Abstract
Background
To accelerate the healing of diabetic wounds, various kinds of growth factors have been employed. It is the short half-life of administered growth factors in hostile wound beds that have limited wide-spread clinical usage. To overcome this limitation, growth factor gene therapy could be an attractive alternative rather than direct application of factors onto the wound beds. We administered two growth factor DNAs, epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) into a cutaneous wound on diabetic mice. We compared the different characteristics of the healing wounds.
Methods
Streptozotocin was injected intraperitoneally to induce diabetes into C57BL/6J mice. The ultrasound micro-bubble destruction method with SonoVue as a bubbling agent was used for non-viral gene delivery of EGF828 and VEGF165 DNAs. Each gene was modified for increasing efficacy as FRM-EGF828 or minicircle VEGF165. The degree of neoangiogenesis was assessed using qualitative laser Doppler flowmetry. We compared wound size and histological findings of the skin wounds in each group.
Results
In both groups, accelerated wound closure was observed in the mice receiving gene therapy compared with non treated diabetic control mice. Blood flow detected by laser doppler flowmetry was better in the VEGF group than in the EGF group. Wound healing rates and histological findings were more accelerated in the EGF gene therapy group than the VEGF group, but were not statistically significant.
Conclusion
Both non-viral EGF and VEGF gene therapy administrations could improve the speed and quality of skin wound healing. However, the detailed histological characteristics of the healing wounds were different.
Keywords: Epidermal growth factor, Gene therapy, Non-viral, Skin wound, Vascular endothelial growth factor
INTRODUCTION
Wound healing, which proceeds through a series of consecutive, but overlapping stages, is characterized by the sequential movement of different cell populations into the wound site [1].
At the molecular level, the acute wound healing response is characterized by changes in the composition and organization of the extracellular matrix (ECM) as well as the local profile of growth factors [2,3].
Blood components are released into the wound site activating the clotting cascade. The resulting clot induces hemostasis and provides a matrix for the influx of inflammatory cells. Platelets degranulate releasing alpha granules, which secrete growth factors such as: epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-β) [4].
With the assistance of platelet released vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), endothelial cells proliferate and angiogenesis ensues. This process is essential for the synthesis, deposition, and organization of a new ECM [5].
EGF, originally reported by Dr. Stanley Cohen [6,7], is secreted by platelets, macrophages, and fibroblasts. This growth factor acts in a paracrine fashion on keratinocytes [8,9]. In vitro studies have shown that EGF is up-regulated after acute injury significantly accelerating reepithelialization [10] and increasing tensile strength of the wound [11].
VEGF is produced by endothelial cells, keratinocytes, fibroblast, smooth muscle cells, platelets, neutrophils, and macrophages [12-17]. It is important in wound healing because it promotes the early events in angiogenesis, particularly endothelial cell migration [18-20] and proliferation [21-25] as seen in several in vitro studies. Chronic wounds have areas of local skin ischemia making VEGF a possible therapeutic modality.
However, high blood glucose levels impair both granulocyte and neutrophil function, and chemotaxis, resulting in increased risk of infection. Inflammation is prolonged, angiogenesis is impaired and there is decreased synthesis of collagen [1].
There are changes in the cellular infiltrate and extracellular matrix, with prolonged expression of fibronectin. Finally, there are fewer T cells and more macrophages persisting beyond the initial stages of healing [3].
We have recently shown that the minicircle-VEGF165 is effective for the healing of the skin wound of the diabetic mice [26].
After the above study, we administered EGF using another gene therapy method. Therefore, this study was thus designed to report the different characteristics of two genes on the skin wound of diabetic mice.
