Abstract
A few studies suggest that non-invasive ventilation (1) and gastric tube (G-tube) may have a positive impact on survival but the effect on functional decline is unclear. Confounding by indication may have produced biased estimates of the benefit seen in some of these retrospective studies. The objective of this study was to evaluate the effects of G-tube and NIV on survival and functional decline using advanced statistical models that adjust for confounding by indications. A database of 331 subjects enrolled in previous clinical trials in ALS was available for analysis. Marginal structural models (MSM) were used to compare the mortality hazards and ALSFRS-R slopes between treatment and non-treatment groups, after adjusting for confounding by indication. Results showed that the placement of a G-tube was associated with an additional 1.42 units/month decline in the ALSFRS-R slope (p < 0.0001) and increased mortality hazard of 0.28 (p = 0.02). The use of NIV had no significant effect on ALSFRS-R decline or mortality. In conclusion, marginal structural models can be used to adjust for confounding by indication in retrospective ALS studies. G-tube placement could be followed by a faster rate of functional decline and increased mortality. Our results may suffer from some of the limitations of retrospective analyses.
Keywords: G-tube, PEG, NIV, Bipap, ALS, marginal structural models
Introduction
Swallowing difficulty and respiratory insufficiency are common symptoms of ALS that frequently lead to choking, aspiration, weight loss, dehydration, respiratory failure, and death (2). Two prospective non-randomized studies suggest that people with ALS who chose to have G-tube placement survive longer (3,4). However, retrospective studies that looked at the effects of G-tube placement on survival had inconsistent results (5–9). Non-invasive ventilation (NIV) has been shown to have a positive impact on survival in non-bulbar ALS in one small randomized trial (10) and in a few small or uncontrolled studies (11–14). The effect of NIV and G-tube on disease functional decline is unknown.
In the absence of randomization, it is challenging to estimate the effects of a treatment intervention (NIV or G-tube) on survival or functional decline because the decisions to initiate these treatments are, at least in part, based on factors related to disease severity and prognosis. For example, people with more aggressive disease may be more likely to choose these treatments and people with milder disease may tolerate these treatments better and tend to use them more effectively. Thus, using standard statistical methods to estimate the effects of NIV and G-tube on survival may give biased estimates because of this confounding by indication.
Marginal structural models (MSM) are a new class of causal models that estimate, from observational data, the causal effects of a time-dependent exposure in the presence of time-dependent covariates that may simultaneously be confounders and intermediate variables (15,16). The MSMs are capable of adjusting for the confounding by indication using weights that represent the probability of receiving the treatment at each visit. The standard approaches to estimate the effect of a time-varying exposure or treatment have been to model the probability of an outcome as a function of past exposure and past confounder history using stratified analysis and its parametric analogs such as logistic and proportional hazards regression models. These standard approaches may be biased when there is a time-dependent covariate 1) that is a risk factor for the treatment of interest and also predicts the subsequent outcome; and 2) when past treatment history predicts subsequent level of the outcome. For example, the time-dependent covariate ALSFRS-R is both an independent predictor of subsequent ALSFRS-R score and of the initiation of G-tube treatment, and may itself be influenced by prior G-tube treatment. This situation is true in many observational studies, particularly those in which there is a potential for confounding by indication (15).
The objective of this study was to estimate the effects of NIV and G-tube on survival and on functional decline in 331 people with ALS after adjusting for confounding by indication.
Material and methods
Study design and population
This was a post-hoc analysis of prospectively collected data from two ALS clinical trials. Study population included subjects who participated in the clinical trials of celecoxib and coenzyme Q10 (CoQ10) (17,18). Subjects had a clinical diagnosis of sporadic or familial ALS (19). The inclusion-exclusion criteria were similar in the two trials including vital capacity (VC) ≥50 in CoQ10 and ≥60% in celecoxib, time from symptom onset <5 years, either not taking riluzole or on a steady dosage of riluzole, and no significant unstable medical condition. ALSFRS-R and vital capacity (VC) were obtained either monthly or bi-monthly. The initiation of G-tube or NIV treatment was recorded as a major study event and survival was defined as death, tracheostomy, or permanent assisted ventilation. Subjects were followed for eight and 12 months in the CoQ10 and Celebrex trials, respectively.
