Abstract
Multiple health benefits of calorie restriction (CR) and alternate day fasting (ADF) regimens are widely recognized. Experimental data concerning the effects of calorie restriction on cardiac health are more controversial, ranging from evidence that ADF protects heart from ischemic damage but results in developing of diastolic dysfunction, to reports that CR ameliorates the age-associated diastolic dysfunction. Here we investigated the effects of chronic CR on morphology and function of the cardiovascular system of aged rats and cardioprotective effect of CR against ischemic damage in the experimental rat model of MI.
Cardiovascular fitness of 24-mo old Fisher 344 rats maintained through life on ad libitum (AL) or CR diets was extensively evaluated via echocardiography, dobutamine stress test, pressure-volume loop analyses, pulse wave velocity measurements, and histology. Groups of 2-mo old AL and 29-mo old CR rats were studied for comparison. Myocardial infarction (MI) was induced by a permanent ligation of the anterior descending coronary artery in 5-mo old rats maintained for 3 months on CR or AL. MI size was evaluated histologically 24 hrs following coronary ligation. Cardiac remodeling was followed-up via echocardiography.
Age-associated changes in 24-mo old rats consisted of 33% increase of fibrosis in the myocardium and more than 2 fold increase of the collagen in the tunica media of the aorta. There was a significant decrease in the density and total number of cardiomyocytes, while their size was increased. These morphological changes were manifested in a decline of systolic and diastolic cardiac function, increase of left ventricular and aortic stiffness, and arterio-ventricular uncoupling. Tachycardic response to dobutamine challenge was absent in the old rats. Compared to AL rats, 24-mo old CR rats had reduced levels of cardiac and aortic fibrosis, increased density of cardiomyocytes that were smaller in size, attenuated diastolic dysfunction, normal systolic function and arterio-ventricular coupling. Tachycardic response to dobutamine was also intact in CR 24-mo old rats and aortic stiffness was reduced. Adjustment for body weight differences through ratiometric or allometric scaling did not affect the overall pattern of differences between AL and CR rats. Attenuation of morphological and functional age-associated changes in 24-mo old CR rats either was not observed at all or was smaller in 29-mo old CR rats. Size of MI induced by a permanent coronary ligation as well as post-MI cardiac remodeling and function were similar in CR and AL rats.
CR does not increase tolerance of myocardium to ischemic damage, but attenuates the age-associated changes in the heart and major vessels. The attenuation of age-associated changes by CR cannot be explained by the effect of lower body weight but are attributable to more intimate cellular mechanisms of CR itself. Attenuation of age-associated changes by CR waned with advancing age, and is consistent with the idea that CR postponed senescence.
Keywords: Aging, calorie restriction, cardioprotection, myocardial infarction, cardiac function, arterial stiffness
Introduction
Multiple health benefits of calorie restriction (CR), either through daily reduction of calorie intake (20-40% less than ad libitun (AL) food consumption), or via implementing an alternate day fasting regimen (ADF) have been reported in animal experiments and several human trials [1-8]. CR is the only proven way to prolong life span, at least in the rodents and lower life forms [9-12].
The salutary effects of both CR and ADF on the known risk factors for cardiovascular diseases have also been demonstrated and universally accepted [3, 8, 13-15]. Nevertheless, with respect to cardioprotective properties of long-term CR/ADF and their effects on general fitness of the cardiovascular system, the experimental data are not uniformly in accord. We have reported that ADF increased myocardial tolerance to ischemic damage [16]: Compared with AL animals the size of myocardial infarction (MI) induced by permanent ligation of a coronary artery in rats was significantly smaller in the cohort that had been maintained on the ADF regimen during the six preceding months [16]. ADF rats also had reduced level of apoptosis in the peri-infarct area. Continued ADF after coronary ligation resulted in attenuation of post MI remodeling and functional decline. We have also reported, however, that long-term ADF in rats resulted in development of myocardial fibrosis associated with diastolic dysfunction and diminished cardiac reserve [17].
Our findings that chronic ADF promotes diastolic dysfunction and fibrosis contrast with results of a simultaneously published report on amelioration of age-associated diastolic dysfunction in rats maintained on chronic CR [18]. To address this apparent controversy we investigated the effects of chronic CR on morphology and function of the cardiovascular system of aged rats and cardioprotective effects of CR against ischemic damage in the experimental MI rat model.
Methods
Animals and experimental design
2-mo old male Fisher344 rats, obtained from Charles River Laboratories (Wilmington, MA), were placed in the NIA vivarium and housed and studied in conformance with the NIH Guide for the Care and Use of Laboratory Animals, Manual 3040–2 (1996), with Institutional Animal Care and Use Committee approval. The diagram of experiments is presented in the supplement Figure 1. Upon arrival all animals were divided into a control group fed ad libitum NIH-07 (7022) diet (AL) and a calorie restricted (CR) group, whose daily calorie intake was reduced by 40%. Both groups had unlimited access to water. Forty five animals from each diet group were set aside for longevity study and were euthanized when found in the moribund condition during routine daily inspection. The rest of the rats were placed into different experiments at different time points. (1) After 3 months on their respective, CR or AL diets, 29 rats from each diet group (5-mo old at the time) were randomly selected and subjected to a coronary ligation to study the cardioprotective effects of CR against ischemic damage. (2) At 24-mo of age (after 22 months on respective diets) 12 rats from CR (24CR group) and 21 rats from AL (24AL group), were subjected to extensive evaluation of cardiovascular structure and function via echocardiography, dobutamine stress test, hemodynamic assessment using pressure-volume loop analyses, measurement of pulse-wave velocity and histological evaluation of the heart. Additional groups, 10 2-mo old AL rats (2AL group) and 15 29-mo old CR (29CR group) rats (27 months on CR) were subjected to the same vigorous evaluation.
