Summary
Objective
To establish the performance of location specific computer measures of radiographic joint space width (JSW) compared to measurements of minimum joint space width (mJSW) for the assessment of medial compartment knee osteoarthritis (OA). The study also investigated the most disease-responsive location for measuring medial compartment JSW.
Methods
Serial bilateral Posterior Anterior (PA) conventional radiographs acquired with a fixed flexion protocol were obtained 36 months apart in 118 persons with knee OA participating in the Health, Aging and Body Composition (Health ABC) Study. Measurements of medial compartment mJSW and JSW at seven fixed locations were facilitated by the use of semi-automated software that delineated the femoral and tibial margins of the joint. A human reader operated custom software to verify and correct the software-drawn margins where necessary. Paired images were displayed with the reader blinded to the chronological order. The amount of joint space narrowing was measured and the standardized response mean (SRM) was used as a metric to quantify performance.
Results
For all subjects, the mJSW SRM value was 0.42 while, for the most responsive location specific measure of JSW, it was SRM = 0.46. For subjects with a Kellgren–Lawrence (KL) score less than or equal to 1, mJSW (SRM = 0.40) was more responsive than the new measures (Maximum SRM = 0.30). For KL = 2 or 3, SRM = 0.49 for mJSW, and SRM = 0.74 for the most responsive location specific measure of JSW. Improved responsiveness was observed in the more central portion of the joint on the more diseased knees.
Conclusions
Location specific computer measures of JSW are feasible and potentially provide a superior method to assess radiographic OA for more diseased subjects. This new measure has the potential to improve the power of clinical studies that use a fixed flexion protocol.
Introduction
Osteoarthritis (OA) currently affects a significant fraction of the US residents and is becoming increasingly prevalent as the population ages1,2. A 2007 study estimated the cost of arthritis to the United States economy to be over $116 billion in 2003 dollars3. Medical imaging provides a quantifiable method to observe and measure structural changes due to OA progression. Radiography provides a proven low-cost method to monitor OA progression and is currently the accepted modality for monitoring the progress of OA4. Radiography is used in several large OA studies such as the Health, Aging and Body Composition (Health ABC)5 and Multicenter Osteoarthritis (MOST)6 Studies. A large cohort study of OA, the Osteoarthritis Initiative7,8, will acquire approximately 48,000 radiographic images of the knee. The cost of medical imaging is not just in the radiographic acquisition, but in analysis of the images as well since the human factors involved in the reading and data output for such a large number of images are considerable. While current methods are valuable and widely used in studies of OA, improvements to the surrogate outcome measures could increase the power and lower the cost of clinical studies of OA.
Radiographically visible structural changes from OA include damage to the articular cartilage, subchondral sclerosis, and osteophytes. Since cartilage is not visible on a radiograph, the loss is measured indirectly by observing the narrowing of the adjacent bones in a joint or loss of the joint space width (JSW). Knee radiographs can be assessed using the Kellgren–Lawrence (KL) semi-quantitative scoring method9, which assigns scores based on joint narrowing and osteophytes.
Radiographic JSW loss has been shown to correlate with cartilage measured by magnetic resonance imaging (MRI)10. Minimum joint space width (mJSW) between the projected femur and tibia margins on a knee radiograph is the currently accepted metric11 to assess OA longitudinally. mJSW can be quantified by visually determining the location of the minimum distance while viewing the film with a handheld graduated lens to make the measurement12. More recently, software-based measurements of mJSW have been introduced13–16 that use image analysis software to delineate the femur and tibia bone margins, and measure mJSW semi-automatically (Fig. 1).
Fig. 1.
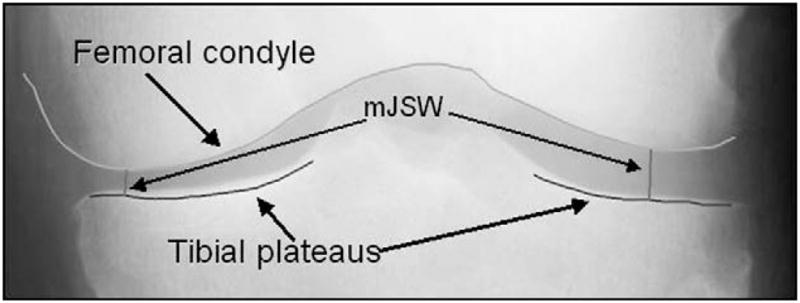
Typical output of knee radiograph analysis software showing the delineation of the femoral condyle and the tibial plateaus, and the mJSW locations.