METHODS
Plasmid construction
Human EGF cDNA was a kind gift from Dr. Siu-Yuen Chan, University of Hong Kong. The pβ-EGF159 was constructed by using human mature EGF plasmid and human full length EGF. We did not construct the minicircle DNA (cDNA) by using of pβ-EGF159. In the case of VEGF165, p2øC31-β-VEGF165 was constructed for the production of minicircle DNA. The DNA fragment only contains the chicken β-actin promoter, VEGF165 cDNA. The SV40 poly adenylation signal sequence was excised with BglII and ClaI from the pβ-VEGF165, and then bluntly ligated between the attB and attP sites of the p2øC31 plasmid, which was a kind gift from Dr. Mark A. Kay (Department of Genetics, Stanford University, CA, USA).
Preparation of DNAs
E. coli DH5α (Invitrogen, Carlsbad, CA, USA) was transformed by p2øC31-β-VEGF165. A single colony of the transformants was grown at 37℃ overnight (OD600=4.5-5.0). The 1 L of bacterial culture in the steady state was spun down in a clinical centrifuge (rotor JA-14, J2-MC centrifuge; Beckman, Fullerton, CA, USA) at 20℃, 1,300×g for 15 minutes. The pellet was re-dissolved with 1 L of fresh LB broth (pH 7.0) containing 1% L-(+)-arabinose. The resuspended bacteria were incubated at 30℃ with constant shaking at 225 rpm for 2 hours. Subsequently, 1 L of fresh LB broth (pH 9.0) containing 1% L-(+)-arabinose was added to the culture and the bacteria were cultivated for additional 2 hours at 37℃ for the activation of the restriction enzyme I-SceI. Super-coiled minicircle DNA was prepared from the culture, using plasmid purification kits from the Qiagen (Valencia, CA, USA). The contaminated endotoxin in the DNA preparation was removed by the AffinityPak Detoxi-Gel (Pierce, Rockford, IL, USA).
Cell culture and in vitro transfection
The human embryonic kidney (HEK293) cells, were purchased from ATCC (Manassas, VA, USA). HEK293 cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a 5% CO2 incubator.
SonoVue
SonoVue microbubbling echo contrast agent that utilizes sulfur hexafluoride was purchased from Bracco Diagnostics, Inc. (Bracco, UK).
For the assessment of transfection by sonoporation, the cells were detached by trypsin-EDTA, washed twice in phosphate-buffered saline, and resuspended with serum free media at 2.0×105 cells/well in 48-well plate (Nunc, Rochester, NY, USA). The diameter of each well was 12.0 mm. After addition of microbubble, each plasmid encoding human EGF and VEGF was mixed with the cell supernatant (2 µg/well). Immediately after the addition of the mixture of plasmids and microbubbles, the cells were exposed to ultrasound with a 20% duty cycle (Sonitron 1000; Rich Mar Inc., Inola, OK, USA) for 30 seconds, using a 6.0 mm in diameter ultrasound probe. The ultrasound probe was immersed directly into the cell suspension without any contact to the surface of well. After exposure, the cell suspensions were harvested, separated by centrifugation, and plated in 6-well dishes. The cells were incubated for 48 hours before ELISA.
Comparison of minicircle VEGF with a typical plasmid with bacterial backbone
To assay the efficiency of transfection by minicircle VEGF, branched polyethylenimine (BPEI, 25kDa; Sigma-Aldrich, St. Louis, MO, USA) was used as a gene carrier. The plasmid/BPEI complexes were prepared at a 10/1 N/P ratio and incubated for 30 minutes at room temperature. The cells were washed twice with serum-free medium, and then 2 mL of fresh serum-free medium was added. The plasmid/BPEI complex was added to each well. The cells were then incubated for 4 hours at 37℃ in a 5% CO2 incubator. After 4 hours, the transfection mixtures were removed and 2 mL of fresh medium containing FBS was added. The cells were incubated in a CO2 incubator for 48 hours. The cells and media were harvested for ELISA.
ELISA
EGF protein was measured using Quantikine human EGF ELISA kit (R&D Systems, Minneapolis, MN, USA). EGF protein was measured using Quantikine human EGF ELISA kit (R&D Systems).