Statistical analysis
Marginal structural models (MSMs) were used to account for confounding by indication when analyzing the effect of treatments (NIV or G-tube) on the rate of decline of ALSFRS-R and on survival. The parameters of a MSM were consistently estimated using a new class of estimators, the inverse-probability-of-treatment weighted (IPTW) estimators (16).
To fit marginal structural models with survival or ALSFRS-R as outcomes, first we fitted a model that includes longitudinal measures as well as baseline patients’ characteristics to estimate the probability of when and whether each patient would receive the treatment assignment (NIV or G-tube), their ‘treatment history’. At the next step, each patient's data were weighted by the inverse probability of their particular treatment history (IPTW) (15) (Appendix of SAS Macro). The appendix is available in the online version of this article: http://www.informaworld.com/ (DOI:10.3109/17482968.2011.577786).
For instance, if a patient with a certain history of clinical and prognostic factors has twice the chance of receiving a G-tube at the third visit than a patient with a different set of factors, then the data for this patient is down-weighted by a factor of two compared to the other patient. As a result, the known factors that lead to treatment do not play a role in the comparison of the outcome after treatment.
Treatment groups characteristics
Baseline characteristics including age, site of onset, ALSFRS-R, VC and riluzole treatment were compared between participants who received NIV or G-tube and those who did not. Student's t-tests were used to compare continuous variables and Fisher's exact tests were used to compare categorical variables. Vital capacity and ALSFRS-R at the visit before receiving the NIV or G-tube treatment were recorded. Mean time of follow-up after receiving NIV or G-tube treatments was estimated.
Estimating weights
A logistic regression model was fitted in order to capture the covariates that predicted receiving the treatments of G-tube or NIV. The regression model to estimate G-tube weights included potential indications of this treatment based on changes from baseline of the following variables: vital capacity (VC), relevant ALSFRS-R questions (swallow, speech, saliva), and change in weight since baseline. The regression model to estimate NIV weights included VC and relevant ALSFRS-R questions (dyspnea, orthopnea, speech, saliva, swallowing). Only significant predictors were then included in calculating the inverse-probability-of-treatment weighted (IPTW) estimators. At each given visit, we estimated the probability (weight) of each subject of receiving the treatment at that particular visit based on values of the above mentioned predictors of treatment.
Fitting the MSM and GEE models
Marginal structural models (MSM) were fitted using IPTW estimators to identify the affects of G-tube and NIV treatments on ALSFRS-R slope and survival. Generalized estimating equation regression models (GEE) were fitted without weights for comparison. In order to fit the GEE and MSM models we followed the statistical methods described in detail by Robins et al., using routine statistical analysis software (SAS) (Appendix) (15). Questions of the ALSFRS-R that are expected to change automatically because of receiving either treatment (G-tube or NIV) were excluded from the total score. Specifically, question 5 was excluded from G-tube analysis and question 12 was excluded from NIV analysis.
Results
Complete data were available on 300 out of 329 subjects who participated in the clinical trials of celecoxib and coenzyme Q10. Baseline and treatment groups characteristics are summarized in Table I. The percentage of subjects who received G-tube and NIV treatments were 13% and 21%, respectively. People who received G-tube were older (p = 0.03), had higher percentage of bulbar onset (p < 0.0001) and lower baseline vital capacity (p < 0.0001). People who used NIV had lower baseline vital capacity (p < 0.0001). The average follow-up period after inserting a G-tube or starting NIV was 3.8 and 4 months, respectively. Mean ALSFRS-R score at the time of initiating either of the treatments was 32 ± 7 and mean vital capacity was 62 ± 16 before inserting a G-tube and 60 ± 17 before starting NIV treatment (Table I).
