Abstract
Exposure to an acute stressful event facilitates classical eye-blink conditioning in the male rat. The facilitation persists for days after the stressor and its induction is prevented by antagonism of the N-methyl-d-aspartate (NMDA) type of glutamate receptor. To determine whether NMDA receptor antagonists prevent the facilitated conditioning by activity in the amygdala, a competitive antagonist, AP5, was injected bilaterally into the lateral/basolateral versus central nuclei of the amygdala. Local injection of d,l-2-amino-5-phosphonovaleric acid (AP5) into the lateral/basolateral nucleus before stressor exposure prevented the facilitated learning 24 hr later, whereas antagonism in the central nucleus before stressor exposure did not. To determine when the necessary activation occurs, AP5 was injected into the lateral/basolateral nucleus before versus after exposure to the acute stressful event. Blockade of NMDA receptors before but not after stressor exposure prevented the facilitated acquisition of the conditioning in response to stress. These results suggest that exposure to a stressful event transiently activates NMDA receptors in basolateral/lateral nuclei of the amygdala and thereby induces a persistent enhancement of associative learning.
It is well known that memories are heavily influenced by emotions and specifically that exposure to stressful and emotional events can affect the acquisition of new memories. Several years ago we reported that rats exposed to an aversive and presumably emotional event in one context learn to associate discrete pieces of information at a facilitated rate in a different context (Shors et al. 1992; Servatius and Shors 1994; Shors and Servatius 1995, 1997). In the study, rats were exposed to a stressor of restraint and intermittent tail shocks. Twenty-four hours later, they were trained on the classically conditioned eye-blink response, in which an auditory conditioned stimulus (CS) predicts the occurrence of an aversive unconditioned stimulus (US) to the eyelid. Rats previously exposed to the stressor exhibited a rapid acquisition of the conditioned response (CR). The effect was not directly attributable to sensitization or pseudoconditioning, because stressed rats exposed to explicitly unpaired presentations of the CS and the US did not respond to the CS alone, yet they exhibited facilitated learning upon their first exposure to paired training (Servatius and Shors 1994). In subsequent studies it was determined that the minimum stressor necessary to evoke the enhanced acquisition consisted of 30 1-mA tail shocks (Shors and Servatius 1997), although 20 min of inescapable swim stress will also suffice (T.J. Shors and M.P. Pacynski, in prep.). In addition, the stress-induced facilitation of classical conditioning is not limited to eye-blink conditioning but extends to classical fear and heart-rate conditioning (Wilson et al. 1975; Maier 1990).
Over the past several years, our goal has been to identify the brain structures and neural mechanisms that are responsible for the induction and expression of the stress-induced facilitation of classical eye-blink conditioning in the male rat. To date, we have established that its induction can be prevented by blocking access to the N-methyl-d-aspartate (NMDA) type of glutamate receptor (Shors and Servatius 1995). A peripheral injection of a competitive NMDA receptor antagonist delivered before the stressor exposure prevented the facilitation without altering acquisition 24 hr later. Because the antagonist was administered peripherally, we did not know where in the brain the induction occurred. Based on the literature, a number of brain structures are implicated: the cerebellum, owing to its necessary role in acquisition of the basic conditioned eye-blink response (McCormick et al. 1982; Skelton 1988; Krupa et al. 1993), and the hippocampus and amygdala, owing to their ability to modify acquisition of the CR (Solomon and Moore 1975; Solomon et al. 1986; Moyer et al. 1990; Kapp et al. 1991; Weisz et al. 1992; Schmajuk et al. 1994). Both the amygdala and the hippocampus are sensitive to the consequences of stress. For example, exposure to a stressor of inescapable tailshock induces immediate-early gene expression, enhances α-amino-3-methylisoxazole-4-propionic acid (AMPA) receptor binding (Schreiber et al. 1991; Tocco et al. 1991), and impairs long-term potentiation in the hippocampus (Shors et al. 1990; Shors et al. 1997b). In the amygdala, exposure to restraint stress increases corticotropin-releasing hormone (CRH) mRNA expression (Hsu et al. 1998), footshock increases norepinephrine levels (Galvez et al. 1996), and subordinate rats have higher levels of 5-HIAA, but not 5-HT, relative to their dominant cohorts (Blanchard et al. 1991).
In the present experiments, we tested whether NMDA receptor activation in the amygdala was critically involved in the stress-induced facilitation of classical eye-blink conditioning. Lesions to the amygdala are reported to impair acquisition of the conditioned eye-blink response (Kapp et al. 1991; Whalen and Kapp 1991; Weisz et al. 1992) and NMDA receptor activation in the amygdala is necessary for acquisition in several conditioning paradigms (Miserendino et al. 1990; Campeau et al. 1992; Kim and McGaugh 1992). More specifically, Fanselow and Kim (1994) reported that NMDA receptor antagonism in the basolateral nucleus but not the central nucleus prevented contextual fear conditioning. In addition, exposure to the stressor that facilitates eye-blink conditioning enhances the binding of [3H]PDBu ([3H]phorbol 12,13-dibutyrate), a marker for protein kinase C, in the basolateral nucleus of the amygdala (Shors et al. 1997a). The stress-induced increase in phorbol ester binding in the amygdala, like the increase in classical eye-blink conditioning, can be prevented by blocking NMDA receptors with a competitive antagonist before stressor exposure.
Here, we hypothesized that exposure to the stressor activates NMDA receptors in the lateral/basolateral nucleus of the amygdala which, in turn, induces facilitated acquisition of the conditioned eye-blink response. We also hypothesized that activation of NMDA receptors occurred during, and not after, exposure to the stressor. To test these hypotheses, we injected the NMDA receptor antagonist into the lateral/basolateral nucleus of the amygdala before versus after stressor exposure. To determine whether the effect of the stressor on acquisition of the CR was specifically induced by neuronal activity within the basolateral versus other nuclei in the amygdala, we also injected the competitive NMDA receptor antagonist AP5 into the central nucleus of the amygdala before stressor exposure. Twenty-four hours later, stressed and unstressed rats were trained on the classically conditioned eye-blink response.
