj.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 76.Billy D, Speijer H, Willems G, Hemker HC, Lindhout T. Prothrombin activation by prothrombinase in a tubular flow reactor. J Biol Chem. 1995;270:1029–1034. doi: 10.1074/jbc.270.3.1029. [DOI] [PubMed] [Google Scholar]
- 77.Hall CL, Taubman MB, Nemerson Y, Turitto VT. Factor Xa generation at the surface of cultured rat vascular smooth muscle cells in an in vitro flow system. J Biomech Eng. 1998;120:484–490. doi: 10.1115/1.2798018. [DOI] [PubMed] [Google Scholar]
- 78.Haynes LM, Dubief Y, Orfeo T, Mann KG. The effects of flow on the activation of bovine prothrombin by prothrombinase at physiologically relevant shear rates. FASEB J. 2010;24 835.834. [Google Scholar]
- 79.Beutler E. Platelet transfusions: the 20,000/microL trigger. Blood. 1993;81:1411–1413. [PubMed] [Google Scholar]
- 80.Butenas S, Branda RF, van't Veer C, Cawthern KM, Mann KG. Platelets and phospholipids in tissue factor-initiated thrombin generation. Thromb.Haemost. 2001;86:660–667. [PubMed] [Google Scholar]
- 81.Mazzaferri EL, Jr, Young JJ. Abciximab: a review and update for clinicians. Expert Rev Cardiovasc Ther. 2008;6:609–618. doi: 10.1586/14779072.6.5.609. [DOI] [PubMed] [Google Scholar]
- 82.Butenas S, Cawthern KM, van't Veer C, DiLorenzo ME, Lock JB, Mann KG. Antiplatelet agents in tissue factor-induced blood coagulation. Blood. 2001;97:2314–2322. doi: 10.1182/blood.v97.8.2314. [DOI] [PubMed] [Google Scholar]
- 83.Shah I, Khan SO, Malhotra S, Fischell T. Eptifibatide: The evidence for its role in the management of acute coronary syndromes. Core Evid. 2010;4:49–65. doi: 10.2147/ce.s6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Blood coagulation at the site of microvascular injury: effects of low-dose aspirin. Blood. 2001;98:2423–2431. doi: 10.1182/blood.v98.8.2423. [DOI] [PubMed] [Google Scholar]
- 85.Undas A, Brummel-Ziedins KE, Mann KG. Antithrombotic properties of aspirin and resistance to aspirin: beyond strictly antiplatelet actions. Blood. 2007;109:2285–2292. doi: 10.1182/blood-2006-01-010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swords NA, Mann KG. The assembly of the prothrombinase complex on adherent platelets. Arterioscler.Thromb. 1993;13:1602–1612. doi: 10.1161/01.atv.13.11.1602. [DOI] [PubMed] [Google Scholar]
- 87.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002 Jul 1;100:148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 88.Undas A, Szuldrzynski K, Brummel-Ziedins KE, Tracz W, Zmudka K, Mann KG. Systemic blood coagulation activation in acute coronary syndromes. Blood. 2009;113:2070–2078. doi: 10.1182/blood-2008-07-167411. [DOI] [PMC free article] [PubMed] [Google Scholar]