Table I.
Baseline characteristics.
| +NIV (n = 63) |
−NIV (n = 238) |
p-value | +G-tube (n = 38) |
−G-tube (n = 262) |
p-value | |
|---|---|---|---|---|---|---|
| Age, years (STD)* | 57 (19) | 54 (19) | NS** | 58 (19) | 53.9 (19) | 0.03 |
| Bulbar onset (%) | 22 | 16 | NS | 55 | 11 | <0.0001 |
| Riluzole (%) | 73 | 68 | NS | 71 | 68 | NS |
| Baseline ALSFRS-R score (STD) | 38 (5) | 40 (5) | NS | 39 (5.5) | 39.5 (5) | NS |
| Baseline VC% predicted (STD) | 79 (19) | 90 (16) | <0.0001 | 78 (14) | 89 (16) | <0.0001 |
| ALSFRS-R score at initiating treatment (STD) | 32 (7) | 32 (7) | ||||
| VC% predicted at initiating treatment (STD) | 60 (19) | 62 (16) | ||||
| Months of follow-up after initiating treatment (STD) | 4 (2.3) | 3.8 (2.9) |
STD: Standard deviation.
NS: not significant.
Estimating weights (IPTW)
Weights for G-tube analysis
Increased swallowing difficulty, lower vital capacity, increased saliva, and worse dysarthria were all significant predictors of G-tube placement and were used to estimate weights for the G-tube analysis. Decreased body weight was not a significant predictor of G-tube placement.
Weights for NIV analysis
Lower total ALSFRS-R score, lower vital capacity, worse dyspnea, and orthopnea sub-scores were significant predictors of NIV treatment and were used to estimate weights for the NIV analysis. Changes in speech, saliva and swallowing were not significant predictors of NIV onset.
Generalized estimating equation models (GEE)
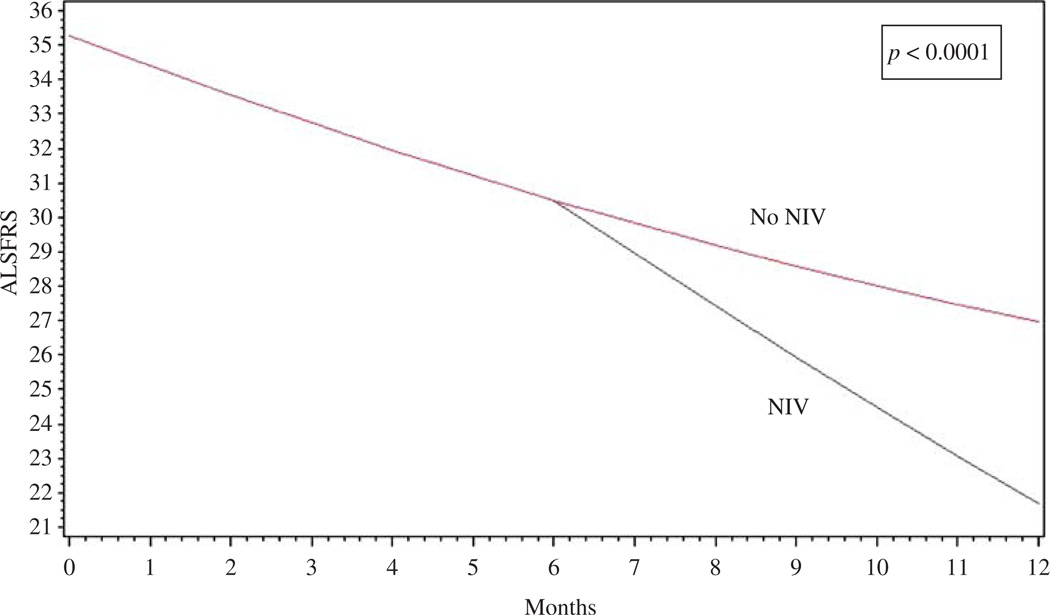
Using the standard approach (GEE), G-tube placement resulted in −1.418 additional decline in ALSFRS-R score (p < 0.0001) and +0.36 increase in the hazard of death (p < 0.001). Similarly, NIV treatment was associated with −0.99 additional decline in ALSFRS-R scores (p < 0.0001) and a +0.17 trend towards increased risk of death (p = 0.059). Figure 1 is an illustrative example of estimating the effect of NIV without correcting for confounding by indication.