Echocardiography (Echo)
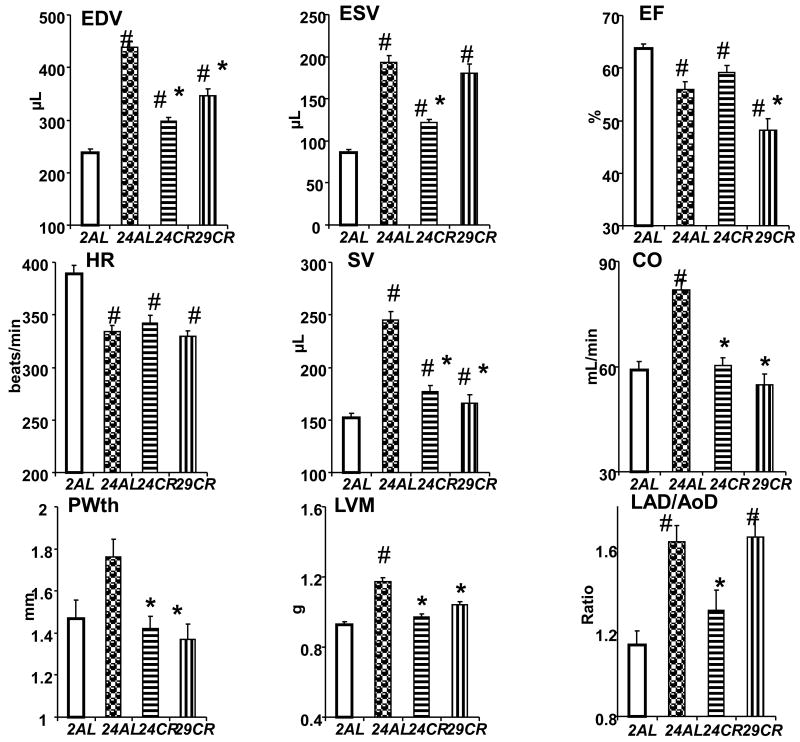
All rats were subjected to Echo before invasive measurements (pressure-volume loop analyses) or surgery, as previously described [17]. Briefly, under Isoflurane anesthesia (2% in oxygen) via face mask, a 12-MHz transducer (HP Sonos 5500, Hewlett-Packard Inc) was used to obtain 2D images of the left ventricle (LV) at long and short axes and 4 chamber views. End-diastolic and end-systolic LV areas were calculated from endocardial area tracings in the 2D mode (short and long axis views) on digital images captured on cineloops, using the leading edge method. End-diastolic volume (EDV) and end-systolic volume (ESV) were calculated by a Modified Simpson's method. Ejection fraction (EF) was then derived as EF = (EDV - ESV)/EDV ×100. Stroke volume (SV) was calculated as EDV - ESV and Cardiac output (CO) - as SV × Heart Rate (HR). LV posterior wall thickness (PWth) was measured from the M-mode LV long-axis tracings. LV mass (LVM) was estimated from the M-mode tracing of LV in long axis. Left atrial dimension (LAD) and aortic dimension (AoD) were measured from long axis M-mode tracings of basal aorta and left atrium at end-diastole, as recommended by the American Society of Echocardiography, and LAD/AoD was used to normalize LAD. Thoracic aorta (TA) was scanned just above the diaphragm using a 30 MHz transducer (707B, Visual Sonics, Toronto, Canada). 2D images and M-mode tracings were obtained in long and short axis views. TA lumen diameter at maximum (TA max) and minimum (TA min) dimensions were measured off-line from M-mode tracings during each cardiac cycle using NIH imaging software. TA lumen fraction expansion was calculated as (TA max – TA min) / TA min. All reported echocardiographic indices are presented as absolute (Figure 2) or normalized for body weight (BW), either ratiometrically or allometrically [17, 19, 20]. All measurements were averaged over three to five consecutive cardiac cycles and made by a single observer blind to the identity of the tracings. Reproducibility of measurements of given observer had been tested on a subgroup of animals and variability did not exceed 5%.
Figure 2.
Echo indices in different age groups of AL and CR rats. # p<0.05 vs 2AL; * p<0.05 vs 24AL (Bonferroni correction for multiple comparison).
Following a baseline Echo, 24-mo old rats were injected i.p. with 10 μg/kg of Dobutamine and the Echo was repeated 5 min later.
Hemodynamics
Prior to euthanasia pressure-volume analyses were performed as previously described [17]. Briefly, under Isoflurane anesthesia (2% in oxygen), rats were intubated and mechanically ventilated. A bilaterial thoracotomy in 4th and 5th intercostal space was performed. After opening the pericardium, a 2F conductance catheter (Millar Instruments Inc., Houston, TX) was inserted into the LV from the apex. LV end-diastolic pressure (EDP), EDV, ESV, SV, +dP/dt, - dP/dt, isovolumic relaxation time (tau) and arterial elastance (Ea) were determined in 10-20 digitally averaged cardiac cycles while the ventilator was stopped. LV end-systolic elastance (Ees), preload recruitable stroke work (PRSW) and end-diastolic stiffness (Eed) were measured using a graded preload reduction technique. Arterio-ventricular (AV) coupling was calculated as Ea/Ees. The test was concluded by advancing the catheter into thoracic aorta to measure arterial blood pressure. All hemodynamic measurements for 24-mo old groups were reported either as is or scaled for body weight differences ratiometrically or allometrically [17, 19, 20].
Pulse Wave Velocity (PWV)
PWV was measured in 12 rats from the 24CR group and 11 from the 24AL. Under Isoflurane anesthesia (2% in oxygen), rats were intubated and ventilated. Left femoral artery was isolated, ligated, and a 1F conductance catheter was inserted and advanced to the thoracic aorta (approximately 100 mm). After recording of several pressure waves and corresponding ECG, the catheter was withdrawn for exactly 50 mm and data recording was repeated. Using the R wave of the ECG as a time marker, the average time between R waves and starting points of five corresponding pressure waves at thoracic and abdominal sections of aorta were measured. The transit time of the pressure wave from upper thoracic aorta to lower abdominal aorta was calculated as the time difference between two measurements. Since the distance between two points of measurement was known (exactly 50 mm), the PWV was calculated as 50 mm / difference between transit times.
Gross Pathology and Histological Assessment
Histological staining and analyses were performed as described previously [17]. Briefly, the hearts and lungs were removed and weighed (wet weight). Hearts were further cut into two pieces through the short axis. The basal half was fast frozen and stored, and the apical half was used for histological analysis. Myocardial tissue sections were subjected to Masson's trichrome and hematoxylin-eosin staining. Myocyte cell size and density were measured in H&E-stained sections. Only myocytes which nuclei were clearly identified were counted. Myocyte diameter was measured as the shortest distance across the nucleus in transverse cell sections. Diameters of 100 myocytes from 5 randomly selected microscope fields (×200 magnification) from the LV posterior wall were averaged to represent the myocyte diameter of a given specimen. Myocyte density was calculated from the same area in the same fashion. Myocardial tissue fibrosis was measured in Masson's trichrome-stained sections and was expressed as a fraction of a microscopic field (×100 magnification) of the LV posterior wall. An average of 5 randomly selected fields represented results of a given specimen. Collagen content in the thoracic aorta wall was measured on 7μm fresh frozen sections stained with Masson's trichrome. Digital images of stained sections were obtained from light microscopy and analyzed using a digital imaging analysis system (MCID, InterFocus Imaging Ltd, Cambridge, UK). The collagen content in aortic wall was calculated as a percentage of the total wall thickness or tunica media. The person assessing all histological slides was blinded to a grouping.