While mJSW is an established metric, we have observed difficulties with achieving a consistent measurement for this metric on some knee radiographs. In Fig. 2(a) the software has successfully delineated the joint margins, however, the femur and tibia contours continuously converge such that the location of mJSW cannot be established consistently. In such cases, since no local minimum exists, mJSW is placed at the extreme limits of the software delineation and the measurement can be susceptible to variation due to the exposure of the radiographic acquisition. The location of the mJSW can also vary for cases with joint attrition [Fig. 2(b)]. mJSW provides a measurement at one location only and does not take advantage of the full delineation of the joint surfaces provided by the software techniques. While we have observed these problems in images acquired with a fixed flexion protocol17, radiographs using fluoroscopic guidance may be less affected.
Fig. 2.
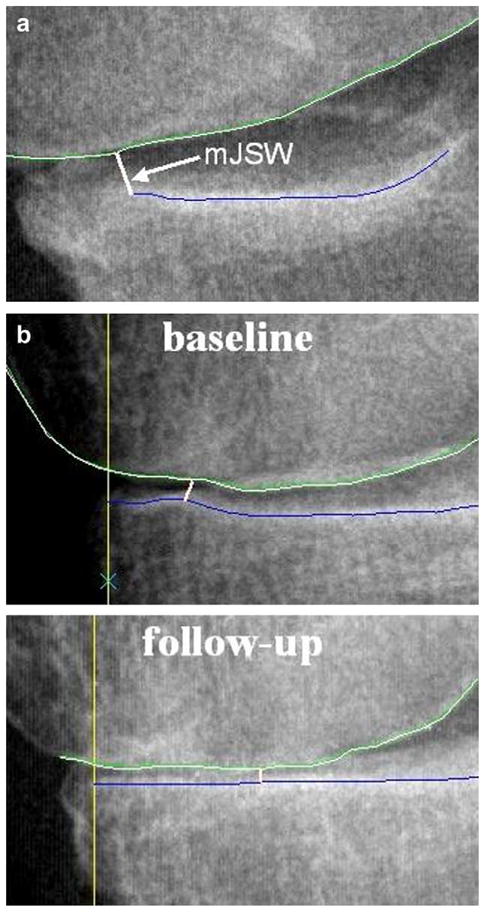
Examples of problematic medial compartment knee radiographs from our data where the location specific JSW may provide a more robust metric. In (a) sub optimal patient positioning causes the femur and tibia margins to continuously converge resulting in no local minimum. In (b) joint attrition causes different locations for mJSW in the baseline and follow-up images.
Previous studies have examined joint space area (JSA) as a metric to quantify OA progression18. For our current longitudinal study we are examining JSW at specific locations, JSW(x), aided by the establishment of a robust coordinate system. Unlike JSA, which is an average over a continuous set of positions, JSW(x) probes the joint at single specific locations. Published work established improved reproducibility of JSW(x) compared to mJSW19 using duplicate knee radiographs. For our current study we hypothesized that location specific computer measures of radiographic JSW, JSW(x), would be more responsive than mJSW for the assessment of medial compartment knee OA based on an analysis of the mean change and the standardized response means (SRMs). An additional goal of the study was to determine the optimal location along the joint interface to measure JSW(x).
Participants and methods
Serial bilateral Posterior Anterior (PA) conventional radiographs were obtained 36 months apart in persons with mild to severe knee OA participating in the Health ABC study, a community based, multi-center cohort study of 3075 white and black men and women aged 70–79 at enrollment. All subjects had risk factors for OA, although many had no radiographic evidence as defined by the KL score. KL scoring was performed by Dr. Hunter. More details about the Health ABC Study and the subject population can be found in a separate publication20. We randomly selected a sample of 136 (272 knee pairs) participants who underwent a bilateral knee radiographs at both the baseline and follow-up (36 month) time points using a fixed flexion subject positioning protocol17.