Figure 1.
Generalized estimating equation models (GEE): an illustration of the effect of NIV on ALSFRS-R slope without controlling for confounding by indication.
Marginal structural models (MSM)
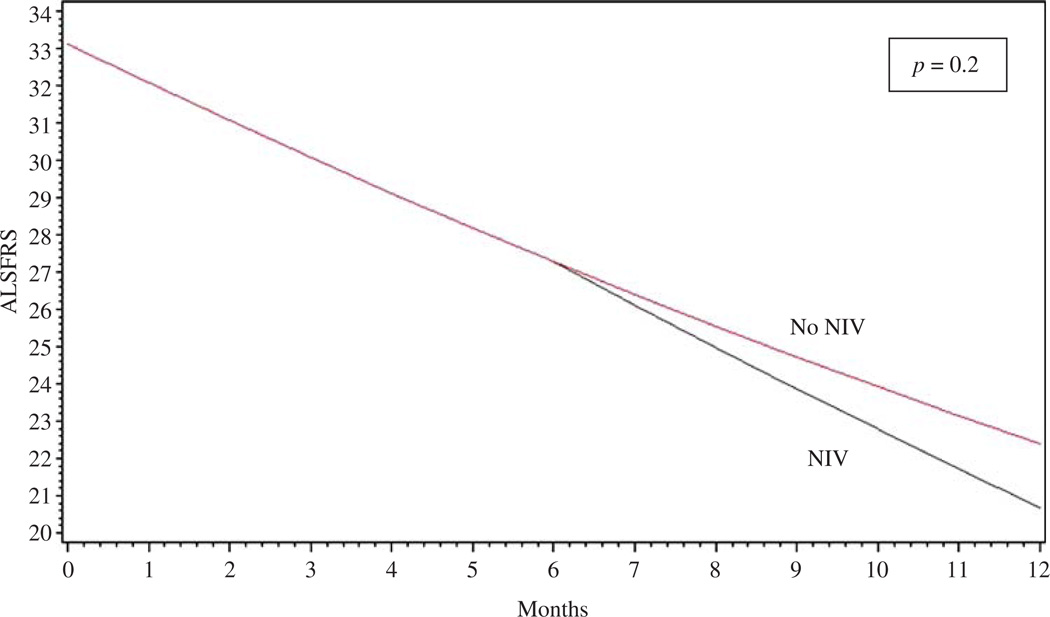
Fitting MSM with the estimated weights as described above, G-tube placement resulted in −1.421 additional decline in ALSFRS-R score (p < 0.0001) and +0.28 increase in mortality hazard (p = 0.02). On the other hand and contrary to the GEE results, NIV treatment had no significant impact on ALSFRS-R decline (p = 0.2) or on mortality hazard (p = 0.5) after adjusting for confounding by indication (Figure 2).
Figure 2.
Marginal structural models (MSM): an illustration that the negative effect of NIV seen using the routine GEE models is no longer there after correcting for confounding by indication.
Discussion
The American Academy of Neurology (AAN) practice parameters for patients with ALS concluded that G-tube placement is probably effective in prolonging survival in ALS based on the results of two class II studies (20). The AAN practice parameters also concluded that there are insufficient data to support or refute specific timing of G-tube insertion and the effect of G-tube placement on functional decline was unclear. In addition, a recent Cochrane review suggests that there is not enough evidence to support or guide G-tubes placement in people with ALS (21). On the other hand, patients with non-bulbar ALS using NIV experienced a median survival benefit of 205 days and a decrease in vital capacity decline in subgroup analysis of one small randomized controlled trial (10). The effects of NIV on functional decline are also unclear.