Evaluation of Cardioprotective Effect of CR
Following the baseline Echo 58 5-mo old rats were subjected to an open chest surgery under Isoflurane anesthesia (2% in oxygen) - either coronary artery ligation or sham operation [16]. The following 4 groups were created: MI-AL, ad lib and ligated (n=26); Sh-AL, ad lib and sham operated (n=3); MI-CR, calorie restricted and ligated (n=23); and Sh-CR, calorie restricted and sham operated (n=6). Twenty-four hours after coronary artery ligation, 9 rats from each group were euthanized for histological evaluation of infarct size. 3 mL of 5% Evans blue dye (Sigma) was rapidly injected into the aorta via a 16-gauge tube to distinguish the perfused area (blue staining) from the unperfused area (no staining). The atria and great vessels were dissected away from the heart, and the heart was cut transversely into 4 slices from base to apex. A section from the midpapillary muscle level was immediately stored in LN2 for later histological analysis. The other 3 samples were incubated at 37°C with 4% triphenyltetrazolium chloride (TTC, Sigma) for 30 minutes to distinguish the infarct area (unstained) from the area at risk (AAR, brick red staining) in the unperfused area. All images were analyzed with NIH Image software. MI size was expressed as a percentage of the unperfused area or LV. Myocardial sections (7 μm thick) were cut from the frozen samples and stained with hematoxylin and eosin (H&E, Sigma) and terminal dUTP nick end-labeling (TUNEL; Cardio TACS, R&D Systems). Inflammatory cells (neutrophils and macrophages) were counted and averaged from 5 different fields of the AAR in H&E-stained sections from each heart. Apoptosis was assessed from TUNEL-stained sections of the AAR.
Remaining surviving animals were subjected to a serial Echo at one and ten weeks after surgery, at which time they were euthanatized, and hearts isolated and weighed. The heart weight (HW) to BW ratio (HW/BW) was calculated. Myocardial sections (7 μm thick) were obtained from the midpapillary muscle level. MI size was measured in Masson's trichrome stained sections as average ratios of epicardial and endocardial segments of MI to respective LV circumferences, expressed as a percentage of the LV.
Scaling for Body Weight Differences [17, 19, 20]
The effect of the body weight differences between 24AL and 24 CR groups on cardiovascular variables was assessed using linear regressions in which a given parameter of interest was regressed on body weight among all animals of both groups at given time point. If the linear regression did not differ from zero, the body weight differences could not affect a parameter of interest and any adjustment to differences in body weight was not necessary. On the contrary, if the linear regression were significantly different from zero, the body weight differences might affect the results of measurement and scaling was needed. In such cases we used two consecutive approaches to adjust for body weight differences. At first, the ratiometric scaling was employed, i.e., a variable of interest was divided by body weight. The derived variable was again plotted against body weight. If the resulting slope were not different from zero, the scaling was satisfactory and further correction was not necessary. If, however, the slope of the regression was still statistically different from zero after ratiometric scaling, a more sophisticated allometric scaling procedure was applied. In this procedure the parameter of interest was divided by a body weight raised to the power required to abolish the slope of the regression of the unadjusted variable against body weight. This procedure certainly excluded any effect of body weight. All echocardiographic and hemodynamic variables were reported before and after scaling for body weight differences.
Statistical Analysis
All data are expressed as the mean ± SEM. Kaplan-Meier survival curves were compared using logrank test. Differences among groups were tested either by a Student t-test comparison, or by a 1-way analysis of variance with a Bonferroni correction for multiple comparisons. Indices of serial Echos were analyzed using 2-way ANOVA for repeated measurements. Group differences at specific time points were tested by Bonferroni post-hoc test. P < .05 was considered statistically significant.
Results
Longevity
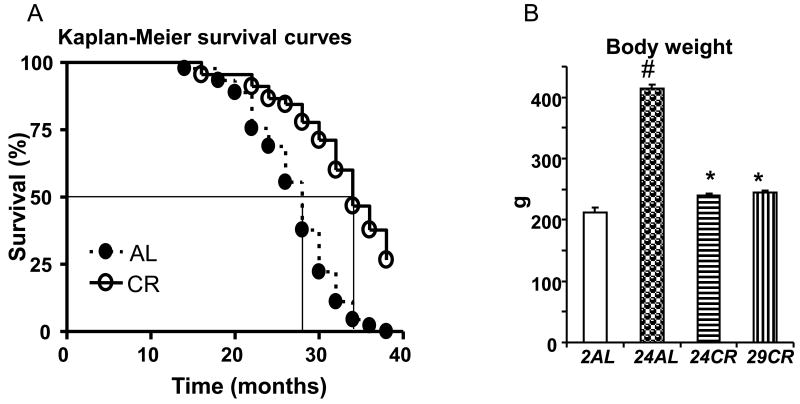
Kaplan-Meier survival curves for AL and CR animals are presented on Figure 1A. Logrank analysis revealed that longevity of these two groups were significantly different (p<0.001). The median life-span of AL group was 28 months. Lifespan of CR animals was extended by 4-5 months.
Figure 1.
A - Kaplan-Meier survival curves from CR and AL rats. * - p<0.001 (Logrank comparison of the curves); B - Body weight by age groups of AL and CR rats. # p<0.05 vs 2AL; * p<0.05 vs 24AL (Bonferroni correction for multiple comparison).
Body weight
Figure 1B illustrates differences in the age-related body weight gain among rats maintained on AL or CR diet. At the age of 24 months the AL rats doubled their body weight compared to 2-mo old AL rats. In contrast, body weight of 24-mo old CR rats only slightly exceeded the body weight of 2-mo old AL rats (p>0.05). The lower body weight continued to be at 2-mo level in 29-mo old CR rats (p>0.05 vs 2AL).
Echocardiography
Results of Echo assessment of the heart in different age/diet groups unadjusted for differences in the body weight are presented in Figure 2. Table 1 presents the comparison of echocardiographic indices for 24AL and 24CR rats scaled for body weight differences and normalized for the body weight of 24AL rats prior to scaling. In AL rats, the LV volumes were, as expected by body growth, substantially larger at 24-mo compared to 2-mo old (by 84% and by 124% for EDV and ESV respectfully, p<0.001 for both), resulting in 13% reduction of EF in 24AL (p<0.01 vs 2AL). Calorie restriction significantly attenuated the age-associated LV expansion: The age associated increase in EDV was significantly reduced in both 24CR and 29CR compared to 24AL, but still was greater than in 2AL (p<0.05); ESV was reduced in 24CR, but in 29CR was not different from 24AL (Fig. 2). Even after correction for body weight differences (Table 1), all Echo indices of LV volumes and CO remained significantly reduced in 24CR compared to 24AL with the exception of ESV, where reduction in 24CR was abolished after correction for body weight. The reduction in LV volumes in CR rats did not translate into improvement of EF, which was similar in 24-mo old AL and CR rats and was even significantly reduced in 29CR rats (p<0.05 vs 24AL).