The secreted VEGF protein in the media was measured using Quantikine human VEGF ELISA kit (R&D Systems).
Animals
All animal procedures were approved by the Experimental Animal Committee at the Inje University. Two-week-old male C57BL/6J mice (20 to 30 g) were purchased from the Samtako (Animal Breeding Center, Osan, Korea) and housed in the pathogen-free condition and had ad libitum access to water and the standard mouse chow. Diabetes mellitus was induced by the single intraperitoneal injection (200 mg/kg) of streptozotocin (STZ) [27]. Random blood glucose levels were measured 1 week after the first STZ injection using a glucometer (Accu-Check; Roche Diagnostics, Indianapolis, IN, USA). Mice that showed blood glucose levels over 200 mg/dL were considered as diabetic [28]. The mice were divided into four groups: control group (n=5), diabetic control group (n=5), EGF treated group (n=5), and VEGF treated group (n=5), and the process was performed in triplicate.
Skin wounding
Each animal was subjected to wounding (6 mm in diameter) by punch biopsy (Stiefel, Bad Oldesloe, Germany) on the skin of the back of the mouse. Briefly, prior to wounding, general anesthesia was induced by the administration of ketamine hydrochloride (100 mg/kg). The hair was shaved with the standard blade (no. 10). Then, the skin was disinfected with the povidone-iodine solution and wiped with sterile water.
Gene delivery into diabetic mouse wound
Diabetic C57BL/6J mouse with the back skin wound received a subcutaneous injection of PBS containing the mixture of minicircle-VEGF165 (20 µg) and pβ-EGF828 (20 µg) with microbubble solution (100 µg). Immediately after the injection, ultrasound (frequency, 1.0 MHz; duty, 20%; intensity, 2.0 W/cm2; time, 30 seconds) was applied to the injection site of the wound edge using a Sonitron 2000 ultrasonicator (Rich-Mar, Inola, OK, USA) with a probe of diameter 6 mm.
Measurement of wound area and laser doppler imaging (LDI)
The areas of the wounds were evaluated by densitometry (Multi Gauge V3.0 software; Fujifilm Life Science, Tokyo, Japan). The percentage wound closure was calculated as a ratio of final wound area to initial wound area. For the estimation of the skin blood flow, the wounds were scanned with a Laser Doppler Imager (Periscan PIM II Laser Doppler Perfusion Imager; Perimed AB, Järfälla, Sweden) under the brief general anesthesia with inhalation of 1% to 2% isoflurane on the days 2, 6, and 12. The mice were placed on a light absorbing dark green background. The distance between the scanner head and the object was 20 cm. The Min and Max values were set at 0 and 8 V, respectively. The perfusion scan image color scale displayed the lowest value in dark blue and the highest value in red, which represent the relative amount of skin perfusion. All mice were placed on a warming pad to maintain core body temperature between 36.8℃ and 37.8℃ during the scanning.
Immunohistochemistry
The tissue samples adjacent to the wounds were embedded in OCT compound (Tissue Tek; Sakura Finetek, Torrance, CA, USA). Transverse sections of 5 µm thickness were placed on polyl-lysine-coated slides.
Histology
On day 12, wound tissues were fixed in 4% phosphate buffered formalin and then embedded in paraffin. From the paraffin-embedded tissue blocks, 5 µm sections were cut and stained with hematoxylin and eosin (H&E) for the histological analysis.
Statistical analysis
All the presented data were expressed as mean±standard deviation. The statistical significance was analyzed by the Student's t-test and ANOVA using the SPSS PC program (SPSS Inc., Chicago, IL, USA).
