Abstract
Diabetes mellitus is a serious and growing health problem worldwide and is associated with severe acute and chronic complications. Moreover, epidemiologic evidence suggests that people with diabetes are at significantly higher risk for many forms of cancer. Several studies indicate an association between diabetes and the risk of liver, pancreas, endometrium, colon/rectum, breast, and bladder cancer. Mortality is also moderately increased in subjects with diabetes. Common risk factors such as age, obesity, physical inactivity and smoking may contribute to increased cancer risk in diabetic patients. Hyperinsulinemia most likely favors cancer in diabetic patients as insulin is a growth factor with pre-eminent metabolic as well as mitogenic effects, and its action in malignant cells is favored by mechanisms acting at both the receptor and post-receptor level. The effect of diabetes treatment drugs, aside from metformin, on cancer is not conclusive. In order to fight the perfect storm of diabetes and cancer, strategies to promote primary prevention and early detection of these conditions are urgently needed.
Keywords: Diabetes mellitus, Cancer, Mortality
INTRODUCTION
Diabetes mellitus (DM) is a serious and growing health problem worldwide and is associated with severe acute and chronic complications. Today, 250 million people live with diabetes globally, and this figure is expected to reach 366 million in 2030 [1]. Therefore, if diabetes is associated with an increase in the risk of cancer, this may have a tremendous impact on health worldwide. The industrialization and economic growth accompanied by the so-called 'westernization' of lifestyle, characterized by a high-calorie diet, obesity, and physical inactivity may explain this diabetes epidemic. As a result of this change, the mortality of lifestyle-related diseases such as cancer, diabetes, and cardiovascular disease has increased in many countries including South Korea. Worldwide, cancer is the 2nd and diabetes is the 12th leading cause of death [2]. According to the recent Statistics Korea report for 2009 [3], cancer is the leading cause of death and DM is the 5th most common cause of death.
Epidemiologic evidence suggests that people with diabetes are at significantly higher risk for many forms of cancer [4-7]. Park et al. [8] found that death by malignancy was markedly increased (from 4.7% to 21.9%) in Korean type 2 diabetes patients during the past 10 years. Type 2 diabetes and cancer share many risk factors, but potential biologic links between the two diseases are not completely understood.
In this review, we will discuss Korean and foreign evidences of an association between diabetes and cancer, and the possible mechanisms involved. The link between diabetes treatment and cancer risk will also be reviewed.
INCREASED CANCER INCIDENCE IN DIABETIC PATIENTS
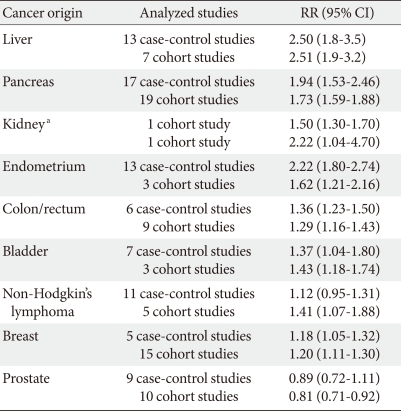
Many longitudinal and case-control studies, often pooled in meta-analyses, have explored the association between diabetes and a large array of different neoplasms. Recently, the results of several studies have been combined for a meta-analytic study [4,9] and the results are shown in Table 1. The relative risks imparted by diabetes are greatest (about twofold or higher) for cancers of the liver, pancreas, and endometrium, and lesser (about 1.2 to 1.5 fold) for cancers of the colon and rectum, breast, and bladder. Other cancers (such as lung) do not appear to be associated with diabetes, and the evidence for some (e.g., kidney, non-Hodgkin's lymphoma) is inconclusive. Diabetes is associated with a lower risk of prostate cancer only.
Table 1.
Meta-analysis on the relative risk (RR) for cancer in different origins of diabetic patients (Modified from Vigneri P, et al. Endocr Relat Cancer 2009;16:1103-23, with permission from Society for Endocrinology) [4]
CI, confidence interval.
aKidney cancer data was not obtained from meta-analysis.