Any knee with a total joint replacement (1 knee), and any joint that was independently assessed to have lateral compartment OA (54 knees) were excluded from the analysis. Determination of lateral compartment OA was based on a non-zero joint space narrowing (JSN) score in the lateral compartment. The study sample was 51% female, 41% African American, with an average age of 74.3 ± 2.8 years (mean ± standard deviation). The baseline KL scores of the eligible 217 knee baseline follow-up pairs (from 118 subjects) were distributed as follows: 93 (KL = 0), 38 (KL = 1), 13 (KL = 2), 55 (KL = 3), and 18 (KL = 4). The radiography protocol called for an extremity detail film cassette (Agfa Ortho Fine), with a 122 cm film to focus distance. The radiography technique was 70 kVp with a variable mAs (6–16 mAs). The radiographic films were digitized using a Vidar (Herndon, VA) film digitizer with a pixel spacing of 0.085 mm and transferred to a personal computer for analysis.
We have previously developed and documented a software tool written using the C programming language16,19 to delineate the tibiofemoral joint on digitized knee radiographs and enable measurements of JSW(x) and mJSW. A previous study established the reproducibility of the technique by measuring the root mean square standard deviation (RMSSD) as 0.16 mm (normal knees) and 0.18 mm (OA knees) for mJSW16 measured on duplicate radiographs. The reproducibility study used the software in a fully automated mode, with no manual correction, however, to improve the value of the software tool we also developed an accompanying graphical user interface using the Interactive Data Language (IDL), (Kodak Inc., Boulder, CO) application. A human user operates the tool on a cropped image of the knee joint, examines the software-generated contours that delineated the joint, and can then use semi-automated editing tools to verify and correct the software-drawn margins where necessary16.
mJSW was measured as the minimum distance between the delineated femur and tibia margins in the medial compartment. Baseline and follow-up images were displayed as pairs with the reader (GN) blinded to the chronological sequence. Image files were prepared by a different researcher (JD), and randomly assigned filenames ensured that the reader had no knowledge of the correct time sequence. The reader judgment was employed to correct any improper software delineated joint margins and to provide a consistent mJSW measurement from baseline to follow-up for cases such as the examples in Fig. 2.
To enable consistent measures of JSW(x), a coordinate system was created based on anatomical landmarks (Fig. 3). The x-axis, defined as the line tangent to both femoral epicondyles, was placed automatically by the software. The x variable represented the position of the JSW(x) measurement along the projected surface of the joint. The y-axis was placed manually as a line perpendicular to the x-axis and tangent to the greatest prominence of the medial epicondyle. The line x = 1, was defined as the tangent to the greatest prominence of the lateral epicondyle of the knee. The variable x is a dimensionless quantity which can be considered to approximately represent the fraction of the total width of the femoral condyle. The use of this variable and the coordinate system is a potential strength of our method since the JSW measurement location can be reproducibly and consistently determined for different subjects and for different visits of the same patient assuming perfectly consistent knee positioning. In principle, x is independent of knee size, pixel spacing, magnification, and other factors that affect the location along the joint. In practice, x can vary due to knee rotation and patient positioning; one goal of our study was to determine whether these effects reduced the responsiveness of the technique.
Fig. 3.
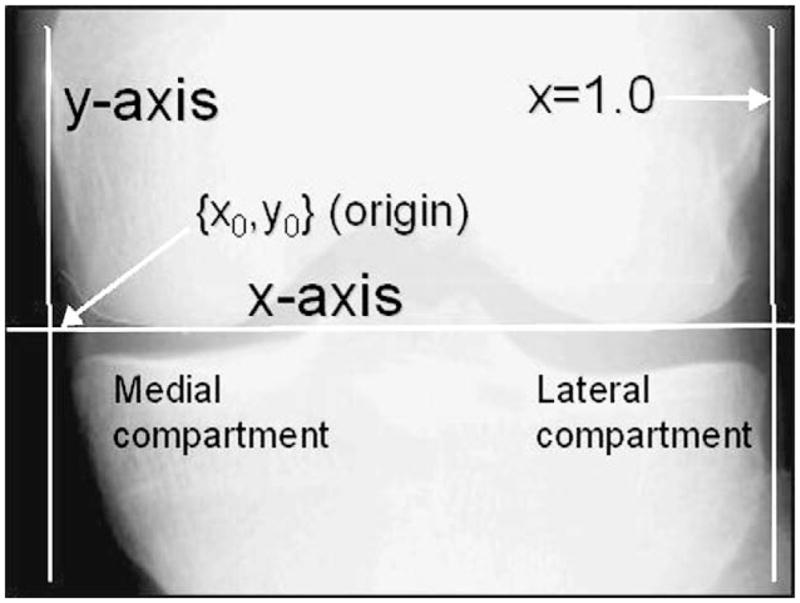
Landmarks and definition of coordinate system.
