Abstract
Background
Chemerin is a novel adipokine that is associated with inflammation and adipogenesis. However, it remains unclear whether chemerin is involved in patients with cardiovascular disease. We investigated whether the serum chemerin levels of Korean patients with coronary artery disease correlated with specific cardiometabolic parameters.
Methods
In total, 131 patients, all of whom had coronary artery stenosis exceeding 50%, participated in this study. Their serum chemerin levels and cardiometabolic parameters were measured. The serum chemerin levels of two groups of patients were compared; those with one stenotic vessel (n=68) and those with multiple stenotic vessels, including left main coronary artery disease (n=63).
Results
Serum chemerin levels correlated positively with the degree of coronary artery stenosis and fasting glucose, triglyceride, total cholesterol, low density lipoprotein cholesterol, and high sensitive C-reactive protein levels. The group with multiple stenotic vessels, including left main disease, had higher chemerin levels than the group with one stenotic vessel (t=-2.129, P=0.035). Multiple binary logistic regression showed chemerin was not an independent risk factor of multiple vessel disease (odds ratio, 1.018; confidence interval, 0.997 to 1.040; P=0.091).
Conclusion
Serum chemerin levels have a significant correlation with several cardiometabolic risk factors and the degree of coronary artery stenosis in Korean patients with coronary artery disease. However, multiple binary logistic regression showed chemerin was not an independent risk factor of multiple vessel disease. Additional investigations are necessary to fully elucidate the role of chemerin in cardiovascular disease.
Keywords: Chemerin, Coronary artery disease, Metabolic syndrome
INTRODUCTION
Due to the essential role obesity plays in the pathogenesis of metabolic syndrome, a syndrome that is associated with cardiovascular morbidity, considerable interest has arisen in understanding the role obesity plays in cardiovascular disease [1]. Adipose tissue is no longer thought to only store excess energy; rather, it appears to be an active endocrine organ that produces many cytokines, which are known as adipokines [2-6]. These adipokines appear to have systemic effects on the brain, liver, muscles, β-cells, gonads, lymphoid organs, and vasculature [7]. Many studies have examined the role adipose tissue plays in the development of coronary artery disease in patients with metabolic syndrome [8-13], and it is now understood that adipose tissue can cause vascular complications either directly by causing atherosclerosis and inducing inflammatory responses, or indirectly by promoting the development of insulin resistance [14]. These atherosclerosis-inducing pathways are mediated by various adipokines, including adiponectin, leptin, retinol binding protein-4 and resistin [8-12]. Analyses of additional adipokines may help us to further understand the pathogenesis of insulin resistance and its cardiovascular complications.
Chemerin is a recently discovered 16 kDa chemoattractant protein that acts as a ligand for the G-protein receptor CMKLR1 (ChemR23 or DEZ) [15,16]. It is secreted by adipose tissue as prochemerin (18 kDa) [16] and is transformed into an active protein by serine protease cleavage of its C-terminal fragment [17]. In its active form, chemerin regulates the immune system and participates in inflammation by promoting the recruitment of tissue macrophages and plasmacytoid dendritic cells [15,18]. Chemerin is also associated with adipogenesis, as studies with Psammomys obesus have shown that while both chemerin and CMKLR1 are expressed by all tissues, their expression is particularly high in the liver, kidney, and adipose tissue [19]. Moreover, while undifferentiated adipose tissue only expresses low levels of chemerin and CMKLR1, both molecules are expressed at progressively higher levels by differentiated adipocytes [15]. The knockdown of chemerin or CMKLR1 expression in pre-adipocytes severely impairs their differentiation and reduces the expression of genes involved in glucose and lipid metabolism [15]. These observations suggest that chemerin may participate in insulin resistance and its cardiovascular complications.