Previous meta-analyses are mainly derived from Western populations. However, a 10-year prospective cohort study of 1.3 million Koreans aged 30 to 95 years [10] found that cancer incidence is generally elevated for persons with diabetes or an elevated fasting serum glucose level greater than 125 mg/dL (6.9 mmol/L) compared with those without hyperglycemia. The association was strongest for pancreatic cancer, comparing the highest and lowest strata in men (hazard ratio [HR], 1.91; 95% confidence interval [CI], 1.52 to 2.41) and in women (HR, 2.05; 95% CI, 1.43 to 2.93). Significant associations were also found for cancers of the esophagus, liver, and colon/rectum in men and of the liver and cervix in women. There were also significant trends with glucose level and cancers of the esophagus, colon/rectum, liver, pancreas, and bile duct in men and of the liver and pancreas in women. We also have reported that 30% of pancreatic cancer patients have diabetes [11]. Findings similar to those of the Korean cohort study were seen in the Japan Pubic Health Center-based Prospective (JPHC) study of 110,000 Japanese aged 40 to 60 years [12].
The large majority of the epidemiologic data on cancer incidence and mortality has been obtained from type 2 diabetic patients. Because of the different physiology between the two subtypes of diabetes, these findings cannot be extended to type 1 diabetic patients. However, the risk of cancer among patients with type 1 diabetes (T1DM) has been investigated in two cohort studies. A Swedish study of over 29,000 T1DM patients revealed an overall 20% increase in the risk of cancer [13]. In particular, the risks of stomach, uterine cervix, and endometrium neoplasm were almost doubled. A UK study involving 28,900 individuals showed that the risk of ovarian cancer and the mortality associated with this neoplasm was more than two-fold higher in subjects with T1DM than in controls [14]. No other significant associations were found.
INCREASED CANCER MORTALITY IN DIABETIC PATIENTS
Besides its role as an independent risk factor for the development of several tumors, diabetes can also have impact on cancer prognosis. Several studies have documented increased cancer mortality in subjects with diabetes. A recent meta-analysis of 23 studies comparing overall survival in cancer patients with and without preexisting diabetes showed that diabetes was associated with an increased mortality HR of 1.41 (95% CI, 1.28 to 1.55) compared with normoglycemic individuals across all cancer types [15]. Subgroup analyses by cancer type showed an increased risk for cancers of the endometrium (HR, 1.76; 95% CI, 1.34 to 2.31), breast (HR, 1.61; 95% CI, 1.46 to 1.78), and colorectum (HR, 1.32; 95% CI, 1.24 to 1.41). A cohort study including Korean individuals [10] of both genders revealed a linear trend of increasing mortality with increasing fasting serum glucose levels, even in the non-diabetic range. The linear association between fasting serum glucose levels and cancer mortality was particularly evident for malignancies of the pancreas, colon/rectum, liver, esophagus, and biliary tract among men and the pancreas, liver, and uterine cervix in women.
COMMON RISK FACTORS FOR BOTH CANCER AND DIABETES
Diabetes and cancer represent common health concerns, and they often coexist in the same individuals. Overall, 8% to 18% of individuals suffering from cancer also have diabetes, and prevalence rates vary according to tumor sites [16].
Age
Over 60% of cancer diagnoses are made in individuals aged 65 years or more [17]; since the prevalence of diabetes reaches 17% in this age class, the coexistence of diabetes and cancer is destined to raise as life expectancy increases.
Obesity
Type 2 diabetes is strongly related to overweight and obesity, two conditions associated with an increased risk of several cancers [18]. Cancers most consistently associated with overweight and obesity are breast (in postmenopausal women), colon/rectum, endometrium, pancreas, adenocarcinoma of the esophagus, kidney, gallbladder, and liver. Moreover, obesity is associated with higher cancer incidence and mortality [19,20].
Physical inactivity
Evidence from observational epidemiologic studies consistently shows that higher levels of physical activity are associated with a lower risk of the colon, postmenopausal breast, and endometrial cancer [21,22]. Prevention of diabetes with increased physical activity has been proven by numerous trials [23-26].