The software displayed cropped images of the epicondyles for both visits simultaneously so that the reader could verify consistent landmark placement for both baseline and follow-up images. All images of the knee joint were placed in a consistent orientation with the medial compartment on the left (x < 0.5) and the lateral compartment on the right (x > 0.5). Measurements of medial compartment mJSW and JSW(x) at seven fixed locations (x = 0.125, x = 0.15, x = 0.175, x = 0.2, x = 0.225, x = 0.25, and x = 0.275), were made using the software tool described above (Fig. 4). JSW(x) was defined as the distance between the femur and tibia joint margins in a direction parallel to the y-axis of our coordinate system.
Fig. 4.
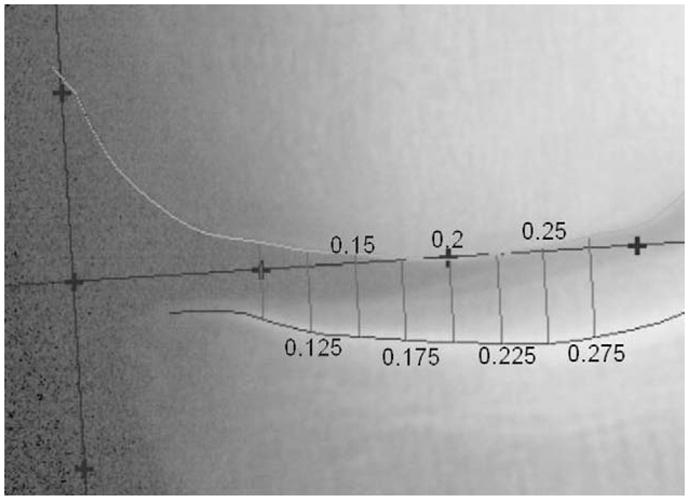
Location specific measurement of JSW, JSW(x), (x = 0.125–0.275), in the medial compartment. Measurements of JSW(x) are made at the x-locations defined by the coordinate system.
We compared medial compartment JSW(x) to mJSW. As a metric to quantify performance we used the SRM, or the ratio of the mean loss to the standard deviation of the loss. We report the SRM values along with the average baseline JSW, the average change, and the standard deviation for each JSW measure.
Results
Table I gives values for each the KL score and in Table II we divide the subjects into two groups, KL ≤ 1 and KL grade equal to 2 or 3. The average change in KL grade was 0.14 with a standard deviation of 0.46, however, no change in KL score was observed for 88% of the knees. An analysis with ANOVA revealed no difference for the longitudinal change values across mJSW and the seven JSW(x) measures (P = 0.25).
Table I.