Materials and Methods
SUBJECTS
Male Sprague–Dawley rats (280–320 grams) were obtained from the colony maintained at Princeton University in the Department of Psychology. Rats were housed in groups of five before surgery and individually postoperatively to prevent damage to the head stage. They had unlimited access to Purina laboratory chow and water and were maintained on a 12:12-hr light/dark cycle.
SURGERY
Before surgery, animals were injected with 0.2 mg/kg of Atropine (Elkins–Sinn) intramuscularly and anesthetized with 50 mg/kg interperitoneal injections of Nembutal (Abbott). Bilateral guide cannulae (26 grams; Small Parts, Inc.) were implanted stereotaxically above the basolateral nucleus (2.4 mm posterior, 5.1 mm lateral, and 8.7 mm ventral to Bregma; Paxinos and Watson 1986) and central nucleus (2.2 mm posterior, 4.3 mm lateral, and 8.1 mm ventral; Paxinos and Watson 1986). The cannulae and headstage were held in place with dental acrylic (Yates and Bird) and anchored to the skull. From the headstage, four 0.005-inch stainless steel wires were implanted subcutaneously and drawn through the eyelid. The tips were deinsulated (2–3 mm) to allow contact with the muscles in the eyelid for measurement of the electromyographic (EMG) response. Cannula patency was maintained with internal stylets (32 grams, Small Parts, Inc.) protruding 1 mm beyond the cannula tip. An acrylic cap surrounded the setup to ensure that the spatial positioning of the cannulae was not altered.
After surgery, 0.4 ml of penicillin (300,000 U/ml; Butler) was administered intramuscularly, and the animal was kept warm and under observation until recovery from anesthesia. Postoperatively, rats were provided with 24-hr access to acetaminophen (IDE) diluted 1:100 in the drinking water for 3–4 days. Rats recuperated for 5–7 days before experimentation.
HISTOLOGY
After experimentation, rats were anesthetized with the inhalation anesthetic methoxyflurane (Pittman–Moore). Brains were extracted, frozen in 2-methyl butane, and stored at −80°C. Coronal sections (40 μm) were cut at −18°C with a cryostat, placed on gelatin-coated slides, and stained with Nissl. Cannula placements were reconstructed using an atlas of the rat brain (Paxinos and Watson 1986).
CONDITIONING APPARATUS
Headstages were connected to a shielded, grounded, IBM-compatible coiled keyboard cable that allowed free movement within the conditioning chamber. Of the four implanted electrodes, two delivered the periorbital shock as an US and two transmitted the EMG signal. Eyelid EMG was filtered to pass 0.3–1.0 KHz and amplified (10K) with a differential AC amplifier, which was passed to a 16-bit A/D card (DAS, 1600; Keithley-Metrabyte, Tauton, MA). The CS (82 dB, 320 msec) was a white noise burst with a 5-msec rise and fall time, and the US was a 0.7-mA, 80-msec shock to the eyelid. The CS overlapped and coterminated with the US.
NMDA RECEPTOR ANTAGONIST
d,l-2-amino-5-phosphonovaleric acid (AP5; Sigma) was dissolved in artificial cerebrospinal fluid (ACSF) and adjusted to physiological pH. Bilateral intra-amygdala infusions were made with 32-gauge injectors (Small Parts, Inc.) and connected via Tygon tubing (Fisher Scientific) to 10-μl Hamilton syringes. Flow rate was 0.5 μl/min and controlled with a Razel syringe pump (Model A-99). Each rat was injected with 0.5 μl/side (2.5 μg/side). Injectors were left in place for 1 min after infusion, after which the stylets were replaced. Vehicle-injected controls were exposed to the same procedure except ACSF (adjusted to physiological pH) was injected without the antagonist.
STRESS PROCEDURE
Rats were habituated to the conditioning chamber for 45 min, and spontaneous blink rate was recorded. Rats were removed from the chamber and injected with AP5 or the vehicle. Rats in the stressed groups were exposed to the tailshock stress 30 min after injection. Stressed rats were taken into a separate room from the conditioning environment, placed in a white soundproof box, and loosely restrained in Plexiglas holders. They were exposed to 30 1-sec, 1-mA tail shocks that were delivered 1/min for 30 min and returned to their home cages for 24 hr. Unstressed rats were returned to their home cages immediately after habituation to the conditioning chamber.
CONDITIONING PROCEDURE
In the first study, eight groups of rats were injected with a competitive NMDA receptor antagonist in the basolateral nucleus either before or after exposure to the stressor (Table 1). For the first four groups, the antagonist was injected 30 min before the stressor to ensure complete blockade of the NMDA receptors during stressor exposure. For the second four groups, the antagonist was injected 20 min after stressor cessation to avoid manipulation of the rat during the grooming that occurs after stressor cessation. Of the eight groups, two groups were infused with AP5 into the basolateral nucleus, one of these was exposed to the stressor 30 min later, and the unstressed group was returned to its home cage; two groups were infused with ACSF into the basolateral nucleus, one of these was exposed to the stressor 30 min later, and the unstressed group was returned to its home cage; two groups were stressed and injected with AP5 or vehicle 20 min after stressor cessation; two groups were infused with either AP5 or vehicle in the basolateral nucleus and returned to their home cage.
Table 1.