The goals of this study were to explore the benefits of using advanced statistical methods, called marginal structural models (MGM), to infer causality from observation data, and to use these methods to estimate the effects of G-tube and NIV on functional decline in people with ALS. Our analyses suggest that G-tube placement could be associated with increased functional decline and shorter survival after the procedure. The onset of NIV, on the other hand, was not associated with significant impact on functional decline or survival.
This study demonstrates that using the usual generalized linear models that ignore confounding by indication may result in biased conclusions when trying to infer causality in retrospective analysis. Generalized estimating equation models (GEE) and marginal structural models (MSM) produced similar results to the negative impact of G-tube placement on functional decline and survival in our cohort, suggesting that there was no significant confounding by indication in this particular analysis. On the other hand, GEE analysis concluded that using NIV results in worsening of function and survival, an effect that was absent after correcting for confounding by indication using marginal structural models (Figures 1, 2).
G-tube placement is a relatively invasive procedure that requires hospitalization, fasting, and anesthesia, and has potential complications such as bleeding and infection. At the time when G-tube is recommended to people with ALS, the disease is usually fairly advanced, respiratory function is low, and prognosis is poor. The results of our study suggest that G-tube placement may not be well tolerated in this population and that it may have a negative impact on function and survival.
The negative survival effect of G-tube placement in our study contradicts the results of two nonrandomized prospective studies that compared people who chose to have a G-tube placed and those who refused G-tube insertion. One possible explanation is that the people who chose to have a G-tube placement in the previous studies had less advanced disease and better prognosis. The other possibility is that the survival benefit was delayed for more than six months, as suggested by Mazzini et al., and thus was not captured in the average four months follow-up time in our cohort (4). Finally, our cohort represents an ALS population that participated in clinical trials over the past 10 years, and although there were no major changes in the practice parameters of using G-tube and NIV treatments (20,22), it is possible that the application of these standards might have changed due to improved patient care and increased awareness and experience using these treatments.
Although we attempted to account for all possible predictors or indications of G-tube placement when estimating the weights for the marginal structural models, in the absence of strictly followed universal G-tube placement guidelines, it is possible that some predictors were not included in our model. The marginal structural model assumes that there are no unmeasured confounders. Missing such confounders from our prediction model of treatment assignment could happen if we did not know about the confounder (or indications of G-tube or NIV treatments) and did not include them in the model or if they were wrongly excluded from the model (mismodeling). We included the AAN guidelines for starting NIV or G-tube placement in our models and our analysis depends on the fact that ALSFRS-R accurately captures most of patients’ symptoms that predict the use of NIV or G-tube. Clearly, the assumption of having ‘no unmeasured confounders’ is rarely completely true; however, we attempted to account for all major confounders so the residual or unmeasured confounding is expected to be minor.
The use of NIV had no impact on functional decline or survival in our cohort. One small randomized study suggested a survival benefit of NIV when used by a subgroup of 11 people with ALS who had no bulbar involvement (10). Finally, the impact of NIV on functional decline measured by ALSFRS-R has not been reported before. The results of our analysis suggest that using NIV may not have a significant impact on disease functional decline.
When randomized trials of certain interventions are unavailable, marginal structural models are powerful statistical tools that can correct for confounding by indication and help improve inference of true treatment effect. The conflicting results of the available non-randomized studies including ours suggest the need of large randomized clinical trials to assess the ideal timing and true benefit of these treatments. The challenge that we would have in conducting such a trial is that there is general agreement that these interventions are not indicated early in a patient's disease and are necessary for patient comfort for advanced disease. Thus, randomization would need to be geared to the relatively narrow window where we have equipoise about whether to initiate them (23). Despite attempting to control for all known confounders, our results may suffer from some of the limitations of retrospective analyses.