Table 1.
Echocardiographic indices at 24 months rats maintained on AL and CR diets adjusted for body weight differences.
| 24AL | BWad | 24CR | |
|---|---|---|---|
| HR (beats/min) | 334±6 | ˆ-0.02 | 338±7 |
| EDV (μL/min) | 440±11 | ˆ0.21 | 335±7* |
| ESV(μL/min) | 193±8 | ˆ1 | 209±5 |
| EF (%) | 56±1.3 | ˆ-0.30 | 50±1* |
| SV (μL) | 331±11 | ˆ-0.10 | 226±8* |
| CO (ml/min) | 82±3 | ˆ-0.12 | 57.5±2* |
| LVM (g) | 1.17±0.02 | ˆ0.14 | 1.05±0.02* |
| PWth (mm) | 1.76±0.08 | ˆ0.08 | 1.48±0.06* |
| LAD (mm) | 5.6±0.22 | ˆ-0.32 | 3.47±022* |
| AoD (mm) | 3.46±0.05 | ˆ0.14 | 3.48±0.11 |
| LAD/AoD | 1.63±0.07 | ˆ-0.46 | 1.01±0.07* |
Data are means ± SEM. BWad (body weight adjustment): ˆ1 – adjusted to a body weight at power 1 (ratiometric); ˆx – adjusted to a body weight at calculated power (allometric).
- p<0.05 vs AL.
HR – heart rate; EDV – end-diastolic volume; ESV – end systolic volume; EF – ejection fraction; SV – stroke volume; CO – cardiac output; LVM – left ventricular mass; PWth – posterior wall thickness; LAD – left atrium dimension; AoD – aortic diameter.
Heart rate was significantly reduced in 24AL compared to 2AL (Fig. 2), while SV increased (P<0.01) resulting in 39% higher CO in 24AL rats (p<0.01). Calorie restriction did not affect the age-associated reduction in HR – it was similar in all three aged groups (p>0.05), and below the level of 2AL (p<0.05). HR was not affected by a body weight correction (Table 1) and remained similar in 24AL and 24CR rats. Calorie restriction, however, attenuated the age-associated increase in SV, which was smaller in both 24CR and 29CR than in 24AL (p<0.05), but still greater than in 2AL. The CO was smaller in both 24CR and 29CR than in 24AL (p<0.01) and did not differ from 2AL.
Age-associated thickening of posterior wall in 24AL rats compared to 2AL (Fig. 2) was not statistically significant, but LVM was greater by 27% (p<0.05 vs 2AL). Calorie restriction significantly attenuated the increase in older rats of both PWth and LVM (p<0.05). The LAD/AoD ratio was significantly higher in 24AL than in 2AL (p< 0.01) due to a larger expansion of left atrium in 24AL. Calorie restriction significantly reduced this ratio in 24CR, but not in 29CR rats, and the differences in LAD/AoD were due to differences in LAD. Lower LAD/AoD in 24CR vs 24AL was not affected by body weigh correction (Table 1).
Pressure-volume loop analyses
Results of pressure-volume loop analyses for all groups presented in Table 2. Additionally, results for 24CR group are also presented after correction for body weight differences and normalization for body weight of 24AL animals prior to correction. Body weight and HR among groups are similar to those reported for Echo test: age-associated body weight increase is attenuated by CR, and calorie restriction does not affect the age-associated decline in the HR. Parameters of arterial blood pressure did not differ among groups with the exception of pulse pressure - its age-related increase was attenuated by CR. HR and parameters of blood pressure were not affected by body weight differences.
Table 2.
Hemodynamic indices for 2-, 24-, and 29-mo old rats maintained on CR or AL diets and 24-mo old CR rats adjusted for the effect of body weight if required.
| 2AL (n=10) | 24AL (n=22) | 24CR (n=11) | 29CR (n=15) | BWAd | 24CRad (n= 11) | |
|---|---|---|---|---|---|---|
| BW (g) | 212±8 | 414±7# | 240±4* | 245±2* | U | N/A |
| HR (beats/min) | 320±8 | 256±6# | 274±5# | 249±7# | U | N/A |
| BPs (mmHg) | 102±4 | 107±5 | 117±3 | 98±4 | U | N/A |
| BPd (mmHg) | 75±3 | 73±4 | 87±3 | 66±4 | U | N/A |
| BPm (mmHg) | 84±4 | 84±4 | 97±3 | 77±4 | U | N/A |
| PP (mmHg) | 27±1 | 33±1# | 30±1 | 31±1 | U | N/A |
| PVR (mmHg/ml/min) | 2.04±0.21 | 1.50±0.13 | 1.96±0.13 | 1.91±0.11 | U | N/A |
| Systolic function | ||||||
|
| ||||||
| ESP (mmHg) | 99±1 | 97±3 | 105±2 | 98±4 | A/BWˆ0.87 | 171±3† |
| (+)dP/dt (mmHg/sec) | 6787±201 | 6875±295 | 7168±299 | 6313±280 | A/BWˆ0.3 | 6077±240 |
| PRSW (mW/μl) | 88.8±4.4 | 52.1±4.2# | 82.6±6.1* | 71.6±6.4* | A/BW/ˆ-0.02 | 81.6±6† |
| Diastolic function | ||||||
|
| ||||||
| EDP (mmHg) | 5.7±0.4 | 5.7±0.3 | 4.3±0.3* | 4.1±0.2* | A/BWˆ-0.09 | 4.1±0.2† |
| (-)dP/dt (mmHg/sec) | 6890±214 | 6251±394 | 7074±316 | 5762±351 | A/BWˆ0.02 | 7155±319 |
| τ (msec) | 10.4±0.6 | 12.7±0.3 | 12.2±0.4 | 13.4±0.3 | A/BWˆ0.02 | 12.3±0.4 |
| Eed (10-3 mmHg/μl) | 13.7±2.5 | 38.9±4.2# | 21.8±2.0#* | 31.5±5.2# | A/BWˆ1 | 38.34±3.9 |
| Arterio-ventricular coupling | ||||||
|
| ||||||
| Ees (mmHg/μl) | 0.26±0.03 | 0.29±0.03 | 0.53±0.07#* | 0.6±0.07#* | A/BWˆ1 | 0.3±0.03 |
| Ea (mmHg/μl) | 0.34±0.03 | 0.47±0.04 | 0.56±0.04# | 0.82±0.11# | A/BWˆ1.83 | 1.56±0.12† |
| Ea/Ees | 1.43±0.15 | 1.85±0.17# | 1.16±0.09#* | 1.58±0.27 | A/BWˆ1 | 2±0.13 |
BW – body weight; BWAd – corrected for body weight differences either ratiometrically (ˆ1) or allometrically (ˆx); U – BWad is not required; BP – arterial blood pressure; PP – pulse pressure; PVR – peripheral vascular resistance; ESP – end-systolic pressure; PRSW – preload recruitable stroke work; EDP – end-diastolic pressure; Eed – end-diastolic stiffness; Ees – end-systolic elastance; Ea – arterial elastance.