RESULTS
Plasmid construction
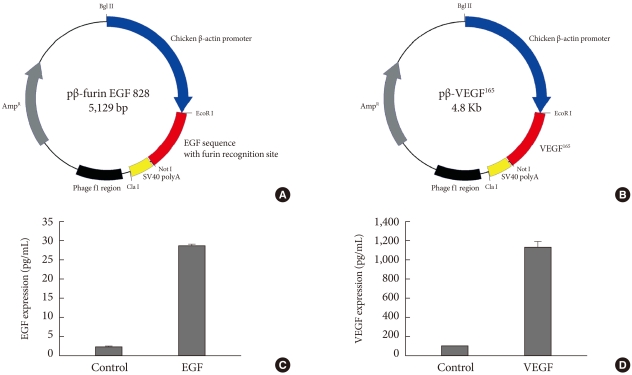
The cDNA sequence of human mature EGF was inserted into pβ-plasmid, which had a chicken β-actin promoter and a SV40 polyadenylation sequence. For the secretion of translated EGF, we added a furin protease recognition site to the N-terminal of EGF sequence (Fig. 1A). Likewise, we cloned the cDNA sequence of human VEGF165 to generate pβ-VEGF (Fig. 1B). All plasmids DNA were transformed into DH5α, cultured to liter scale, and prepared using DNA maxi-prep kit (Qiagen, Valencia, CA, USA).
Fig. 1.
Plasmid constructions and their expressions by ultrasound mediated gene delivery in human embryonic kidney (HEK) 293 cells. (A) cDNA encoding human epidermal growth factor (EGF) with a N-terminal furin recognition sequence was inserted between EcoR I and Not I restriction sites under chicken β-actin promoter. (B) cDNA of human vascular endothelial growth factor 165 (VEGF165) was inserted to produce pβ-VEGF165. (C, D) Ultrasound mediated gene delivery in HEK 293 cells. The cells were exposed to 1 MHz US in the presence of 2 µg of each plasmid with a concentration of microbubble (10 mg/mL). Ultrasound intensity was 2.0 W/cm2; ultrasound exposure time was 30 seconds; duty cycle was 20%.
Gene delivery to HEK293 cells by sonoporation
The human embryonic kidney (HEK) 293 cells in the presence of 2 µg of each plasmid were exposed to 1 MHz ultrasound with microbubble (10 mg/mL). With proper exposure intensity (2.0 W/cm2) and duty (20%) the cell membrane was caused to be in a transiently porous state under ultrasound irradiation called "sonoporation." The gene delivery by ultrasound has a major advantage of safety to the cell or tissue that exert the therapeutic effect of transgene. As shown in Fig. 1C and D, EGF and VEGF were measured from cell culture media (25 pg/mL and 1.2 ng/mL, respectively) 48 hours after gene delivery in HEK293 cells.
Production of minicircle-VEGF
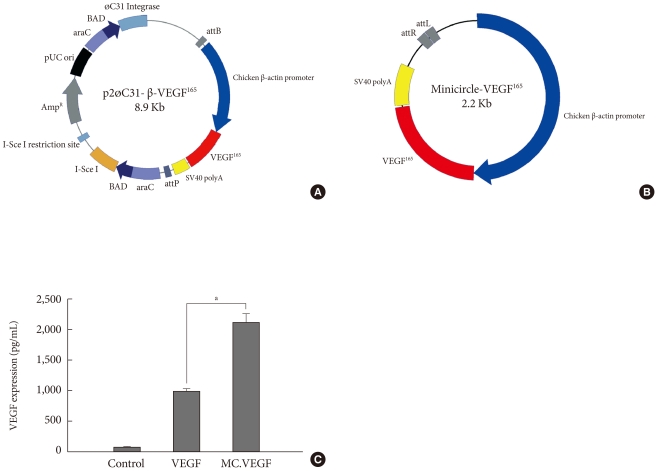
For the healing of chronic diabetic wound model, the initial rapid generation of blood circulation is known to be critical for the attraction of neutrophiles and monocytes to advance the repair process [29]. In the case of gene delivery of VEGF, we hypothesized that the earlier blood vessel formation and the initial increase of blood perfusion in the wound tissue would be necessary to accelerate the whole wound healing process. Therefore, we constructed a parent plasmid (p2øC31-VEGF) to induce a minicircle form of DNA encoding VEGF that has minimal size of expression machinery to transgene expression of VEGF (Fig. 2A). By adding L-(+)-arabinose and adjusting pH and temperature to pH 9.0 and 37℃, respectively, minicircle-VEGF DNA was recombinated out of the parent plasmid in DH5α. The remaining circular bacterial backbone DNA including an antibiotics resistance gene and a replication origin is linearized by homing endonuclease I-Sce I and degraded by bacterial exonucleases. The outcome of these processes is the production of supercoiled minicircle-VEGF DNA (2.2 kb) that has largely reduced the size in comparison with a typical form of plasmid pβ-VEGF (4.8 kb) (Fig. 2B). The transgene expression using minicircle-VEGF was 2 to 3 fold higher than a typical form of plasmid pβ-VEGF (Fig. 2C). This is considered to provide more rapid movement of minicircle DNA across the cytosol to the nuclear membrane than previously possible. Furthermore, minicircle DNA has no CpG island that stimulates unnecessary immune responses resulting in transgene quiescence.