Experiment design
| Stress | Drug | Targeta | Time of injection | No. analyzed/ total rats | |
|---|---|---|---|---|---|
| Study 1 | yes | AP5 | BLN | 30 min before stress | 7/12 |
| no | AP5 | BLN | 30 min before stress | 10/12 | |
| yes | ACSF | BLN | 30 min before stress | 9/11 | |
| no | ACSF | BLN | 30 min before stress | 9/12 | |
| yes | AP5 | BLN | 20 min after stress | 7/11 | |
| no | AP5 | BLN | 20 min after stress | 7/10 | |
| yes | ACSF | BLN | 20 min after stress | 8/10 | |
| no | ACSF | BLN | 20 min after stress | 6/10 | |
| Study 2 | yes | AP5 | CN | 30 min before stress | 6/9 |
| no | AP5 | CN | 30 min before stress | 5/8 | |
| yes | ACSF | CN | 30 min before stress | 5/8 | |
| no | ACSF | CN | 30 min before stress | 5/8 |
Procedural information includes manipulations, time courses, and number of rats that were tested vs. analyzed.
(BLN) Basolateral nucleus; (CN) central nucleus.
In the second study, four groups of rats were injected with the vehicle or the NMDA receptor antagonist in the central nucleus of the amygdala before stressor exposure. Of the four groups, two groups were infused with AP5 into the central nucleus, and two groups were infused with ACSF into the central nucleus. Thirty minutes later, one group of rats was either injected with the antagonist or the ACSF was stressed. All rats were returned to their home cage. Twenty-four hours later, rats were taken into the conditioning room and placed in the conditioning apparatus for approximately 20 min while the spontaneous blink rate was again recorded. To evaluate the potential effect of the stressor or the drug on sensitization to the CS, rats were exposed to 10 white noise stimuli (320 msec, 82 dB) before any paired training, and eye blinks to the CS alone were recorded. Rats were then exposed to paired stimuli using a delayed conditioning paradigm. [It is noted that we sustain a relatively low learning rate in unstressed controls to observe a facilitation of the CR. To do this, we present the CS at a relatively low intensity of 82 dB. In previous studies we have shown that enhancing the intensity of the CS to 85 dB induces rapid acquisition in the rat (Servatius and Shors 1996).] The intertrial interval (ITI) was randomized at 20 ± 10 sec. All rats were exposed to 300 trials in 1 day. The CS consisted of a 320-msec, 82-dB white noise stimulus that overlapped and coterminated with a US that consisted of an 80-msec, 0.7-mA shock to the eyelid. A sequence of 10 trials consisted of a CS alone, 4 paired trials, a US alone, and 4 paired trials.
Each trial began with a 240-msec baseline recording period before stimulus presentation. To be considered an eye blink, the EMG response had to exceed the maximum value of the prestimulus baseline in addition to four times its standard deviation. An eye blink was scored as a CR if the blink began 80 msec after CS onset. An eye blink was scored as a sensitized response if the blink occurred in the first 80 msec after CS onset. Performance on paired trials was computed as a percentage of CRs produced in trials in which a CS was delivered. For statistical purposes, the 300 trials were arbitrarily divided into three blocks of 100 trials. These three blocks were used as the dependent variable in an ANOVA with the three blocks as the repeated measures. Newmann Keuls post hoc analyses were used to verify significance between individual groups.
Results
HISTOLOGICAL OBSERVATIONS
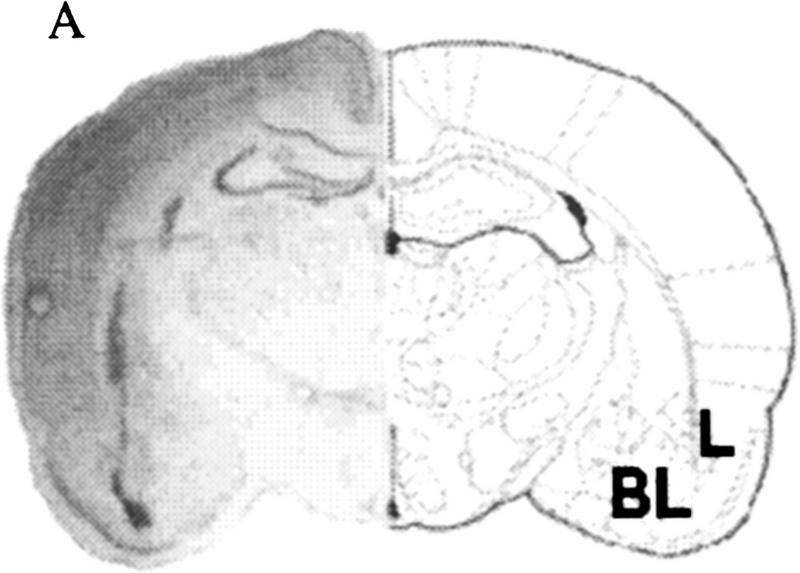
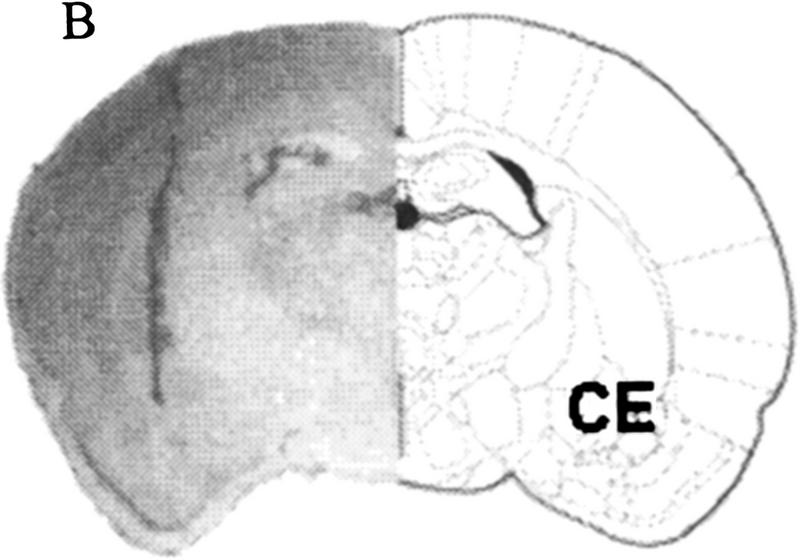
In study 1, only brains with cannulae placement <0.75 mm from the center of the lateral/basolateral amygdaloid nucleus with no overlap into the central amygdaloid nucleus were included. In study 2, brains with the cannulae placement <0.75 mm from the center of the central nucleus with no overlap into the lateral/basolateral amygdaloid nucleus were included. Because the antagonist was released from the tip of the cannula, tip placement was aimed above the target nuclei. Because the lateral nucleus is positioned above the basolateral nucleus, we included rats with cannulae tip placement in the lateral nucleus as inclusive with those in the basolateral group. Representative sections from a brain that was injected with AP5 within the target region of the lateral/basolateral nuclei and the central nucleus of the amygdala and subsequently stained with Nissl are shown in Figure 1, A and B. From a total 121 rats, 84 had injection sites within the target zone, and they were used for all subsequent analysis (Fig. 2).