- p<0.05 vs 2 mo AL (after post hoc Bonferroni correction);
- vs 24 mo AL (after post hoc Bonferroni correction);
- p< 0.05 vs 24 mo AL (t-test).
The age-associated decline of systolic function manifested as a reduction of PRSW was attenuated by calorie restriction. This attenuation remained even after correction for body weight differences. The age-associated decline of diastolic function manifested by a 3-fold increase of Eed was attenuated in 24CR rats, but was not affected by CR in 29-mo old. The reduction of Eed in 24CR group was due to differences in body weight as it was abolished after ratiometric correction for body weight. On the other hand, EDP was reduced in both aged CR groups, and this reduction was not affected by correction for body weight differences. Arterio-ventricular coupling was increased with age and attenuated in 24CR, however this attenuation was not observed after ratiometric correction for body weight.
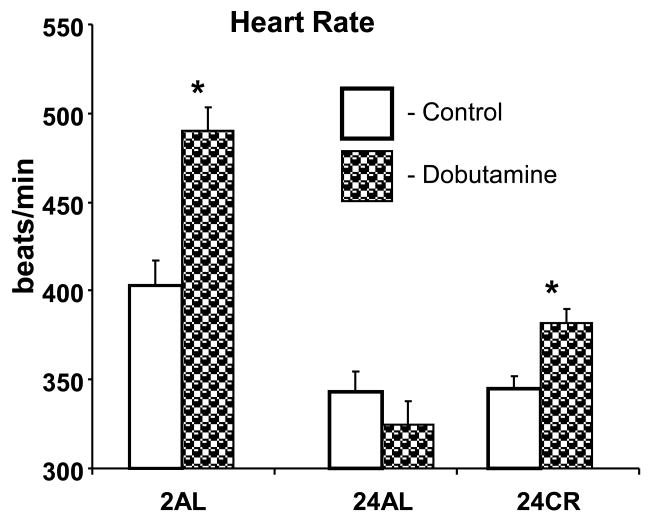
Dobutamine stress test
Figure 3 illustrates the HR responses to dobutamine challenge in different groups. 2AL rats responded to dobutamine injection with a strong (22%, p<0.01) acceleration of the HR. This effect was completely absent in 24-mo old AL rats, but partially restored in 24CR group (11%, p<0.05).
Figure 3.
Heart rate response to a dobutamine challenge in different age groups of AL and CR rats. * p<0.05 dobutamine vs control in the same group (Bonferroni correction for multiple comparison).
Histological assessment of the heart
Results of histological evaluation of the hearts are presented on the Figure 4. Representative slides of myocardium from different groups (Fig. 4A) shows an excess of interstitial fibrosis in the myocardium in 24AL, and to a lesser extent in the myocardium of 24CR rats in comparison with 2AL. In fact, fibrotic tissue in myocardium (Fig. 4B) increased nearly 33 fold (p<0.001) in 24AL vs 2AL. This increase was significantly attenuated in 24CR rats (p<0.01), but the level of fibrosis remained significantly above that of 2AL (p<0.05). The attenuation of the extent of fibrotic tissue in myocardium was not observed in 29CR rats where is was similar with 24AL. The effect of age and calorie restriction on cardiomyocyte density represents a mirror image of their effect on myocardial fibrosis (Fig. 4C) – cardiomyocyte density is significantly reduced in 24AL compared with 2AL (p<0.05), this reduction is attenuated in 24CR (p<0.05 vs 24AL), but reduced again in 29CR. Age associated increase in cardiomyocyte transverse diameter (p<0.05 vs 2AL) is also attenuated in 24CR (p<0.05 vs 24AL) and increased in 29CR (Fig. 4D). The total number of cardiomyocytes (Fig. 4E) progressively declined with age (2>24>29) and this age-associated reduction is not affected by CR.
Figure 4.
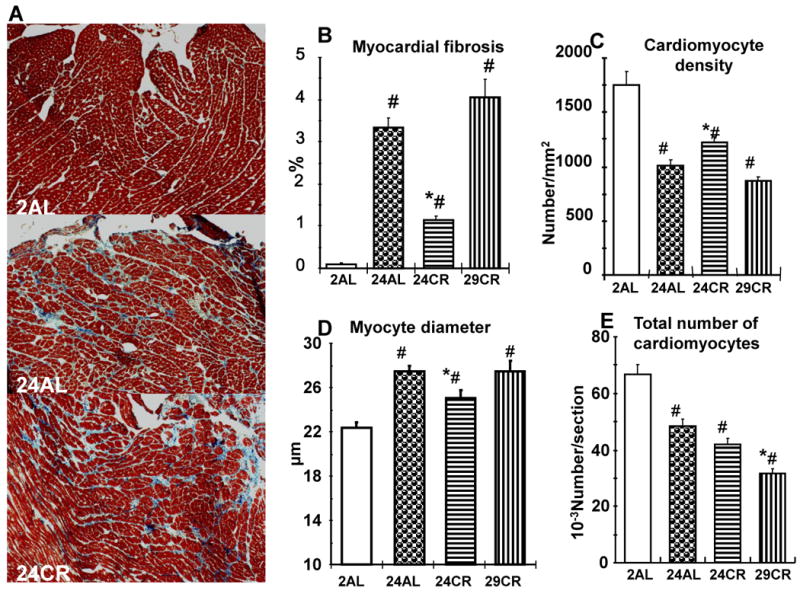
Histological assessment of the myocardium in different age group of AL and CR rats. A – Representative H&E stained slides of myocardium of 2AL, 24AL, and 24CR groups illustrating an increased fibrosis in 24AL and its reduction in 24CR groups; B - Myocardial fibrosis, expressed as a percent of myocardium, in different age groups of AL and CR rats; C – Cardiomyocyte density in different age groups of AL and CR rats; D – Myocyte diameter (short axis) in different age groups of AL and CR rats; E – Total number of cardiomyocytes in different age groups of AL and CR rats; # p<0.05 vs 2AL; * p<0.05 vs 24AL (Bonferroni correction for multiple comparison).