As mentioned above, chemerin is associated with inflammation, adipogenesis, glucose, and lipid metabolism, all of which may contribute to the development of diabetic cardiovascular complications, especially atherosclerosis [15,18,19]. Therefore, we hypothesized that chemerin levels might be related to other cardiometabolic markers and that an increase in chemerin might reflect the degree of atherosclerotic burden. To further address this question, we examined whether the chemerin levels in Korean patients with coronary artery disease correlate with specific cardiometabolic parameters.
METHODS
Subjects
Of the patients who underwent coronary angiography at Keimyung University Dongsan Hospital, Daegu, Korea from November 2006 to April 2007, 131 were proven by coronary angiography to have meaningful stenosis (≥50%) and were recruited. Patients who did not have infections or renal or liver failure were eligible for the study. The medical history of each patient was determined by a questionnaire. The present study was approved by the local Ethical Review Committee of the Keimyung University School of Medicine. All participants gave informed consent.
Anthropometric, blood pressure, and biochemical measurements
All patients provided their personal information, and their height, weight, and waist circumference were measured. Body mass index (BMI) was calculated (kg/m2). Blood pressure was measured by an electronic device (FA-94H; Fanics, Korea) after sitting for 5 minutes. Blood was drawn in the morning after a 12-hour fast. The fasting serum glucose levels were measured by using the glucose oxidase method (Cobas Integra 800; Roche, Basel, Switzerland). Fasting serum insulin concentrations were measured by a radioimmunoassay kit (Insulin Myria; TechnoGenetics, Milano, Italy). The homeostasis model of assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin×(fasting glucose/22.5). The levels of the following biochemical markers were measured by enzymatic methods (Cobas Integra 800): serum triglyceride, total cholesterol, and high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol. High sensitive C-reactive protein (hsCRP) concentrations were measured using the latex method (TBA-200FR; Tosiba, Tokyo, Japan). Serum homocysteine concentrations were measured using an Axsym (Abbott, Abbott Park, IL, USA). The separated sera were stored at -70℃ until the chemerin levels could be measured using a commercially available ELISA (R&D, Minneapolis, MN, USA) as previously described [19]. The capture antibody and detection antibody were used. Intra- and inter-assay coefficients of variance for the chemerin ELISA were 5.0% and 10.0%, respectively. The sensitivity of the ELISA assay was 1 to 10 ng/dL, and the midrange of the assay was 5 ng/dL. This kit can detect human chemerin concentration by 0.5 ng/dL at least.
Coronary angiogram
Angiographic images were reviewed by an experienced cardiologist who was unaware of the patient's biochemistry results. As outlined in the Duke coronary artery index [20,21], we defined significant stenosis as >50% of the lumen diameter. The degree of stenosis of the diseased vessels served as a severity index. Patients were divided into two groups; those with one stenotic vessel (n=68) and those with multiple stenotic vessels, including left main disease (n=63).
Statistical analysis
The data are expressed as mean±standard deviation. We performed Student's t-test to compare chemerin levels and other metabolic factors in patients divided according to the number of stenotic vessels, and checked multiple binary logistic regression analysis (95% confidence interval) on risk of multiple vessel stenosis. We performed Pearson's correlation analyses between chemerin and cardiometabolic factors using SPSS version 12.0 software for Windows (SPSS Inc., Chicago, IL, USA). P values of less than 0.05 were considered statistically significant.