Figure 1.
(A) Localized microinjection of AP5 bilaterally into the lateral/basolateral amygdala. (Left) A photomicrograph of a Nissl-stained section (40 μm) obtained from a rat injected with the competitive NMDA receptor antagonist AP5 (0.5 μl) into the lateral/basolateral nuclei (BL) of the amygdala. (Right) A coronal section from the rat atlas of Paxinos and Watson (1986) that corresponds to the cannula location. (Bregma) −2.56 mm; interaural 6.44 mm. (B) Localized microinjection of AP5 bilaterally into the central amygdala. (Left) A photomicrograph of a Nissl-stained section (40 μm) obtained from a rat injected with the competitive NMDA receptor antagonist AP5 (0.5 μl) into the central nucleus (CE) of the amygdala. (Right) A coronal section from the rat atlas of Paxinos and Watson (1986) that corresponds to the cannula location. (Bregma) −1.80 mm; interaural 7.20 mm.
Figure 2.

Coronal sections from the brain atlas by Paxinos and Watson (1986) illustrating the sites of drug (AP5) and vehicle (ACSF) injection. A site with injections representing more than one rat is labeled with only one point on the diagram. (A) Lateral/basolateral amygdala injection sites before stressor exposure. Slices shown are −3.14, −2.80, −2.56, −2.30, and −2.12 mm relative to Bregma. (B) Lateral/basolateral amygdala injection sites after stressor exposure. Slices shown are −3.14, −2.80, −2.56, −2.30, and −2.12 mm relative to Bregma. (C) Central amygdala injection sites before stressor exposure. Slices shown are −3.14, −2.80, −2.56, −2.30, and −2.12 mm relative to Bregma.
EFFECTS OF NMDA RECEPTOR ANTAGONISM IN THE LATERAL/BASOLATERAL NUCLEUS
Exposure to stressor or injection of the NMDA receptor antagonist in the lateral/basolateral nucleus did not interact and alter the spontaneous blink rate [F(1,55) = 0.061, P = 0.81]. Nor did they elicit sensitized responses to the CS before training [F(1,55) = 0.911, P = 0.34]. When injected into the lateral/basolateral nucleus, NMDA receptor antagonism during the stressor prevented the facilitated learning in response to the stressor (Fig. 3A). Using stressor exposure (yes or no), drug injection (AP5 or vehicle), time (before vs. after stressor exposure), and trials of training (repeated measures of three blocks of 100 trials each) as the independent variables and CRs as the dependent variable, there was a four-way interaction between stressor exposure, drug injection, time, and trials of training [F(2,110) = 3.35, P < 0.04]. Post hoc analysis using Newmann Keuls revealed that prevention of the facilitation only occurred when the antagonist was administered before the stressor and not after the stressor. As expected, rats that were injected with the vehicle and exposed to the stressor were facilitated in their rate of acquisition when compared with unstressed rats injected with the vehicle. The increase was evident during the last 200 trials (P = 0.02 and 0.002, respectively). Rats injected with AP5 before the stressor were not significantly different in their responses during any of the three blocks of 100 trials when compared with the rats that were injected with AP5 and not exposed to the stressor (P = 0.96, 0.96, and 0.74, respectively). This same group was significantly different from rats injected with AP5 after the stressor during the last 200 trials of training (P = 0.05 and 0.01, respectively) (Fig. 3B). Rats injected with AP5 after the stressor exhibited enhanced acquisition of the CR relative to those that were not stressed and injected with vehicle during the first 200 trials (P = 0.0003 and 0.05, respectively). In summary, the results suggest that NMDA receptor blockade in the lateral/basolateral nucleus of the amygdala prevents the stress-induced facilitation of classical eye-blink conditioning during and not after exposure to the stressor.
Figure 3.