Aortic stiffness and anatomy
Representative microphotographs of Masson-trichrome stained slides of thoracic aorta from different groups are shown on Figure 5A. Collagen content, measured as a percent of total aortic wall (Fig. 5B) or as a percent of tunica media (Fig. 5C), was significantly higher in 24AL than in 2AL rats (p<0.001). It was significantly reduced in 24CR rats (p<0.01 vs 24AL), with less reduction in 29CR. Reduction of the collagen fraction in the wall of thoracic aorta in 24CR rats was translated into functional improvement: while fractional expansion of aorta's wall (Fig. 5D) did not increase in 24CR compared to 24AL, it became significantly higher (p<.01) after scaling for body weight differences (24CRsc). Ultimate improvement of wall function, however, is demonstrated in Figure 5E: pulse wave velocity was reduced by 25% in 24CR rats compared to 24AL (p<0.01). The effect remained (p<0.01) even after scaling for body weight differences (CRsc).
Figure 5.
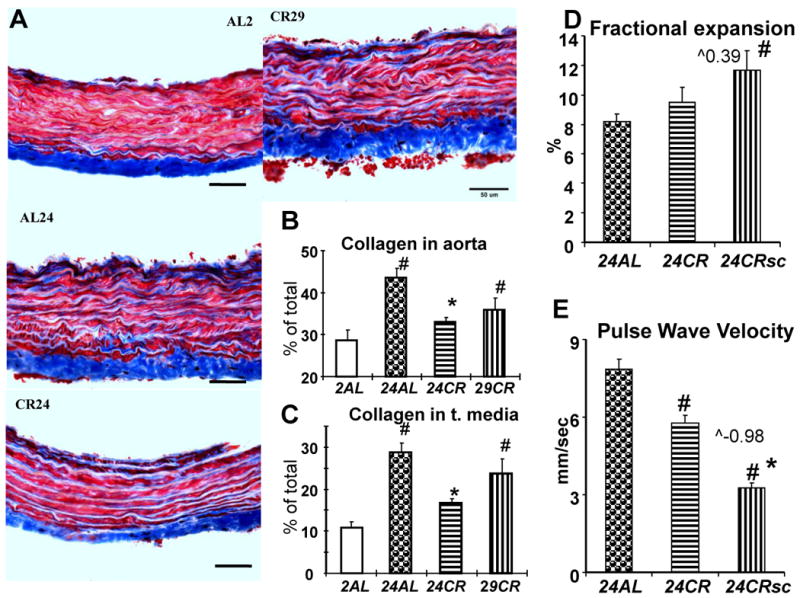
Arterial stiffness. A – representative histological slides of thoracic aorta (Masson trichrome) from different age/diet groups; B, C – content of collagen in the aortic wall expressed as a percent of the total wall area (B) or tunica media (C), # - p<0.05 vs 2AL, * - p<0.05 vs 24AL; D – sonographic measurement of fractional expansion of aortic wall, # - p<0.05 vs 24AL, ˆ0.39 – calculated power of body weight for scaling procedure; E - pulse wave velocity in descending aorta of AL and CR 24-mo old rats. * p<0.05 CR vs AL (Student's t-test).
Cardioprotection by calorie restriction
The effects of CR on the myocardial tolerance to ischemic damage are presented on Figures 6 and 7. Fig. 6A presents photographs of myocardium of a subset of CR and AL rats 24 hrs after permanent ligation of anterior descending coronary artery, perfused by Evans blue then incubated in TTC. Area at risk, shown as area of myocardium colored in red on Fig. 6A, was similar in CR and AL rats (Fig. 6B). Size of MI (uncolored area of myocardium on Fig. 6A) measured as a percent either of area at risk, or LV was also similar in both groups.
Figure 6.
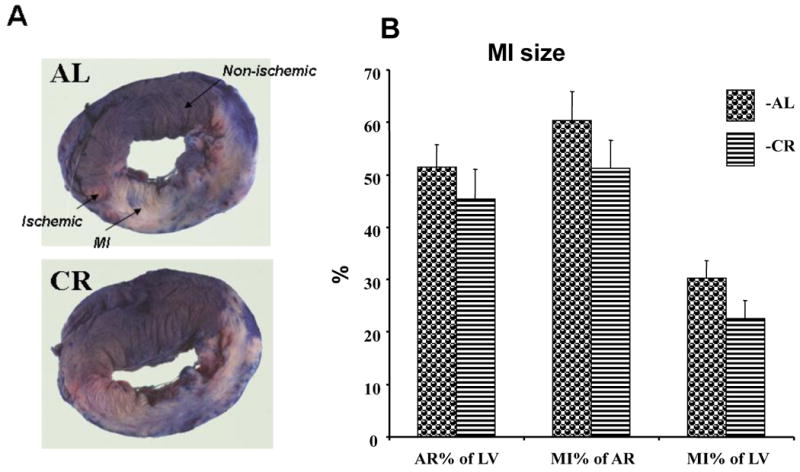
Myocardial infarction and area at risk 24 hrs after permanent ligation of a coronary artery in the hearts of AL and CR rats. A – representative sections of the hearts after Evans blue parfusion and TTC staining. B – Area at risk and MI sizes in AL and CR rats.
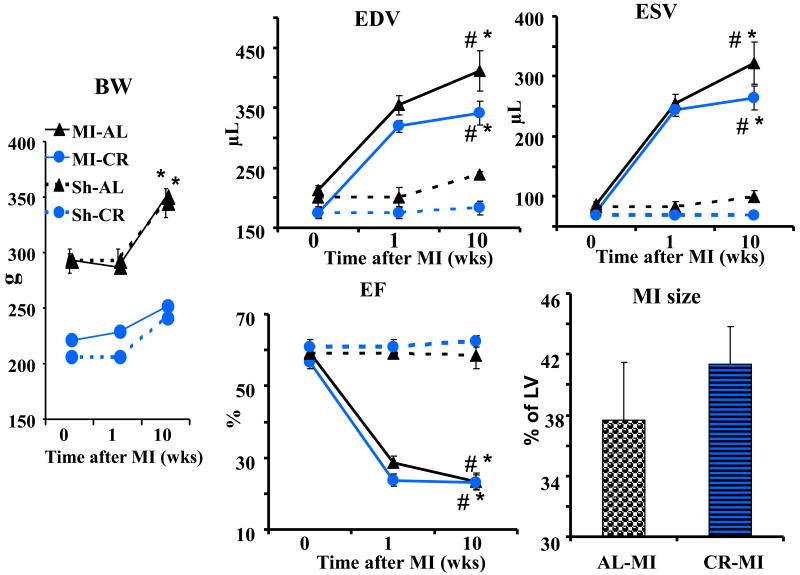
Figure 7.