Smoking
It is estimated that worldwide, cigarette smoking accounts for 71% of all trachea, bronchus, and lung cancer deaths [27]. Other cancers strongly associated with smoking are cancers of the larynx, upper digestive tract, bladder, kidney, pancreas, liver, stomach, uterine cervix, and leukemia. Studies suggest that smoking is also an independent risk factor for the development of diabetes [28,29].
POSSIBLE MECHANISMS UNDERLYING DIABETES AND CANCER
The role of insulin in promoting cancer growth was first recognized by studies in experimental animals as early as 1972 [30,31]. Diabetes is generally characterized by hyperglycemia and hyperinsulinemia, which are often coupled with a reduced metabolic effect of insulin (insulin resistance) in peripheral tissues. Chronic hyperinsulinemia, however, is a possible factor favoring cancer initiation and progression in diabetic patients due to the mitogenic effect of insulin, which has been reviewed in detail [32]. In brief, an increase in insulin levels leads to a decrease in the liver synthesis and blood levels of insulin-like growth factor binding protein 1 (IGFBP1), and is associated with a decrease in IGFBP2 in the blood. These effects in turn result in an increase in bioavailable IGF1. Insulin and IGF1 signal through insulin receptors and IGF1 receptors, respectively, to promote cellular proliferation and inhibit apoptosis in many tissue types. These effects might contribute to cancerogenesis.
Many cancer cells have increased insulin receptor (IR) content [33]. In malignant cells, the expression of the isoform (IR-A) is predominant, and its activation elicits more mitogenetic than metabolic effects [34]. By binding to the overexpressed IR-A, insulin may favor cancer progression and facilitate the growth of tumors that would otherwise have likely remained clinically irrelevant.
Insulin mitogenic activity might be enhanced at the cellular level by post-receptor molecular mechanisms, including insulin (or its synthetic analogs) residence time on the receptor [35] and the intracellular up-regulation of the insulin mitogenic pathway.
DIABETES TREATMENT AND CANCER
Most diabetic patients are treated for years or decades with a variety of drugs. The potential role of these drugs in favoring cancer is unclear, but is most likely minor, if any. The data are not conclusive because the large majority of diabetic patients change the drug dosage and/or type many times during the course of the disease. Moreover, many are treated with more than one drug simultaneously. As a result, this issue is not well-studied epidemiologically, and evidence is weak, inconclusive and controversial. The debate about the potential risk of developing cancer associated with insulin use has been ignited by the publication of four observational studies [36-39] in 2009. However, randomized clinical trial data from an open-label 5-year trial of insulin glargine versus NPH insulin did not find any evidence of excess cancer risk with insulin glargine [40]. Due to the methodological limitations of previous studies, which have been broadly debated [41-43], a clear relationship between use of insulin glargine and increased risk of cancer cannot be established.
On the contrary, a mounting body of evidence suggests that metformin may reduce cancer risk [36,44-47] or mortality [48] in diabetic patients. There is probably a dual mechanism for the anticancer effect of metformin. In addition to the lowering of circulating levels of insulin, metformin stimulates expression of AMPK, leading to the rapid inhibition of cellular protein synthesis and growth [49,50].