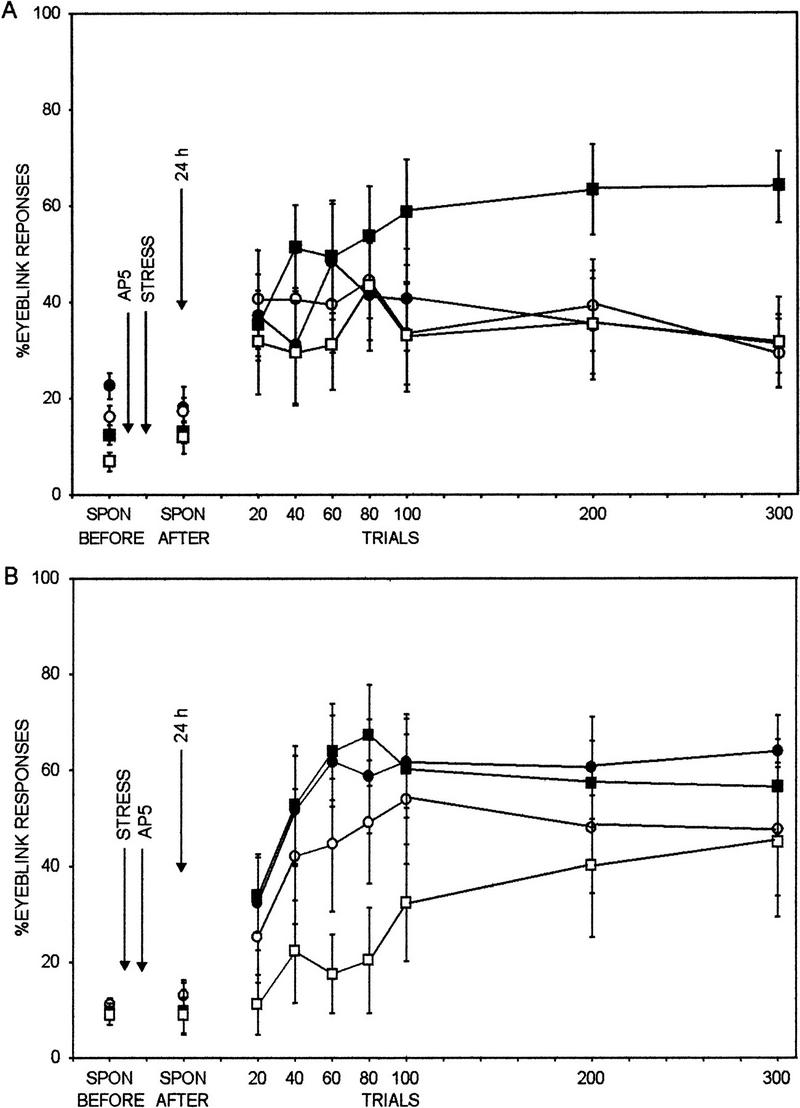
Effect of NMDA receptor antagonism in the lateral/basolateral amygdaloid nucleus before stressor exposure on classical conditioning. Spontaneous (SPON) blink rate before and after stress is shown, followed by percent CRs to the auditory CS (eye blinks that commenced 80 msec after CS onset) across 300 trials of training 24 hr after stressor cessation. NMDA receptor antagonism in the lateral/basolateral amygdaloid nucleus before stressor exposure prevented the stress-induced facilitation of associative learning in male rats (stress/AP5, •) relative to unstressed male rats (no stress/AP5, ○). Neither stressor exposure nor NMDA receptor antagonism affected the spontaneous blink rate. (█) stress/vehicle; (□) no stress/vehicle. (B) Effect of NMDA receptor antagonism in the lateral/basolateral amygdaloid nucleus after stressor exposure on classical conditioning. Spontaneous (SPON) blink rate before and after stress is shown, followed by percent CRs to the auditory CS (eye blinks that commenced 80 msec after CS onset) across 300 trials of training 24 hr after stressor cessation. NMDA receptor antagonism in the lateral/basolateral amygdaloid nucleus after stressor exposure did not prevent the stress-induced facilitation of associative learning in male rats (stress/AP5) relative to unstressed male rats (no stress/AP5). Neither stressor exposure nor NMDA receptor antagonism affected the spontaneous blink rate.
EFFECTS OF NMDA RECEPTOR ANTAGONISM IN THE CENTRAL NUCLEUS ON FACILITATED LEARNING
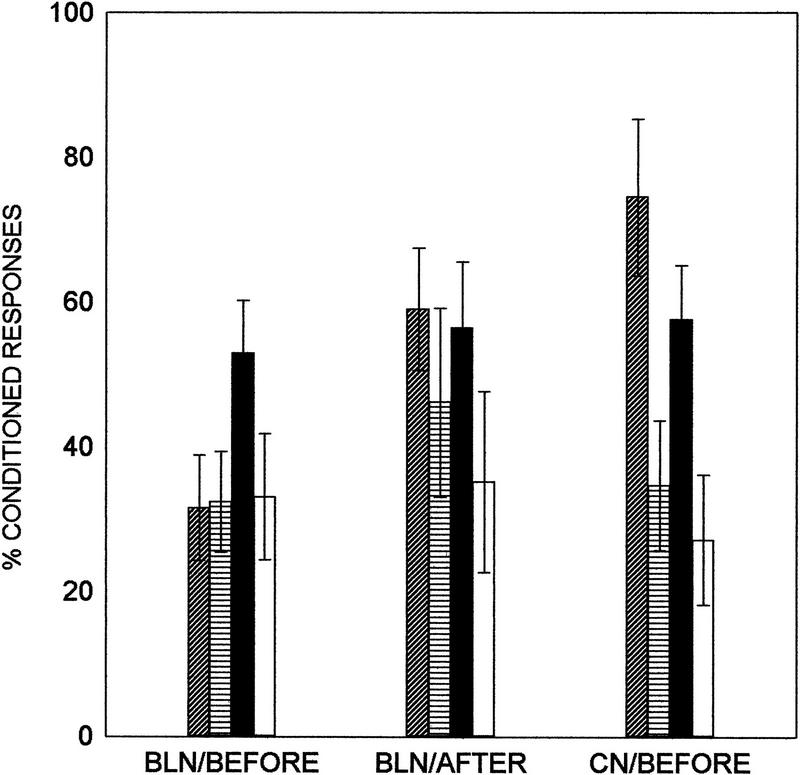
Exposure to the stressor or the NMDA receptor antagonist in the central nucleus did not interact and alter the spontaneous blink rate [F(1,17) = 1.34, P = 0.26] or sensitized eye-blink responses to the CS before training [F(1,17) = 1.32, P = 0.27]. In contrast to the lateral/basolateral nucleus injection, injection of AP5 into the central nucleus before the stressor did not prevent the facilitated acquisition in response to the stressor. Using stress (yes or no) and drug (AP5 or vehicle) and trials of training (three blocks of 100 trials) as independent variables and CRs as the dependent variable, there was only a main effect of stress [F(1,17) = 13.74, P = 0.002] (Fig. 4). In other words, exposure to the stressor enhanced acquisition of the CR across all trials of training irrespective of whether the antagonist or the vehicle were injected into the central nucleus.