Body weight change, cardiac remodeling (Echo) and MI size (histology) 10 weeks following induction of MI in AL and CR rats. # p<0.05 vs pre MI baseline (0 wks); * p<0.05 vs Sh of the same diet group.
The remaining MI-CR (n=12), MI-AL (n=11), Sh-CR (n=6), and Sh-AL (n=3), all 5-mo old, continued on their respective diets (Fig. 7) and were evaluated by Echo at week 1 and 10 after MI induction. This was followed by a histological measurement of MI size at 10 weeks. Eight rats (3 from AL and 5 from CR groups) died perioperatively and were excluded from assessments.
Body weight differences between CR and AL rats persisted through 10 wks of observation regardless of MI induction (p<0.001). LV volumes expanded during post MI observation time above Sh-AL levels for EDV (72%) and ESV (225%) in MI-AL group, and above Sh-CR level by 86% and 282% for EDV and ESV, respectively, in MI-CR group (p<0.001). EF at week 10 fell to 60 and 63% below corresponding levels of Sh groups for MI-AL and MI-CR groups, respectively (p<0.001). There were no differences between MI-AL and MI-CR groups with respect to extent of LV expansion or EF reduction. MI size measured histologically at the end of the experiment did not differ between two diet groups (p>0.05).
Discussion
Age-associated changes in cardiovascular system
Assessment of young and old rats presented a pattern of age-associated changes in the cardiovascular system, some aspects of which had been reported previously [18, 21, 22]. Specifically, we observed an expansion of LV volumes with age in rats on the control (ad libitum) diet (Echo). The larger age-associated expansion of ESV than of EDV resulted in reduction of EF in 24AL rats. These age-related changes in LV volume were accompanied with an increase in CO, due to an increase of SV, while HR became lower, and LVM increased. Echo also revealed an age-associated increase of LAD relative to AoD, suggesting an increase in LV stiffness, i.e., a diastolic dysfunction. Hemodynamic assessment (pressure-volume loop analyses) strengthened the evidence of age-associated decline in diastolic function of the heart showing a substantial, 3-fold, increase of Eed – load independent index of myocardial stiffness, and added the evidence of decline in systolic function indicated by a reduction in PRSW. Moreover, the decline in cardiac function was accompanied with an arterio-ventricular uncoupling. Histological assessment of the myocardium provided a mechanistic basis for age-related changes observed via Echo and hemodynamic analyses: 33 fold increase of fibrosis in the myocardium of old rats was accompanied by decline in cardiomyocyte density and total number, as well as by increase in their size. Increased LV cardiac fibrosis was accompanied by increased deposition of collagen in the aorta, predominantly in the area of tunica media. Also, the normal tachycardic response to dobutamine challenge in 2-mo old AL was absent in the 24AL rats.
Effects of CR on age-related changes in cardiovascular system
Compared to 24AL, 24CR demonstrated an attenuated LV expansion without improvement of EF, reduced the increase in the CO and LVM, and attenuated diastolic dysfunction (Echo). Pressure-volume loop analyses confirmed attenuation of diastolic dysfunction revealed by Echo, and additionally demonstrated a restoration of systolic function and arterio-ventricular coupling in 24CR vs 24AL. These improvements in 24CR were associated with a reduction of myocardial fibrosis, an increase of cardiomyocyte density and a reduction of myocyte size. The tachycardic response to dobutamine, which was absent in 24AL rats, was restored in 24CR rats. CR also decreased the collagen content in the tunica media of aortic wall resulting in the functional improvement: increased fractional aortic expansion (index of distensibility) and decreased pulse wave velocity in 24-mo old rats, suggesting a reduction of age-associated arterial stiffness. Improvement of age-associated cardiac remodeling and diastolic dysfunction by CR in the rodents is in a full agreement with findings that had been reported previously [18, 23, 24]. However, attenuation of the age-associated decline in systolic function observed in the present study is not necessarily in conformance with previous findings. For instance, Shinmura et al [18] reported that CR did not improve age-associated decline in systolic performance of the heart. This discrepancy is probably explained by a more sensitive analysis of systolic function provided by a pressure volume loop technique employed in our study. It is more difficult, however, to explain why the reduction of age-associated fibrosis in the myocardium observed in our study and by others [23] was not confirmed in some other reports [18].
Attenuation of age-associated histologic changes in the myocardium of 24CR did not persist in 29CR group, which was similar with 24AL with respect to fibrosis, cardiomyocyte density and size. On the other hand, the total number of cardiomyocytes was reduced with age and this trend was not affected by CR. Echo and hemodynamic indices in 29CR rats reflect a pattern consistent with that of histology: attenuation of age-associated decline in 24CR was not observed in 29CR. This delay of the onset of age-associated morphological and functional cardiovascular changes is well correlated with a general life-span expansion attributed to CR intervention [9-12]: age-associated changes observed between 2-mo old rats and 24AL were attenuated in 24CR, but reappeared in 29CR, consistent with 5 months median life span extension observed in CR cohort (Fig. 1A).
Ratiometric or allometric corrections of the Echo and hemodynamic indices for lower body weight of 24CR rats used in our study did not affect the general pattern of salutary effects of CR on age-associated changes, for the exception of EF, which was not affected by CR in older animals, but was reduced after correction for body weight. Mechanisms by which CR retards senescence are continuously debated in the literature [22, 25], and their elucidation was not an objective of this study. However, the fact that correction for 60% differences in the body weight between 24CR and 24AL did not change the pattern of salutary effects of CR on cardiovascular aging indicates that these effects of CR are more than simply an effect of prevention of age-associated increase of body weight [26].
Summarizing the effect of long-term CR on cardiovascular system, (1) CR attenuated the age-associated changes in the heart and major vessels; (2) the attenuation of age-associated changes by CR cannot be explained by the effect of lower body weight but are attributable to more intimate cellular mechanisms of CR itself; (3) attenuation of age-associated changes by CR waned between 24- and 29-mo of age, and is consistent with an idea [27, 28] that CR postponed senescence.