Average baseline (BL), average JSW loss, standard deviation of the loss, and SRM values for different KL grades
| BL (mm) | Loss (mm) | SD (mm) | SRM | |
|---|---|---|---|---|
| (a) KL = 0 (N = 93) | ||||
| mJSW | 3.60 | 0.22 | 0.52 | 0.43 |
| JSW(x = 0.125) | 4.46 | 0.16 | 0.60 | 0.27 |
| JSW(x = 0.15) | 4.50 | 0.15 | 0.52 | 0.28 |
| JSW(x = 0.175) | 4.57 | 0.11 | 0.46 | 0.25 |
| JSW(x = 0.2) | 4.71 | 0.12 | 0.51 | 0.24 |
| JSW(x = 0.225) | 4.96 | 0.16 | 0.52 | 0.30 |
| JSW(x = 0.25) | 5.30 | 0.20 | 0.51 | 0.40 |
| JSW(x = 0.275) | 5.69 | 0.19 | 0.57 | 0.34 |
| (b) KL = 1 (N = 38) | ||||
| mJSW | 3.10 | 0.25 | 0.69 | 0.35 |
| JSW(x = 0.125) | 3.79 | 0.23 | 0.72 | 0.32 |
| JSW(x = 0.15) | 3.82 | 0.20 | 0.82 | 0.24 |
| JSW(x = 0.175) | 3.97 | 0.20 | 0.81 | 0.25 |
| JSW(x = 0.2) | 4.20 | 0.22 | 0.80 | 0.28 |
| JSW(x = 0.225) | 4.44 | 0.20 | 0.85 | 0.24 |
| JSW(x = 0.25) | 4.72 | 0.15 | 0.84 | 0.18 |
| JSW(x = 0.275) | 5.09 | 0.16 | 0.87 | 0.19 |
| (c) KL = 2 (N = 13) | ||||
| mJSW | 3.58 | 0.51 | 0.59 | 0.86 |
| JSW(x = 0.125) | 4.40 | 0.47 | 0.77 | 0.61 |
| JSW(x = 0.15) | 4.35 | 0.40 | 0.66 | 0.61 |
| JSW(x = 0.175) | 4.42 | 0.46 | 0.64 | 0.72 |
| JSW(x = 0.2) | 4.54 | 0.48 | 0.65 | 0.74 |
| JSW(x = 0.225) | 4.83 | 0.60 | 0.74 | 0.81 |
| JSW(x = 0.25) | 5.15 | 0.53 | 0.85 | 0.63 |
| JSW(x = 0.275) | 5.56 | 0.45 | 0.77 | 0.58 |
| (d) KL = 3 (N = 55) | ||||
| mJSW | 1.68 | 0.32 | 0.75 | 0.43 |
| JSW(x = 0.125) | 2.09 | 0.32 | 0.92 | 0.34 |
| JSW(x = 0.15) | 2.16 | 0.33 | 0.83 | 0.40 |
| JSW(x = 0.175) | 2.34 | 0.39 | 0.72 | 0.53 |
| JSW(x = 0.2) | 2.56 | 0.45 | 0.72 | 0.63 |
| JSW(x = 0.225) | 2.82 | 0.51 | 0.77 | 0.66 |
| JSW(x = 0.25) | 3.14 | 0.54 | 0.79 | 0.68 |
| JSW(x = 0.275) | 3.66 | 0.65 | 0.82 | 0.79 |
| (e) KL = 4 (N = 18) | ||||
| mJSW | 0.40 | 0.09 | 0.30 | 0.29 |
| JSW(x = 0.125) | 0.65 | −0.04 | 0.33 | −0.12 |
| JSW(x = 0.15) | 0.64 | −0.04 | 0.42 | −0.09 |
| JSW(x = 0.175) | 0.74 | 0.09 | 0.37 | 0.25 |
| JSW(x = 0.2) | 0.86 | 0.20 | 0.40 | 0.49 |
| JSW(x = 0.225) | 0.97 | 0.36 | 0.40 | 0.90 |
| JSW(x = 0.25) | 1.14 | 0.45 | 0.46 | 0.97 |
| JSW(x = 0.275) | 1.37 | 0.51 | 0.62 | 0.81 |
Table II.