Figure 4.
Effect of NMDA receptor antagonism in the amygdala on classical eye-blink conditioning. Percent CRs to the auditory CS (eye blinks that commenced 80 msec after CS onset) over 300 trials of training are shown for all 12 groups. The first set of four groups was injected with AP5 in the lateral/basolateral nucleus before stressor exposure (BLN/BEFORE). The second set was injected with AP5 in the lateral/basolateral nucleus after exposure to the stressor (BLN/AFTER). The third set was injected with AP5 into the central nucleus before stressor exposure (CN/BEFORE). Only NMDA receptor antagonism in the lateral/basolateral amygdaloid nucleus before stressor exposure prevented the stress-induced facilitation of associative learning 24 hr later. Bars: (Diagonal lines) Stress/AP5; (horizontal lines) no stress/AP5; (solid) stress/vehicle; (open) no stress/vehicle.
Discussion
Results from the present experiments indicate that the stress-induced facilitation of associative learning is prevented by antagonism of NMDA receptors in the lateral/basolateral nucleus of the amygdala. Exposure to a stressor of brief intermittent tail shocks in the presence of a competitive NMDA receptor antagonist, AP5, injected bilaterally and locally into the lateral/basolateral nucleus of the amygdala prevented the facilitated learning 24 hr later, whereas the exposure to the stressor in the absence of the antagonist induced rapid acquisition of the CR (Fig. 3A,B). The effect was specific to the basolateral region of the amygdala, because NMDA receptor antagonism in the nearby central nucleus before stressor exposure did not prevent the facilitated acquisition 24 hr later (Fig. 4). Because the lateral nucleus is positioned above the basolateral nucleus, it is difficult to rule out involvement of the lateral nucleus when injecting into the basolateral nucleus. Thus, the present results support the hypothesis that the facilitated learning induced by exposure to the stressful event is occurring by NMDA receptor activation in the amygdala and the effect is localized to the basolateral/lateral nucleus complex.
In addition to identifying the brain region where the NMDA receptor antagonism prevents the facilitated acquisition in response to stress, the present results also suggested when the antagonism must occur. When the antagonist was injected before exposure to the stressor, the facilitated responding was prevented 24 hr later, but when it was injected after the stressor exposure, the facilitated responding was not prevented 24 hr later (Fig. 3B). Although we did not directly measure NMDA receptor activation, the results suggest that access to these receptors is necessary for the induction of the facilitated learning and access must occur during exposure to the stressful event. Facilitated acquisition of the CR is apparent within 10 min of stressor cessation (T.J. Shors and M.P. Paczynsky, in prep.) but can persist for at least 48 hr (Servatius and Shors 1994; Shors and Servatius 1997). Therefore, the present results suggest that a transient NMDA receptor activation in response to the stressor induces persistent responses that maintain the enhanced acquisition over days.
Persistent changes in neuronal plasticity associated with learning are often mediated through activation of second-messenger systems. Because one consequence of NMDA receptor activation is calcium influx, second-messenger systems activated by calcium are a candidate mechanism for maintaining the enhanced acquisition in response to stress. Exposure to the stressor persistently enhances the binding affinity of [3H]PDBu, a marker for protein kinase C (PKC) (Shors et al. 1997a). Like the effect of stress on associative learning, the enhanced binding was localized to the lateral/basolateral nucleus of the amygdala and was prevented by NMDA receptor antagonism during exposure to the stressor. Consistent with its role in long-term mechanisms of plasticity and memory, calcium-dependent activation of PKC may mediate the persistent enhancement of learning in response to stress. In terms of physiological responses, spontaneous unit activity was significantly and persistently suppressed after exposure to the stressor (M.E. Chachich, P.R. Mathew, and T.J. Shors, unpubl.). This effect, like the facilitated learning and the increase in [3H]PDBu binding, was prevented by NMDA receptor antagonism during exposure to the stressful event. Thus, a number of persistent and NMDA receptor-dependent changes in neuronal plasticity are associated with the facilitated learning. Whether they are necessary will require further study.
In addition to the amygdala, the hippocampus was considered a likely brain region for mediating the effect of stress on learning. This consideration was based on the well-established role of the hippocampus in the acquisition of new information (Squire 1992), its sensitivity to the stressor that facilitates learning (Shors et al. 1990, 1997b; Schreiber et al. 1991), and its ability to modulate acquisition of the conditioned eye-blink response (Solomon et al. 1986). Exposure to the stressor also enhances trace conditioning (Beylin and Shors 1998), a task that is dependent on an intact hippocampus for learning (Solomon et al. 1986; Moyer et al. 1990; Weiss et al. 1998). Despite these associations, lesions to the major output of the hippocampus, the fornix, did not prevent the stress-induced facilitation of learning (T.J. Shors, unpubl.). It is possible that the facilitating effect of stress is mediated via other efferent pathways of the hippocampus besides the fornix (such as those to the entorhinal cortex), but until more discrete lesions are performed, we consider the hippocampus involved but not necessary for inducing the facilitated learning itself.