Fig. 2.
Production of minicircle-vascular endothelial growth factor (VEGF) and the comparison of efficiency to a typical form of plasmid (pβ-VEGF) containing of bacterial backbone using branched polyethylenimine (BPEI) as a gene carrier in human embryonic kidney (HEK) 293 cells. (A) p2øC31-β-VEGF165 the expression cassette from pβ-VEGF165 was excised by restriction enzymes and bluntly ligated between attB and attP site of p2øC31 vector, which contains phi-C31 integrase and I-SceI homing endonuclease. (B) Minicircle-VEGF165. (C) Minicircle-VEGF165 showed 2 to 3 folds higher transfection efficiency in HEK 293 cells. 2×105 cells of HEK293 were treated for 4 hours with plasmid DNA (pβ-VEGF165 or Minicircle-VEGF165) complexed with branched polyethylenimine (BPEI, 25 kDa, N/P ratio 10:1). VEGF concentrations in the culture media were measured by ELISA. aP<0.05.
Measurement of wound area
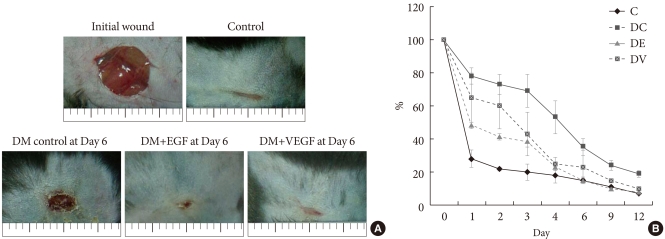
The results for the changes of the percentages of wound closure in both gene therapy groups are shown in Fig. 3. By day 6 post operation, all EGF and VEGF treated groups demonstrated complete closure of the wounds in contrast to diabetic control with a mean percent closure below 70%. The differences among the groups were not statistically significant.
Fig. 3.
Wound size comparison by ultrasound mediated gene delivery of epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF). (A) Growth factor gene delivery by sonoporation enhanced the progress of wound closure in diabetic mice to non-diabetic normal control group. (B) Average areas (in pixels) of wound that received ultrasound mediated gene delivery of EGF and VEGF were significantly reduced to non-diabetic control mice by day 12 post operation. C, control; DC, Diabetic (DM) control; DE, DM+EGF; DV, DM+VEGF.
LDI
The differences of wound perfusion were monitored by non invasive LDI at day 2 and 6 (Fig. 4). In comparison with the EGF group, the minicircle-VEGF DNA treated diabetic mice group showed increased blood flow in the wound area. Skin perfusions were gradually decreased after day 12 (data not shown).
Fig. 4.
Blood perfusion in the wound tissue was significantly increased by ultrasound mediated gene delivery of growth factors to diabetic mice. The laser doppler imager (PeriScan) used in this study was employed for the visualization of blood perfusion. Red color represents high blood perfusion in the wound site. At the 6th day post operation, a significant increase of blood perfusion was observed in diabetic (DM) mice group that received vascular endothelial growth factor (VEGF) gene delivery.