Cardioprotection by CR
Recently we have reported that three months of alternate day fasting (ADF) reduced the size of MI induced by a permanent coronary ligation and attenuated the extent of subsequent cardiac remodeling in young (5-mo old) rats [16]. In other studies both short-term (4 wks) and long-term (6 months) CR attenuated myocardial damage of isolated heart of rat from ischemia-reperfusion injury [29, 30]. In the light of these observations, findings presented in this study that CR does not increase the tolerance of the myocardium to ischemic damage in 5-mo old rats, was counterintuitive. It is difficult to compare results of our experiment, which was conducted on whole animals and the MI was induced by a permanent ligation of a coronary artery, with results of experiments by Shinmura et al [29, 30]conducted on isolated rat hearts using an ischemia-reperfusion model, where mechanisms of myocardial damage are substantially different. However, our previous experiments on the model of ADF employed methodology identical with the present study, but, contrary to results of the present study, demonstrated a strong cardioprotection. Taken together with results of our prior study [17] which demonstrated a development of diastolic dysfunction and reduced systolic reserve in rats after chronic ADF, results of the present study offer some insight into mechanisms of the effects that different modes of calorie restriction have on the cardiovascular system and cardioprotection from ischemic damage. ADF, being one of the forms of CR, accelerates the development of myocardial fibrosis resulting in a “stiff heart” with diastolic dysfunction and diminishing systolic reserve. In contrast, long-term daily CR delays age-associated changes in the cardiovascular system by reducing the rate of collagen development in the myocardium and cardiomyocyte hypertrophy, thus attenuating age-associated decline of cardiac function. Myocardial fibrosis in the ADF rats is associated with a lower capillary density (31), and this might lead to transient myocardial ischemia, which, in turn, can activate the mechanisms of preconditioning (32). The myocardium of rats subjected to long-term CR is less fibrotic than the myocardium of their AL counterparts, not preconditioned to ischemia, and therefore not protected from ischemic damage. Of course, this is an idea that requires further testing.
Study limitations
Young, 2-mo old rats were compared with presenescent, 24- and 29-mo old (normal median life span in this cohort was 28 months). While age-associated changes in heart and major vessels were consistent and clearly demonstrate the salutary effect of CR which postponed age changes in 24-mo old, the lack of cross-sectional evaluation of several intermediate ages precluded the more precise definition of the time of onset of age-associated changes and separation of maturation from senescence.
Supplementary Material
Highlights.
Different modes of calorie reduction have different effects on cardiovascular system
CR delays age-related decline of cardiac function, fibrosis, and arterial stiffness
Chronic calorie restriction (CR) does not protect myocardium from ischemic damage
Long-term alternate day fasting promotes cardiac fibrosis and diastolic dysfunction
At the same time ADF increases myocardial tolerance to ischemic damage
Acknowledgments
This work was fully supported by the Intramural Research Program of the National Institute on Aging, NIH.
List of abbreviations
- CR
Calorie restriction
- ADF
Alternate day fasting
- MI
Myocardial infarction
- LV
Left ventricle
- EDV
End-diastolic LV volume
- ESV
End-systolic LV volume
- EF
Ejection fraction
- PWth
Posterior wall thickness
- LVM
Left ventricular mass
- LAD
Left atrial dimension
- AoD
Aortic dimension
- TA
Thoracic aorts
- ESP
End-systolic pressure
- EDP
End-diastolic pressure
- PV
Pressure-volume loop analyses
- HR
Heart rate
- SV
Stroke volume
- CO
Cardiac output
- Ea
Arterial elastance
- PRSW
Preload recruitable stroke work
- Ees
Ventricular elastance
- Eed
Myocardial stiffness
Footnotes
Disclosures: Authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelley DE. Effects of weight loss on glucose homeostasis in NIDDM. Diabetes Rev. 1995;3:366–77. [Google Scholar]
- 2.Walford RL, Mock D, MacCallum T, Laseter JL. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in Biosphere 2: health, aging, and toxicological perspectives. Toxicol Sci. 1999;52:61–5. [PubMed] [Google Scholar]
- 3.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–9. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 4.Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Calorie restriction improves whole-body glucose disposal and insulin resistance in association with the increased adipocyte-specific GLUT4 expression in Otsuka Long-Evans Tokushima fatty rats. Arch Biochem Biophys. 2005;436:276–84. doi: 10.1016/j.abb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Z, Jiang W, Thompson HJ. Effect of energy restriction on tissue size regulation during chemically induced mammary carcinogenesis. Carcinogenesis. 1999;20:1721–6. doi: 10.1093/carcin/20.9.1721. [DOI] [PubMed] [Google Scholar]
- 6.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–66. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 7.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–42. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Barrows CH, Kokkonen GC. Dietary restriction and life extension, biological mechanisms. In: Moment GB, editor. Nutritional approaches to aging research. Boca Raton, FL: CRC Press Inc; 1982. pp. 219–43. [Google Scholar]
- 10.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, IL: Charles C Thomas Publisher; 1988. [Google Scholar]
- 11.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging N Engl J Med. 1997;337:986–94. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 13.Krizova E, Simek V. Influence of intermittent fasting and high-fat diet on morphological changes of the digestive system and on changes of lipid metabolism in the laboratory mouse. Physiol Res. 1996;45:145–51. [PubMed] [Google Scholar]
- 14.Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17:1133–4. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- 15.Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–7. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- 16.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–21. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 17.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan MI. Chronic alternate-day fasting results in reduced diastolic compliance and diminished systolic reserve in rats. J Card Fail. 2010;16:843–53. doi: 10.1016/j.cardfail.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, et al. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–27. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Batterham AM, George KP, Whyte G, Sharma S, McKenna W. Scaling cardiac structural data by body dimensions: a review of theory, practice, and problems. Int J Sports Med. 1999;20:495–502. doi: 10.1055/s-1999-8844. [DOI] [PubMed] [Google Scholar]
- 20.Chantler PD, Clements RE, Sharp L, George KP, Tan LB, Goldspink DF. The influence of body size on measurements of overall cardiac function. Am J Physiol. 2005;289:H2059–H2065. doi: 10.1152/ajpheart.00022.2005. [DOI] [PubMed] [Google Scholar]
- 21.Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, et al. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med. 2010;48:47–54. doi: 10.1016/j.freeradbiomed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. Caloric restriction delays cardiac ageing in rats: role of mitochondria. Cardiovasc Res. 2010;88:267–76. doi: 10.1093/cvr/cvq273. [DOI] [PubMed] [Google Scholar]
- 23.Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci. 1997;52:B285–90. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- 24.Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol. 2006;41:661–8. doi: 10.1016/j.yjmcc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 26.Gerstenblith G. What we can learn from caloric restriction. J AM Coll Cardiol. 2006;47:403–4. doi: 10.1016/j.jacc.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, et al. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol. 2011;46:23–9. doi: 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–96. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–55. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: role for interstitial fibrosis. Mol Cell Biochem. 1995;147:29–34. doi: 10.1007/BF00944780. [DOI] [PubMed] [Google Scholar]
- 32.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc Res. 2009;83:234–46. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.