In the present studies we considered the amygdala because of its sensitivity to stress (Blanchard et al. 1991; Schreiber et al. 1991; Galvez et al. 1996; Hsu et al. 1998) and, more critically, its involvment in the acquisition and modulation of numerous types of conditioning paradigms (Blanchard and Blanchard 1972; Kapp et al. 1979, 1991; Gentile et al. 1986; LeDoux et al. 1986; Hitchcock and Davis 1987; Gallagher et al. 1990; Davis 1992; Helmstetter 1992; Kim and McGaugh 1992; Maier et al. 1993; McGaugh et al. 1993; Watkins et al. 1993; Fanselow and Kim 1994; Gallagher and Holland 1994; LeDoux 1994; Walker and Gold 1994). What tends to distinguish whether a task is dependent on the amygdala is its emotional nature. Conditioned bradycardia, freezing, inhibitory avoidance, and potentiated startle are considered emotional tasks, and they are dependent on an intact amygdala. Generally, classical eye-blink conditioning is not considered an emotional task, and the amygdala is not necessary for attaining the basic skeletal response (McCormick et al. 1982; Thompson et al. 1987; Skelton 1988). However, during early exposure to the CS and an aversive US, an animal elicits emotional responses to the CS (Rescorla and Solomon 1967), such as bradycardia and freezing. Such emotional responses to the CS can, in turn, modulate discrete conditioned reflexes, such as the conditioned eye-blink response (Wagner and Brandon 1989; Brandon and Wagner 1991). In addition, early responses to training, such as reflex facilitation, are indicative of behavioral arousal and are impaired by amygdala lesions (Weisz et al. 1992), and a short-term latency component of the eye-blink reflex is enhanced by amygdala stimulation (Canli and Brown 1996). Overall, the observation that NMDA receptor activation in the amygdala is necessary for induction of the stress-induced facilitation of classical conditioning is consistent with the growing body of evidence implicating the amygdala in the emotional modulation of associative memory formation, including discrete Pavlovian conditioned reflexes.
One possible substrate for mediating the effects of stress on classical eye-blink conditioning is the stress hormone corticosterone. During eye-blink conditioning, rats exhibit high levels of serum corticosterone (Shors et al. 1992). Interestingly, these hormones are reported to enhance instrumental learning when injected either systemically or directly into the amygdala, and the enhancement is prevented by lesions to the basolateral nucleus (Cahill and McGaugh 1996; Roozendaal and McGaugh 1997). As presented here, rats exposed to an emotional event that elevates glucocorticoids acquire the CR at a facilitated rate, and the effect is mediated within the lateral/basolateral nucleus of the amygdala (Shors et al. 1992; Servatius and Shors 1994; Shors and Servatius 1995). We found recently that removal of the adrenal cortex but not the adrenal medulla prevents the stress-induced enhancement of trace conditioning (A.V. Beylin and T.J. Shors, unpubl.). Thus, it is conceivable that the stress-induced enhancement of classical conditioning is likewise mediated by endogenous glucocorticoids within the amygdala formation.
Even though the present results suggest that induction of the facilitated learning in response to stress occurs in the amygdala, it is likely that the enhanced acquisition of the CR is expressed elsewhere. Numerous studies have indicated that the necessary circuitry for acquisition and expression of the CR resides in the cerebellum (Thompson et al. 1987; Skelton 1988; Krupa et al. 1993). The enhanced responding after stress does not appear to be mediated efferent to the cerebellum, for example, at the level of the motor output. If it was, one would expect enhanced responding irrespective of when the stressor was delivered, yet exposure to the stressor does not enhance responding after animals have acquired the response (Beylin and Shors 1998; T.J. Shors and M.P. Paczynski, in prep.). Thus, the data suggest that induction of the facilitated acquisition occurs in the lateral or basolateral nuclei of the amygdala and expression is mediated either within or efferent to the amygdala and afferent to or within the cerebellum.
There are a number of theories regarding how activation of NMDA receptors contributes to learning and memory processes. We have proposed that their activation before learning enhances the neural representation of cues in the environment, an increase that directs attention to those cues and increases learning when those cues are relevant (Shors and Matzel 1997). Similarly, others have suggested that arousal (conditioned arousal) enhances the detection and processing of sensory information (Whalen and Kapp 1991; Gallagher and Holland 1994), whereas others suggest that arousal reduces the range of cues to which an organism will respond (Easterbrook 1959). A stress-induced increase in the detection of cues within a decreased range could enhance or impair acquisition, depending on the task and whether the cues are (or become) relevant. We maintain that exposure to the stressor enhances the neural representation of cues and thereby enhances associative learning when the cues are discrete and relevant, as in classical eye-blink conditioning.
Acknowledgments
This work was supported by grants to T.J.S. from the National Science Foundation (IBN-9511027) and the Whitehall Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Beylin, A.B. and T.J. Shors. 1998. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav. Neurosci. (in press). [DOI] [PubMed]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Cholvanich P, Blanchard RJ, Clow DW, Hammer RP, Jr, Rowlett JK, Bardo MT. Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res. 1991;568:61–66. doi: 10.1016/0006-8993(91)91379-f. [DOI] [PubMed] [Google Scholar]
- Brandon SE, Wagner AR. Modulation of a discrete Pavlovian conditioned reflex by a putative emotive Pavlovian conditioned stimulus. J Expt Psychol. 1991;17:299–311. [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Modulation of memory storage. Curr Opin Neurobiol. 1996;6:237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of the fear-potentiated startle to a conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- Canli T, Brown TH. Amygdala stimulation enhances the rat eyeblink reflex through a short-latency mechanism. Behav Neurosci. 1996;110:51–59. doi: 10.1037//0735-7044.110.1.51. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;5:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychol Rev. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. The amygdala complex: Multiple roles in associative learning and attention. Proc Natl Acad Sci. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned performance. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gentile CG, Jarrell TW, Teich A, McCabe PM, Schneiderman N. The role of the amygdaloid central nucleus in the retention of differential Pavlovian conditioning of bradycardia in rabbits. Behav Brain Res. 1986;20:263–273. doi: 10.1016/0166-4328(86)90226-3. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ. The contribution of the amygdala to learning and performance of conditioned fear. Physiol Behav. 1992;51:1271–1276. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: Effects of lesions of the amygdala. Physiol Behav. 1987;39:403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventrivular nucleus: An in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JB. Amygdala central nucleus lesions: Effect on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Markgraf CG, Wilson A, Pascoe JP, Supple WF. Contributions of the amygdala and anatomically-related structures to the acquisition and expression of aversively conditioned responses. In: Dachowski L, Flaherty CF, editors. Current topics in animal learning: Brain, emotion and cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. pp. 311–346. [Google Scholar]