Histological findings
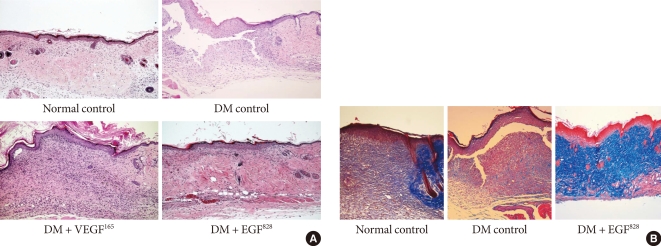
Both EGF and VEGF gene therapy could improve the speed and quality of skin wound healing. The detailed histological characteristics of healing wounds were different. The histology of biopsied wound tissue at day 12 in each group is shown in Fig. 5A. The wound of diabetic control shows severe edema and a disorganized pattern with heavy infiltration of inflammatory cells. In minicircle-VEGF treated group, wound restoration is considered to be caused mainly by new blood vessel formation. Contrary to minicircle-VEGF, the EGF treated group, demonstrated epidermal reorganization that has completely restored the normal wound microarchitectures (Fig. 5B) without observation of excessive increase of blood perfusion (Fig. 4).
Fig. 5.
Histology of wound tissues. (A) Histology of wound tissues at day 12 post operation (H&E stain, ×100). After the generation of skin wound, non-treated diabetic (DM) control showed severe edema, disorganized micro-architectures and the heavy infiltration of inflammatory cells. The wound tissues that received ultrasound mediated gene delivery of epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) showed a highly restored well organized state of tissue compared to the non-diabetic normal control. (B) Tissue reorganization of wound tissues at day 12 post operation (M&T, ×100). In contrast to non-treated diabetic control, the wound tissues that received gene delivery of EGF showed more collagen accumulation and appeared as a organized wound matrix.
DISCUSSION
Diabetic foot ulcers (DFUs), one of the most common complications of diabetes, are a leading cause of hospitalization for diabetic patients. For a patient living with diabetes, the lifetime risk of developing a DFU is estimated to be 25% [30,31]. The prevalence of DFUs causes significant health care resources to be spent on the management of wounds. The interventions include emergency room visits, antibacterial medications, amputations, and a multitude of other therapies directed at non healing wounds. Therefore, many growth factors affecting wound healing are studied.
In 2004, one growth factor, topically applied recombinant PDGF, is currently approved by the US Food and Drug administration for the treatment of diabetic foot ulcers. [30] However, its clinical success is limited in part by the need for daily application. There is a hostile proteolytic wound environment in which the protein is applied. A study about the sustained effect and therapeutic potency is being performed.
There were many previous studies using VEGF and EGF. Angiogenesis is necessary for granulation tissue formation as well as for providing oxygen and nutrition to wounds [32]. Inadequate angiogenesis in diabetic patients inhibits wound healing. VEGF is one of the most potent growth factors in stimulating angiogenesis. It triggers endothelial cell division, chemotaxis, and vascular permeability [33]. VEGF acts in a paracrine manner on dermal microvessels and endothelial cells. It also promotes the production of nitric oxide, which enhances collagen deposition. Previous studies with VEGF have demonstrated its efficacy in ischemic heart disease [34]. However, the increase in reepithelialization by VEGF165-treated wounds has been reported [35]. Data from this study, confirmed by histology, showed the improvement in reepithelialization. In previous studies, the more rapid maturation of granulation tissue in the VEGF165-treated wound was shown to have more organized collagen fiber bundles deposited in granulation tissue. However, in this study, the greater cell density in the granulation tissue and healed tissue was observed in the EGF-treated group.