RESULTS
Clinical and metabolic subject characteristics
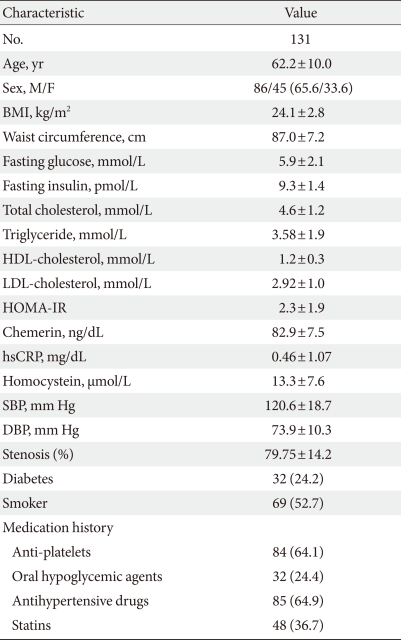
Table 1 shows the clinical characteristics of the 131 study subjects, of whom 86 (65.6%) and 45 (33.6%) were men and women, respectively. In total, 69 (52.7%) patients were smokers, 32 (24.4%) had diabetes and 85 (64.9%) had hypertension. Mean BMI was 24.1±2.8 kg/m2. Thirty-five (26.7%) patients were overweight (BMI 23 to 24.9 kg/m2) and 51 (39.0%) patients were obese (BMI >25 kg/m2). Angiography showed that 68 (49.8%) patients had one vessel disease, 39 (32.2%) had two vessel disease, 16 (12.3%) had triple vessel disease, and 8 (5.8%) had left main disease. In addition, 84 (64.1%) patients were being treated with anti-platelet agents, 32 (24.4%) patients were being treated with an oral hypoglycemic agent, 85 (64.9%) patients were being treated with an antihypertensive drug, and 48 (36.7%) patients were being treated with statins before enrollment.
Table 1.
Clinical and metabolic characteristics of subjects
Data are presented as mean±standard deviation or number (%).
BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostasis model of assessment of insulin resistance; hsCRP, high sensitive C-reactive protein; SBP, systolic blood pressure; DBP, diastolic blood pressure; Stenosis (%), percentage of coronary artery stenosis.
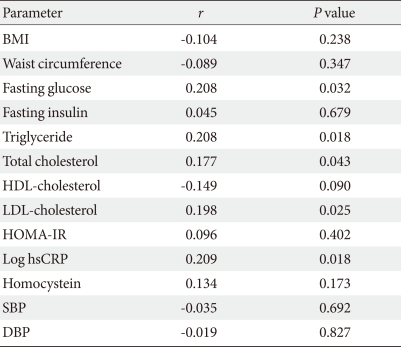
Correlation between serum chemerin concentrations and cardiometabolic parameters
There were significant but weak correlations between serum chemerin concentrations and fasting glucose, triglyceride, total cholesterol, LDL-cholesterol and hsCRP (Table 2). However, serum chemerin concentrations did not correlate significantly with fasting insulin, HDL-cholesterol or homocysteine levels, BMI, waist circumference, HOMA-IR and systolic and diastolic blood pressure.
Table 2.
Correlation between chemerin and cardiometabolic parameters
The P values are from Pearson's correlations.
BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostasis model of assessment of insulin resistance; hsCRP, high sensitive C-reactive protein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Correlation between serum chemerin concentrations and coronary angiographic findings
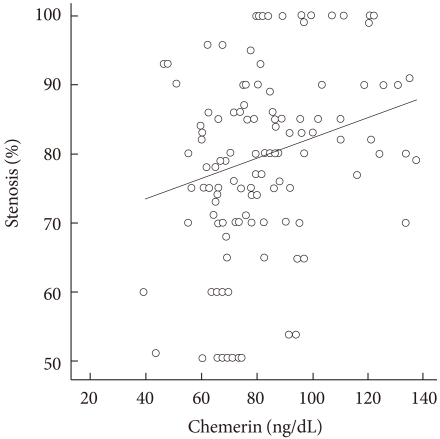
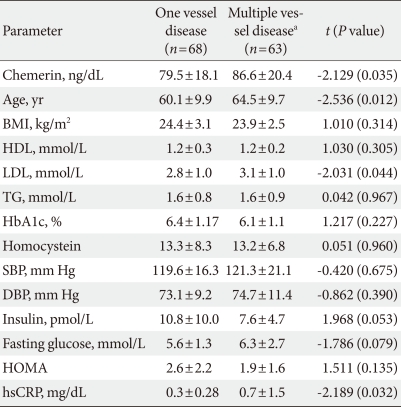
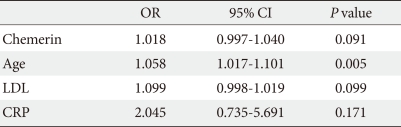
To determine whether serum chemerin concentration is associated with coronary artery disease, we analyzed the correlation between serum chemerin concentrations and coronary stenosis (%) in all participants. Fig. 1 shows how serum chemerin concentrations correlate with the degree of coronary artery stenosis. We measured a significant positive correlation, albeit weak, between these two parameters (r=0.0200, P=0.022). Moreover, the group with multiple vessel disease, including left main disease, showed higher age and chemerin, LDL, and CRP levels than the one vessel disease group (Table 3). However, Table 4 depicts that chemerin level is not an independent risk factor in multiple vessel disease.