Average baseline (BL), average JSW loss, standard deviation of the loss, and SRM values for KL ≤ 1 and for KL grade equal to 2 or 3
| BL (mm) | Loss (mm) | SD (mm) | SRM | |
|---|---|---|---|---|
| (a) KL ≤ 1 (N = 131) | ||||
| mJSW | 3.46 | 0.23 | 0.58 | 0.40 |
| JSW(x = 0.125) | 4.27 | 0.18 | 0.63 | 0.28 |
| JSW(x = 0.15) | 4.31 | 0.16 | 0.63 | 0.26 |
| JSW(x = 0.175) | 4.39 | 0.14 | 0.58 | 0.24 |
| JSW(x = 0.2) | 4.57 | 0.15 | 0.61 | 0.25 |
| JSW(x = 0.225) | 4.81 | 0.17 | 0.63 | 0.27 |
| JSW(x = 0.25) | 5.13 | 0.19 | 0.63 | 0.30 |
| JSW(x = 0.275) | 5.52 | 0.18 | 0.67 | 0.28 |
| (b) KL grade equal to 2 or 3 (N = 68) | ||||
| mJSW | 2.05 | 0.36 | 0.73 | 0.49 |
| JSW(x = 0.125) | 1.69 | 0.35 | 0.90 | 0.38 |
| JSW(x = 0.15) | 2.53 | 0.34 | 0.81 | 0.43 |
| JSW(x = 0.175) | 2.18 | 0.40 | 0.71 | 0.56 |
| JSW(x = 0.2) | 2.58 | 0.46 | 0.71 | 0.64 |
| JSW(x = 0.225) | 2.24 | 0.53 | 0.77 | 0.68 |
| JSW(x = 0.25) | 2.74 | 0.54 | 0.81 | 0.66 |
| JSW(x = 0.275) | 2.34 | 0.61 | 0.82 | 0.74 |
Generally, the baseline JSW increases for higher x (more centrally located) as did the amount of change, and the SRM. The optimal location to measure JSW(x) varies as a function of the KL score. Based on Table I, the optimal x values are x = 0.25 (KL = 0), x = 0.125 (KL = 1), x = 0.225 (KL = 2), x = 0.275 (KL = 3), and x = 0.25 (KL = 4). With the exception of the KL = 1 set, the most responsive location was in the more central portion of the joint. The results in Table II suggest that x = 0.275 should be defined as the optimal location to measure JSW(x).
The results also demonstrated that location specific JSW(x) tended to outperform mJSW in some regions. Generally, JSW(x) showed better responsiveness compared to mJSW in the central portion (x > 0.2) than in the outer portion. The difference in responsiveness between mJSW and JSW(x) as well as between the less central (x < 0.2) and more central portions was enhanced for more diseased knees as defined by the KL score. For the KL = 3 and KL = 4 groups (Table Id and e) there was a clear trend for increased loss of joint space as the measurement was made in the more central region. Since the number of subjects is relatively small for each KL group we provided Table II, which gives the results for two higher powered subgroups of less diseased (Table IIa) and more diseased (Table IIb) subjects. These results suggest that mJSW is the preferred metric for less diseased subjects, while JSW(x) is superior for a more diseased OA population.
Discussion
The data indicate that the more central portion of the knee may be the best location to measure JSW(x) for more diseased subjects. While counterintuitive, we would speculate that the result may be explained by the need to have unambiguous joint margins present on the radiographic image to facilitate consistent delineation of the joint space. The more central regions of an OA knee joint are less likely to exhibit structural damage that can confound joint margin detection or cause inconsistencies from baseline to follow-up. Since the femur is a rigid structure, JSW(x) does provide an indirect measure of joint loss in other areas, and potentially provides more clear and unambiguous images of the joint margins for the software or for a human observer. The data also indicate that, for the most severely diseased subjects (Table Ie, KL = 4), the amount of narrowing for x < 0.2, is much less than in the region defined by x > 0.2. This may suggest a threshold effect whereby once the knee joint approaches bone-on-bone status, JSW change can be better observed away from the point where the bones come into contact.
Our study used radiographs acquired with a fixed flexion protocol that did not include fluoroscopic guidance. Since radiographic JSW is highly influenced by knee positioning, we can make no claims about the generalization of our results to studies using different patient positioning techniques, including fluoroscopically guided methods. The merits of the different knee positioning schemes are debated in the literature20–26. The disadvantage of the fixed flexion protocol is that the knee flexion angle is not optimized for each patient. However, the method can be more conveniently administered, and for this reason it is the primary patient knee positioning method for the Osteoarthritis Initiative and for the Health ABC Study. Despite the potential for suboptimal patient positioning using the fixed flexion technique, our results and others20 demonstrate that such radiographs can be used to detect longitudinal change. The software location specific JSW measurements are potentially very useful for this and other large studies of OA that use the fixed flexion protocol.