Acknowledgements
We would like to thank the Northeast ALS Consortium (NEALS) for providing the data. This work was conducted with support from a Muscular Dystrophy Association (MDA) clinical research training grant (CRTG160611) and NIH training grant (T32NS048005).
Appendix
%macro smm_longitudinal(input,treatmodel,tr eatmodel1, treatment = bipapind, outcome = alsnores, outcomelabel = ALSFRS, censormodel = vc alsfrs, stab = yes, covariates =); /* Input parameters: input = input file treatmodel = list of variables in input file that affect the probability of treatment treatment = idicator for treatment outcome = indicator for outcome outcomelabel = label for outcome censormodel = model for censoring stab = use “stable wieghts” (yes) or (no); covariates = list of variables that affect the probability of the outcome; Assumed variables: visit, visit number 0,1,…. sub = subject number cn = censoring indicator 1 = censored at that visit death = death indicator 1 = death, if outcome is an event it must be death, if outcome isn’t death than death does not need to be in the dataset. Rows with missing data in variables that are used are ignored. */ proc datasets;delete vk vk_score hs;run; *Remove data with missing values; data a1;set &input; array vars &treatmodel &treatment &outcome &censormodel; ff = 0; do i = 1 to dim(vars); if vars[i] = . then ff = 1; end; if ff = 0; run; %let input = a1; proc sort data = a1 out = tmarg;by sub descending visit;run; %local mv censor; proc sql; select mean(visit) into: mv from &input; select sum(cn) into: censor from &input; data vk;set tmarg;by sub; array vars &treatmodel &treatment &outcome &censormodel; visit2 = (visit-&mv)**2; ff = 0; do i = 1 to dim(vars); if vars[i] in (.,999) then ff = 1; end; if ff = 1 then delete; run; *Set up data for fitting treatment model; *flag is 1 at visit before treatment and afterwards flag is used as the indicator for future treatment; data vk;set vk;by sub; retain flag 0 retain nsub 0; if first.sub then do; nsub = nsub + 1; if &treatment = 1 then flag = 1; else flag = 0; end; output ; if &treatment = 0 then flag = 0; run; *output statement; title2 ‘Number of observations’; proc sql; select n(treatment) label = ‘Number of patients’, sum (visit) label = ‘Number of visits’, sum (treatment) label = ‘Number of treated patients’ from (select unique sub,n(sub) as visit,max (&treatment) as treatment from vk group by sub); /* Creates cumulative treatment indicator which starts at visit before treatment to have a different slope */ proc sort data = vk out = vk;by sub visit;run; data vk;set vk;by sub visit; retain newcumtrtA ovisit; if first.sub then do; newcumtrtA = 0; ovisit = 0; end; else do; dvisit = visit-ovisit;*change in visit; ovisit = visit; end; if &treatment = 1 then newcumtrtA = new-cumtrtA + dvisit;*newcumtrtA is the time after the treatment changed; else newcumtrtA = 0; newcumtrt = newcumtrtA; cvisit = visit; drop newcumtrtA ovisit; if last.visit = first.visit;*? removes dublicate visits; run; /* List is variables that are considered in the model for bipap*/; *Poooled logistic regressions for bipap not data after bipap begins is not used; *creates censoring variable; title2 ‘Model for probability of treatment and censoring’; %macro modeltrt; ods listing close; *calculate denominator of weight p_0 p_1; proc logistic data = vk;where &treatment = 0; model flag = visit visit2 &treatmodel; score data = vk out = vk; run; *calculate numberator of weight; proc logistic data = vk(rename = (p_0 = p2) drop = p_1);where &treatment = 0; model flag = visit visit2; score data = vk(rename = (p_0 = p2) drop = p_1) out = vk; run; ods listing; %mend; *Model for censoring; %macro modelcn; %if &censor^ = 0 %then %do; ods listing close; *numerator; proc logistic data = vk(rename = (p_0 = p1) drop = p_1); model cn = visit visit2; score data = vk(rename = (p_0 = p1) drop = p_1) out = vk; run; *denominator; proc logistic data = vk(rename = (p_0 = p3) drop = p_1); model cn = visit visit2 &censormodel; score data = vk(rename = (p_0 = p3) drop = p_1) out = vk; run; data vk;set vk(rename = (p_0 = p4) drop = p_1);run; ods listing; %end; %else %do; %data vk;set vk;p3 = 1;p4 = 1;run; %end; %mend; %modeltrt; %modelcn; /*An internal macro of used to allow for simulation */ /* Creates weights*/ data hs;set vk; retain umult oumult mult omult 1; array p p1–p4; if visit = 0 then do; mult = 1;omult = 1;umult = 1;oumult = 1;end; select; when(flag = 0 and &treatment = 0) do; mult = mult* (p1/p2)*(p3/p4);umult = umult/(p2*p4);end;*No election of bipap; when(flag = 1 and &treatment = 0) do;mult = mult*((1 -p1)/(1 -p2))*(p3/p4);umult = umult/((1 -p2)*p3);end;*first election of pipap; when(&treatment = 1) do;mult = mult*p3/p4; umult = umult/p4;end;*established pipab; end; output; omult = mult;oumult = umult; *weight omult will be output for next observation; run; data hs;set hs;omult = omult*2;oumult = oumult*2;run; /* GEE MODEL */ title2 “Without weights”; proc genmod data = hs descending; ods output GEEEmpPEst = ts1; class sub cvisit; model &outcome = newcumtrt visit visit2 &covariates %if &outcome = death %then %do; /link = logit dist = bin; %end; %else %do; ; %end; repeated subject = sub /type = ind within = cvisit; run; title2 “With weights”; proc genmod data = hs descending; ods output GEEEmpPEst = t1; class sub cvisit; model &outcome = newcumtrt visit visit2 &covariates %if &outcome = death %then %do; /link = logit dist = bin; %end; %else %do; ; %end; %if &stab = yes %then %do; weight omult; %end; %else %do; weight oumult; %end; repeated subject = sub/type = ind within = cvisit; run; %mend; /*Test run of model, replace file with correct path Note: This will not give the exact results of the paper because quadratic terms are not included in the simplified macro. */; proc cimport file = ‘H:\als\nazeem2\mmarg. xpt’; title1 ‘BIPAP’; %smm_longitudinal (input = mmarg, treatmodel = alsfrs vc dyspnea orthopnea alsfrs2, treatment = bipapind,outcome = als_bipap,outcomelabel = ALSFRS_bipap, censormodel = alsfrs vc,covariates =); %smm_longitudinal (input = mmarg, treatmodel = alsfrs vc dyspnea orthopnea alsfrs2, treatment = bipapind,outcome = death, outcomelabel = death, censormodel = alsfrs vc,covariates =);
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Chicago Uo. Ceftriaxone as effective therapy in refractory Lyme disease. The Journal of Infectious Diseases. 1987;155:1322–1324. doi: 10.1093/infdis/155.6.1322. [DOI] [PubMed] [Google Scholar]
- 2.Brooks BR. Natural history of ALS. Neurology. 1996;47 Suppl 2:S71–S82. doi: 10.1212/wnl.47.4_suppl_2.71s. [DOI] [PubMed] [Google Scholar]
- 3.Del Piano M, Occhipinti P, Orsello M, Balare M. Percutaneous endoscopic gastrostomy (PEG) reduces complications and improves survival in amyotrophic lateral sclerosis (ALS) Gastrointest Endosc. 1999;49:192. [Google Scholar]
- 4.Mazzini L, Corrá T, Zaccala M, Mora G, Del Piano M, Galante M. Percutaneous endoscopic gastrostomy and enteral nutrition in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1995;242:695–698. doi: 10.1007/BF00866922. [DOI] [PubMed] [Google Scholar]
- 5.Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P. Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;2:91–96. doi: 10.1080/14660820050515386. [DOI] [PubMed] [Google Scholar]