Fig. 1.
Correlation between chemerin levels and the degree of coronary artery stenosis. Pearson's correlation coefficient=0.21; P<0.022.
Table 3.
Characteristics in patients divided according to the number of stenotic vessels and presence of left main lesions
Data are presented as mean±standard deviation.
Patients were divided according to one stenotic vessel (one vessel disease) or multiple stenotic vessels (multiple vessel disease).
The P values are from Student's t-test.
BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA, homeostasis model of assessment; hsCRP, high sensitive C-reactive protein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
aMultiple vessel disease included two and triple vessel disease and left main disease.
Table 4.
Multiple binary logistic regression on risk of multiple vessel stenosis
OR, odds ratio; CI, confidence interval; LDL, low density lipoprotein; CRP, C-reactive protein.
DISCUSSION
Accumulating evidence has suggested that adipokines play an important role in the development of coronary artery disease in patients with metabolic syndrome [8-13]. Recent studies have shown that chemerin levels associate significantly with metabolic components of the metabolic syndrome [19,22,23]. However, whether chemerin levels are also associated with cardiovascular parameters in patients with coronary artery disease remains to be elucidated. In this study, we investigated whether circulating chemerin concentrations correlate with the cardiometabolic parameters of Korean patients with coronary artery disease. We observed that serum chemerin concentrations were positively correlated with fasting glucose, triglyceride, total cholesterol, LDL-cholesterol, and hsCRP. Serum chemerin concentrations also had a significant correlation with the degree of coronary artery stenosis, although we recognize this association was weak.
Chemerin is a recently identified chemoattractant protein that induces leukocyte migration; in addition, its receptor is expressed by tissue macrophages [16-18]. These observations suggest that chemerin functions in the development of inflammatory diseases, including atherosclerosis. In this study, we found that chemerin levels correlated positively with hsCRP levels. hsCRP is a circulating marker of inflammation that is predominantly produced by the liver in response to IL-6 [24]. Patients with metabolic syndrome have higher CRP levels than healthy individuals, and this high CRP level is an independent risk factor for type 2 diabetes and cardiovascular disease [24-26]. CRP also directly promotes the inflammatory component of atherosclerosis by inducing adhesion molecule expression by human endothelial cells [27]. Recently, Lehrke et al. [22] analyzed the relationship between chemerin and metabolic components in patients with chest pain. In their study, chemerin is associated with coronary plaque burden and the number of non-calcified plaques, but these correlations dropped out after adjusting for other cardiovascular risk factors. In contrast to their study, this report shows that there was a significant but weak correlation between chemerin concentrations and the degree of coronary artery stenosis even after adjusting for other factors. Moreover, the group with multiple vessel disease had higher chemerin levels than the group with one vessel disease. Thus, our study suggests the possibility that chemerin could be associated with coronary artery disease; however our study did not show that chemerin level was not an independent risk factor in multiple vessel disease.