CONCLUSION
Descriptive and analytical evidence obtained to date suggest that cancer risk and cancer mortality are increased in diabetic patients, providing more evidence for some site-specific cancers. The increase in the risk of cancer is likely related to the interplay between obesity, hyperinsulinemia and hyperglycemia. Improved glycemic control guidelines with a wide prescription of statin and aspirin have reduced death from vascular complications and diabetes itself. As a result, death by cancer is rising steeply although cardiovascular disease and infection are still the leading cause of the death. The impressive rate of increase in the incidence of both diabetes and obesity will pose serious problems to many countries including South Korea. In order to fight the perfect storm of diabetes and cancer, preventive strategies to promote primary prevention and early detection of these conditions are urgently needed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Statistics Korea: Statistics of Cause of Death. Available from: http://www.kostat.go.kr. (updated 2010 Sep 9)
- 4.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 5.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47:87–95. doi: 10.1007/s00592-010-0187-3. [DOI] [PubMed] [Google Scholar]
- 6.Tsugane S, Inoue M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci. 2010;101:1073–1079. doi: 10.1111/j.1349-7006.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SK, Park MK, Suk JH, Kim MK, Kim YK, Kim IJ, Kang YH, Lee KJ, Lee HS, Lee CW, Kim BH, Lee KI, Kim MK, Kim DK. Cause-of-death trends for diabetes mellitus over 10 years. Korean Diabetes J. 2009;33:65–72. [Google Scholar]
- 9.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47:87–95. doi: 10.1007/s00592-010-0187-3. [DOI] [PubMed] [Google Scholar]
- 10.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Park YJ, Kim KW, Oh EY, Min YK, Lee MS, Lee MK, Lee JK, Lee KT, Kim YI, Choi YH. Prevalence of diabetes mellitus in pancreatic cancer patients. J Korean Diabetes Assoc. 2001;25:316–322. [Google Scholar]
- 12.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 13.Zendehdel K, Nyren O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95:1797–1800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, Waugh NR, Morris AD, Gatling W, Gale EA, Patterson CC, Keen H. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005;92:2070–2075. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko C, Chaudhry S. The need for a multidisciplinary approach to cancer care. J Surg Res. 2002;105:53–57. doi: 10.1006/jsre.2002.6449. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Cancer survivorship--United States, 1971-2001. MMWR Morb Mortal Wkly Rep. 2004;53:526–529. [PubMed] [Google Scholar]
- 18.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 19.Adami HO, Trichopoulos D. Obesity and mortality from cancer. N Engl J Med. 2003;348:1623–1624. doi: 10.1056/NEJMp030029. [DOI] [PubMed] [Google Scholar]
- 20.Vigneri P, Frasca F, Sciacca L, Frittitta L, Vigneri R. Obesity and cancer. Nutr Metab Cardiovasc Dis. 2006;16:1–7. doi: 10.1016/j.numecd.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132(11 Suppl):3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 22.Lee IM. Physical activity and cancer prevention: data from epidemiologic studies. Med Sci Sports Exerc. 2003;35:1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- 23.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 25.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 26.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67:152–162. doi: 10.1016/j.diabres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Mackay J, Ericksen M, Shafey O. The cancer atlas. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 28.Foy CG, Bell RA, Farmer DF, Goff DC, Jr, Wagenknecht LE. Smoking and incidence of diabetes among U.S. adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2501–2507. doi: 10.2337/diacare.28.10.2501. [DOI] [PubMed] [Google Scholar]
- 29.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 30.Heuson JC, Legros N. Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer Res. 1972;32:226–232. [PubMed] [Google Scholar]
- 31.Heuson JC, Legros N, Heimann R. Influence of insulin administration on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in intact, oophorectomized, and hypophysectomized rats. Cancer Res. 1972;32:233–238. [PubMed] [Google Scholar]
- 32.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 33.Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L, Vannelli GB, Brand R, Goldfine ID, Vigneri R. Elevated insulin receptor content in human breast cancer. J Clin Invest. 1990;86:1503–1510. doi: 10.1172/JCI114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Meyts P, Christoffersen CT, Urso B, Wallach B, Gronskov K, Yakushiji F, Shymko RM. Role of the time factor in signaling specificity: application to mitogenic and metabolic signaling by the insulin and insulin-like growth factor-I receptor tyrosine kinases. Metabolism. 1995;44(10 Suppl 4):2–11. doi: 10.1016/0026-0495(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 36.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 37.Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–1754. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 38.Colhoun HM SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–1765. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenstock J, Fonseca V, McGill JB, Riddle M, Halle JP, Hramiak I, Johnston P, Davis M. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia. 2009;52:1971–1973. doi: 10.1007/s00125-009-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollak M, Russell-Jones D. Insulin analogues and cancer risk: cause for concern or cause célèbre? Int J Clin Pract. 2010;64:628–636. doi: 10.1111/j.1742-1241.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC. Does insulin therapy promote, reduce, or have a neutral effect on cancers? JAMA. 2010;303:446–447. doi: 10.1001/jama.2010.60. [DOI] [PubMed] [Google Scholar]
- 43.Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 44.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol. 2009;46:279–284. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 45.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 47.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, Decensi A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol Biomarkers Prev. 2009;18:701–705. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- 50.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol. 2009;27:3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]