- 6.Mitsumoto H, Davidson M, Moore D, Gad N, Brandis M, Ringel S, et al. Percutaneous endoscopic gastrostomy (PEG) in patients with ALS and bulbar dysfunction. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:177–185. doi: 10.1080/14660820310011728. [DOI] [PubMed] [Google Scholar]
- 7.Mathus-Vliegen LMH, Louwerse LS, Merkus MP, Tytgat GNJ, Vianney de Jong JMB. Percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis and impaired pulmonary function. Gastrointestinal Endoscopy. 1994;40:463–469. doi: 10.1016/s0016-5107(94)70211-x. [DOI] [PubMed] [Google Scholar]
- 8.Shaw A, Ampong M-A, Rio A, Al-Chalabi A, Sellars M, Ellis C, et al. Survival of patients with ALS following institution of enteral feeding is related to pre-procedure oximetry: A retrospective review of 98 patients in a single centre. Amyotroph Lateral Scler. 2006;7:16–21. doi: 10.1080/14660820510012013. [DOI] [PubMed] [Google Scholar]
- 9.Forbes RB, Colville S, Swingler RJ. Frequency, timing and outcome of gastrostomy tubes for amyotrophic lateral sclerosis/motor neuron disease. J Neurol. 2004;251:813–817. doi: 10.1007/s00415-004-0429-9. [DOI] [PubMed] [Google Scholar]
- 10.Bourke SC, Bullock RE, Williams TL, Shaw PJ, Gibson GJ. Non-invasive ventilation in ALS. Neurology. 2003;61:171–177. doi: 10.1212/01.wnl.0000076182.13137.38. [DOI] [PubMed] [Google Scholar]
- 11.Sherman M, Paz H. Review of respiratory care of the patient with amyotrophic lateral sclerosis. Respiration. 1994;61:61–67. doi: 10.1159/000196308. [DOI] [PubMed] [Google Scholar]
- 12.Aboussounan L, Khan S, Meeker D, Stelmach K, Mitsumoto H. Effect of non-invasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Int Med. 1997;127:450–453. doi: 10.7326/0003-4819-127-6-199709150-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kleopa K, Sherman M, Neal B, Romano G, Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci. 1999;164:82–88. doi: 10.1016/s0022-510x(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 14.Pinto A, Evangelista T, Carvalho M, Alves M, Saslue L. Respiratory assistance with a non-invasive ventilator (Bipap) in MND/ALS patients: survival rates in a controlled trial. J Neurol Sci. 1995;129:19–26. doi: 10.1016/0022-510x(95)00052-4. [DOI] [PubMed] [Google Scholar]
- 15.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Hernán MA, Brumback BA, Robins JM. Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Statistics in Medicine. 2002;21:1689–1709. doi: 10.1002/sim.1144. [DOI] [PubMed] [Google Scholar]
- 17.Cudkowicz M, Shefner J, Schoenfeld D, Zhang H, Andreasson K, Rothstein J, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Annals of Neurology. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 18.Ferrante KL, Shefner J, Zhang H, Betensky R, O’Brien M, Yu H, et al. Tolerance of high-dose (3000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- 19.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial ‘Clinical limits of amyotrophic lateral sclerosis ’. J Neurol Sci. 1994;124:96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 20.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice Parameter update. The care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review) Neurology. 2009;73:1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzberg H, Benatar M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2011;19(1) doi: 10.1002/14651858.CD004030.pub3. CD004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller RG, Rosenberg JA, Gelinas DF, Mitsumoto H, Newman D, Sufit R, et al. Practice parameter. The care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1999;52:1311. doi: 10.1212/wnl.52.7.1311. [DOI] [PubMed] [Google Scholar]
- 23.Lavori PW, Dawson R. Dynamic treatment regimes: practical design considerations. Clinical Trials. 2004;1:9–20. doi: 10.1191/1740774s04cn002oa. [DOI] [PubMed] [Google Scholar]