In addition to having pro-inflammatory effects, chemerin is a novel adipokine that has been associated with metabolic syndrome phenotypes [22]. Chemerin has been shown to regulate adipocyte differentiation and the expression of adipocyte genes involved in glucose and lipid homeostasis [19]. While Bozaoglu et al. [19] previously failed to detect a significant difference in the chemerin levels of subjects with normal glucose tolerance and subjects with type 2 diabetes, obese subjects were found to have significantly higher chemerin levels than lean subjects. Moreover, the chemerin levels in normal glucose-tolerant subjects were found to correlate significantly with various metabolic parameters, including BMI, waist circumference, waist-hip ratio, fasting glucose levels, fasting insulin levels, plasma triglyceride levels, and blood pressure [19]. However, this study shows that while serum chemerin concentrations significantly but weakly correlated with fasting glucose, triglyceride, total cholesterol and LDL cholesterol levels, they did not correlate with fasting insulin levels, HOMA-IR, BMI, waist circumference or blood pressure. This discrepancy may be due to differences between the study populations of the previous study and our own. In particular, our study included subjects who were taking antihypertensives, oral hypoglycemia agents and statins.
This study has certain limitations that we will address here. First, we measured the number of significant coronary stenoses without individualizing each coronary stenosis according to the type of lesion involved. If we had done so, we could have obtained a more accurate correlation between chemerin and coronary stenosis (%). Second, we enrolled patients with significant coronary artery stenosis (mentioned in Methods; >50% of patients) without enrolling healthy controls with no coronary artery disease. Thus, we could not determine the odds ratio of various cardiometabolic factors in coronary atherosclerosis. Third, the chemerin ELISA kit used in this study measures full-length chemerin form. Chemerin protein exists as a full-length protein in plasma, but this form of the protein is known to have lower bioactivity than prochemerin [28]. However, despite these shortcomings, the results suggest that chemerin could be useful as a predictor of metabolic syndrome and cardiovascular complications.
In conclusion, we found that circulating chemerin concentrations weakly but significantly correlated with several cardiometabolic parameters as well as the severity of coronary artery stenosis in Korean patients with coronary artery disease. However, multivariate analysis showed chemerin was not an independent risk factor for multiple vessel disease. Additional investigations are necessary to fully elucidate the role chemerin plays in the development and/or progression of cardiovascular disease.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A08-4335-AA2004-08N1-00020B).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 2.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 3.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 4.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 5.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 7.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffens S, Mach F. Adiponectin and adaptive immunity: linking the bridge from obesity to atherogenesis. Circ Res. 2008;102:140–142. doi: 10.1161/CIRCRESAHA.107.170274. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, Kataoka T, Kamimori K, Shimodozono S, Kobayashi Y, Yoshiyama M, Takeuchi K, Yoshikawa J. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 11.Ingelsson E, Sundstrom J, Melhus H, Michaelsson K, Berne C, Vasan RS, Riserus U, Blomhoff R, Lind L, Arnlov J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009;206:239–244. doi: 10.1016/j.atherosclerosis.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13:1764–1771. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 13.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 14.Kowalska I. Role of adipose tissue in the development of vascular complications in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2007;78(Suppl 1):S14–S22. [Google Scholar]
- 15.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 16.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280:34661–34666. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 18.Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol. 2005;174:244–251. doi: 10.4049/jimmunol.174.1.244. [DOI] [PubMed] [Google Scholar]
- 19.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 20.Mark DB, Nelson CI, Califf RM, Harrell FE, Jr, Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR, DeLong ER, Smith PD, Reves JG, Jollis JG, Tcheng JE, Muhlbaier LH, Lowe JE, Phillips HR, Pryor DB. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 21.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 22.Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, Goke B, Leber AW, Parhofer KG, Broedl UC. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161:339–344. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 23.Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O'Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes. 2009;58:1971–1977. doi: 10.2337/db08-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 27.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura T, Oppenheim JJ. Chemerin reveals its chimeric nature. J Exp Med. 2008;205:2187–2190. doi: 10.1084/jem.20081736. [DOI] [PMC free article] [PubMed] [Google Scholar]