- Kim M, McGaugh J. Effects of intra-amygdala injections of NMDA receptor antagonists on acquisition and retention of an inhibitory avoidance task. Brain Res. 1992;585:35–48. doi: 10.1016/0006-8993(92)91188-k. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The amygdala: Contributions to fear and stress. Semin Neurosci. 1994;6:231–237. [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1986;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Expt Psychol Anim Behav Processes. 1990;16:137–149. [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Clark GA, Lavond DG, Thompson RF. Initial localization of the memory trace for a basic form of learning. Proc Natl Acad Sci. 1982;3:293–299. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Introini-Collison IB, Cahill LF, Castellano C. Neuromodulatory systems and memory storage: Role of the amygdala. Behav Brain Res. 1993;58:81–90. doi: 10.1016/0166-4328(93)90092-5. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1986. [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Lam YW, Christiansen BA. Latent inhibition of the rat eyeblink response: Effect of hippocampal aspiration lesions. Physiol Behav. 1994;55:597–601. doi: 10.1016/0031-9384(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Tocco G, Shors TJ, Thompson RF. Immediate early gene induction after acute stress. NeuroReport. 1991;2:17–20. doi: 10.1097/00001756-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats. Behav Neurosci. 1994;108:1101–1106. doi: 10.1037//0735-7044.108.6.1101. [DOI] [PubMed] [Google Scholar]
- ————— Early acquisition, but not retention, of the classically conditioned eyeblink response is N-methyl-d-aspartate (NMDA) receptor dependent. Behav Neurosci. 1996;110:1040–1048. doi: 10.1037//0735-7044.110.5.1040. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. Stress-induced sensitization and facilitation of learning are dependent on NMDA-receptor activation. NeuroReport. 1995;6:677–680. doi: 10.1097/00001756-199503000-00023. [DOI] [PubMed] [Google Scholar]
- ————— The contribution of stressor intensity, duration and context to the stress-induced facilitation of associative learning. Neurobiol Learn Mem. 1997;67:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Matzel LD. Long-term potentiation (LTP): What’s learning got to do with it? Behav & Brain Sci. 1997;20:597–655. doi: 10.1017/s0140525x97001593. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Foy MR, Levine S, Thompson RF. Unpredictable and uncontrollable stress impairs neuronal plasticity in the rat hippocampus. Brain Res Bull. 1990;24:663–667. doi: 10.1016/0361-9230(90)90005-k. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Elkabes S, Selcher JC, Black IB. Stress persistently increases NMDA receptor binding of [3H]PDBu, a marker of protein kinase C, in the amygdala, and reexposure to the stressful context reactivates the increase. Brain Res. 1997a;750:293–300. doi: 10.1016/s0006-8993(96)01369-8. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Gallegos R, Breindl A. Transient and persistent consequences of inescapable stress on long-term potentiation (LTP), synaptic efficacy, theta rhythms and bursts in area CA1 of the hippocampus. Synapse. 1997b;26:209–217. doi: 10.1002/(SICI)1098-2396(199707)26:3<209::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid response in unrestrained rats. Behav Neurosci. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Moore JW. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctologus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol. 1975;89:1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Van der Schaaf ER, Weisz DJ, Thompson RF. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Squire L. Memory and the hippocampus: A synthesis from findings with rats, monkeys and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Donegan NH, Clark GA, Lavond DG, Lincoln JS, Madden J, Mamounas LA, Mauk MD, McCormick DA. Neuronal substrates of discrete, defensive conditioned reflexes, conditioned fear states, and their interactions in the rabbit. In: Gormezano I, Prokasy WS, Thompson RF, editors. Classical conditioning. 3rd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. pp. 371–399. [Google Scholar]
- Tocco G, Shors TJ, Baudry M, Thompson RF. Selective increase of AMPA binding to the AMPA/quisqualate receptor in the hippocampus in response to acute stress. Brain Res. 1991;559:168–171. doi: 10.1016/0006-8993(91)90302-c. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) In: Klein SB, Mowrer RR, editors. Contemporary learning theories. Hillsdale, NJ: Lawrence Erlbaum Associates; 1989. pp. 149–190. [Google Scholar]
- Walker DL, Gold PE. Intraamygdala kinase inhibitors disrupt retention of a learned avoidance response in rats. Neurosci Lett. 1994;176:255–258. doi: 10.1016/0304-3940(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Maier SF. The amygdala is necessary for the expression of conditioned but not unconditioned analgesia. Behav Neurosci. 1993;107:402–405. doi: 10.1037//0735-7044.107.2.402. [DOI] [PubMed] [Google Scholar]
- Weiss, C., H. Boumeester, J.M. Power, and J.F. Disterhoft. 1998. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav. Brain Res. (in press). [DOI] [PubMed]
- Weisz DJ, Harden DG, Xiang Z. Effects of amygdala lesions on reflex facilitation and conditioned response of acquisition during nictitating membrane response conditioning in rabbit. Behav Neurosci. 1992;106:262–273. doi: 10.1037//0735-7044.106.2.262. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS. Contributions of the amygdaloid central nucleus to the modulation of the nictitating membrane reflex in the rabbit. Behav Neurosci. 1991;105:141–153. doi: 10.1037//0735-7044.105.1.141. [DOI] [PubMed] [Google Scholar]
- Wilson LM, Wilson JR, Dicara LV. Facilitation of Pavlovian conditioned cardiodecelerations following preshock in immobilized rats. Physiol Behav. 1975;15:653–658. doi: 10.1016/0031-9384(75)90115-8. [DOI] [PubMed] [Google Scholar]