In addition, there were many studies using recombinant human EGF to enhance the healing effect on the chronic diabetic foot in human [36]. Various routes of administration have been used for delivering genes to the chronic wound. The advent of new topical agents has broadened the treatment approaches to wound healing. However, in the case of a full thickness wound, the wound healing process might be delayed by using only topical application. When an intralesional infiltration of EGF was done, the wound size reduction time was accelerated [36]. Abundant functional capillaries started to emerge and vascular endothelial nuclei were clearly less hypertrophic.
In the present study, the improvement of wound healing time and wound reepithelization were similar in both groups. However, the detailed characteristics were different. In the VEGF treated diabetic group, LDI showed improved blood perfusion at the wound site, but ECM reorganization was more immature than the EGF treated group.
On the other hand, blood perfusion was not different from the control group in the EGF treated group, but ECM reorganization was markedly improved. The EGF treated group demonstrated an increased amount of mature collagen compared to the VEGF group.
Jazwa et al. [37] demonstrated that combined VEGF and EGF gene transfer into the wound improves wound healing in diabetic mice. In this study, authors concluded that VEGF might increase the migration of diabetic fibroblasts cultured in high glucose concentration through FGF4-mediated up-regulation of one of the VEGF receptors, Flt-1 (VEGF-Receptor 1). We did not try the combined gene transfer into the wound, but it needs further evaluation. The combined gene therapy could be explained as a synergistic effect or a side effect.
We used a nonviral gene delivery system. When choosing appropriate biomaterials during the design of a delivery vehicle, several factors must be considered [38,39]. These factors include predictability, accessibility, and safety issues. We focused on the safety issues. The efficacy of nonviral gene delivery is lower than the viral system, but the safety is most important factor.
One of the limitations of our study is that we used different delivery system in each group. There is the structural difference between VEGF and EGF DNAs. Second, we administrated the same dose of DNAs but we could not identify that the dose acts with the same efficacy. Therefore, if we injected the same dose, the effect could be different. Third, we regret that we did not perform the collagen stain in the VEGF group, since we were unable to compare the efficacy of reepithelization between two groups.
In conclusion, VEGF enhances the neoangiogenesis, but was not effective on maturation of organized reepithelization. The neoangiogenesis induced by EGF was not dominant, but it enhanced the maturation of organized reepithelization.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090258).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90:133–146. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 2.Petri JB, Konig S, Haupt B, Haustein UF, Herrmann K. Molecular analysis of different phases in human wound healing. Exp Dermatol. 1997;6:133–139. doi: 10.1111/j.1600-0625.1997.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 3.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111:850–857. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 5.Hantash BM, Zhao L, Knowles JA, Lorenz HP. Adult and fetal wound healing. Front Biosci. 2008;13:51–61. doi: 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Elliott GA. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J Invest Dermatol. 1963;40:1–5. doi: 10.1038/jid.1963.1. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 8.Shiraha H, Glading A, Gupta K, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243–254. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991;45:346–352. doi: 10.1002/jcb.240450407. [DOI] [PubMed] [Google Scholar]
- 10.Brown GL, Curtsinger L, 3rd, Brightwell JR, Ackerman DM, Tobin GR, Polk HC, Jr, George-Nascimento C, Valenzuela P, Schultz GS. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986;163:1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Nordquist R, von Fraunhofer A, Schultz GS. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 1988;208:788–794. doi: 10.1097/00000658-198812000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namiki A, Brogi E, Kearney M, Kim EA, Wu T, Couffinhal T, Varticovski L, Isner JM. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995;270:31189–31195. doi: 10.1074/jbc.270.52.31189. [DOI] [PubMed] [Google Scholar]
- 13.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 14.Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90:4153–4161. [PubMed] [Google Scholar]
- 16.Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jazwa A, Loboda A, Golda S, Cisowski J, Szelag M, Zagorska A, Sroczynska P, Drukala J, Jozkowicz A, Dulak J. Effect of heme and heme oxygenase-1 on vascular endothelial growth factor synthesis and angiogenic potency of human keratinocytes. Free Radic Biol Med. 2006;40:1250–1263. doi: 10.1016/j.freeradbiomed.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yebra M, Parry GC, Stromblad S, Mackman N, Rosenberg S, Mueller BM, Cheresh DA. Requirement of receptor-bound urokinase-type plasminogen activator for integrin alphavbeta5-directed cell migration. J Biol Chem. 1996;271:29393–29399. doi: 10.1074/jbc.271.46.29393. [DOI] [PubMed] [Google Scholar]
- 19.Suzuma K, Takagi H, Otani A, Honda Y. Hypoxia and vascular endothelial growth factor stimulate angiogenic integrin expression in bovine retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 1998;39:1028–1035. [PubMed] [Google Scholar]
- 20.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 21.Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270(1 Pt 2):H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- 22.Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 23.Goto F, Goto K, Weindel K, Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993;69:508–517. [PubMed] [Google Scholar]
- 24.Watanabe Y, Lee SW, Detmar M, Ajioka I, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene. 1997;14:2025–2032. doi: 10.1038/sj.onc.1201033. [DOI] [PubMed] [Google Scholar]
- 25.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 26.Yoon CS, Jung HS, Kwon MJ, Lee SH, Kim CW, Kim MK, Lee M, Park JH. Sonoporation of the minicircle-VEGF(165) for wound healing of diabetic mice. Pharm Res. 2009;26:794–801. doi: 10.1007/s11095-008-9778-x. [DOI] [PubMed] [Google Scholar]
- 27.Ramabadran K, Bansinath M, Turndorf H, Puig MM. The hyperalgesic effect of naloxone is attenuated in streptozotocin-diabetic mice. Psychopharmacology (Berl) 1989;97:169–174. doi: 10.1007/BF00442244. [DOI] [PubMed] [Google Scholar]
- 28.Anjaneyulu M, Ramarao P. Studies on gastrointestinal tract functional changes in diabetic animals. Methods Find Exp Clin Pharmacol. 2002;24:71–75. doi: 10.1358/mf.2002.24.2.677129. [DOI] [PubMed] [Google Scholar]
- 29.Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 30.Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351:48–55. doi: 10.1056/NEJMcp032966. [DOI] [PubMed] [Google Scholar]
- 31.Setacci C, de Donato G, Setacci F, Chisci E. Diabetic patients: epidemiology and global impact. J Cardiovasc Surg (Torino) 2009;50:263–273. [PubMed] [Google Scholar]
- 32.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 34.Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR, Isom OW, Crystal RG. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann Surg. 1999;230:466–470. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, Sheahan CM, Weinberg AD, Woo SL, Ehrlich HP, Tomic-Canic M. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275–2287. doi: 10.1038/jid.2009.26. [DOI] [PubMed] [Google Scholar]
- 36.Acosta JB, Savigne W, Valdez C, Franco N, Alba JS, del Rio A, Lopez-Saura P, Guillen G, Lopez E, Herrera L, Fernandez-Montequin J. Epidermal growth factor intralesional infiltrations can prevent amputation in patients with advanced diabetic foot wounds. Int Wound J. 2006;3:232–239. doi: 10.1111/j.1742-481X.2006.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jazwa A, Kucharzewska P, Leja J, Zagorska A, Sierpniowska A, Stepniewski J, Kozakowska M, Taha H, Ochiya T, Derlacz R, Vahakangas E, Yla-Herttuala S, Jozkowicz A, Dulak J. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010;8:6. doi: 10.1186/1479-0556-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreadis ST, Geer DJ. Biomimetic approaches to protein and gene delivery for tissue regeneration. Trends Biotechnol. 2006;24:331–337. doi: 10.1016/j.tibtech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Vasita R, Katti DS. Growth factor-delivery systems for tissue engineering: a materials perspective. Expert Rev Med Devices. 2006;3:29–47. doi: 10.1586/17434440.3.1.29. [DOI] [PubMed] [Google Scholar]