Direct comparison to other studies using fluoroscopically guided radiography is difficult since our method uses a fixed flexion protocol and the responsiveness is highly dependent on the KL grade. However, the responsiveness of mJSW is similar to other studies18,20,25,26 and our mJSW results are also similar to published study that investigated subjects from the Health ABC Study20.
The decision to study JSW(x) from x = 0.125–0.275 was made based on the results from a cross sectional reproducibility study that demonstrated optimal precision near x = 0.219. Since we have learned from our current longitudinal study that the more central region (x ≥ 0.25) can be superior, future studies of medial compartment JSW(x) should examine even more central locations. We excluded patients with lateral compartment OA in our study, but JSW(x) in the lateral compartment (x > 0.5) could be measured with our method to assess these subjects and to potentially enhance our understanding of medial compartment OA.
There are several limitations to the study and to the methodology we used. Measurements of JSW(x) were more time consuming than for mJSW since additional manual editing was necessary to ensure full joint delineation, and the reader was also required to place landmarks to define the coordinate system. The mJSW measurement was made in a total reader time of less 2 min per knee for our study, while the JSW(x) measurement required approximately twice the amount of reader time. We hope to reduce the reader time though the development of more automated joint delineation software and the implementation of software-generated landmarks. Our study was limited to a single nonflouroscopically guided acquisition method, and it is not obvious that the same trends and comparison to mJSW would be observed for fluoroscopically guided knee radiographs.
In conclusion, location-specific computer measures of joint space are feasible and provide a more responsive method to assess disease progression than mJSW in patients with advanced OA. This method should prove useful to improve the power of clinical studies that use knee radiography with the fixed flexion protocol17.
Acknowledgments
We would like to thank the participants and investigators of the Health ABC study for their support of this project. The authors would also like to thank Dr. John Lynch for his valuable comments on the manuscript. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Aging and NIH/NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106.
Footnotes
Conflict of interest
There are no conflicts of interest of any authors with the work presented in this manuscript.
References
- 1.Schned ES, Reinertsen JL. The social and economic consequences of rheumatic disease. In: Klippel JH, editor. Primer on the Rheumatic Diseases. Atlanta: William M. Otto; 1997. pp. 6–9. [Google Scholar]
- 2.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–99. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,527 patients. Arthritis Rheum. 2003;48(10):2750–62. doi: 10.1002/art.11439. [DOI] [PubMed] [Google Scholar]
- 4.Peterfy CG. Imaging of the disease process. Curr Opin Rheumatol. 2002;14(5):590–6. doi: 10.1097/00002281-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, et al. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci. 2000;904:456–61. [PubMed] [Google Scholar]
- 6.Multicenter Osteoarthritis Study. 2006 http://researchresources.bumc.bu.edu/abstract/5U01AG018820-05.htm. (checked 5/2006)
- 7.Osteoarthritis Initiative. 2008 http://www.niams.nih.gov/ne/oi/index.htm. (checked 12/2008)
- 8.Fawaz-Estrup F. The osteoarthritis initiative: an overview. Med Health R I. 2004;87(6):169–71. [PubMed] [Google Scholar]
- 9.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16(11):494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52(10):3152–9. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 11.Buckland-Wright JC, Macfarlane DG, Williams SA, Ward RJ. Accuracy and precision of joint space width measurements in standard and macroradiographs of osteoarthritic knees. Ann Rheum Dis. 1995;54(11):872–80. doi: 10.1136/ard.54.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lequesne M. Quantitative measurements of joint space during progression of osteoarthritis: ‘chondrometry’. In: Kuettner KE, Goldberg V, editors. Osteoarthritis Disorders. Rosemont: American Academy of Orthopedic Surgeons; 1995. pp. 427–44. [Google Scholar]
- 13.Dacre JE, Coppock JS, Herbert KE, Perrett D, Huskisson EC. Development of a new radiographic scoring system using digital image analysis. Ann Rheum Dis. 1989;48(3):194–200. doi: 10.1136/ard.48.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch JA, Buckland-Wright C, Macfarlane DG. Precision of joint space width measurment in knee osteoarthritis from digital image analysis of high definition macroradiographs. Osteoarthritis Cartilage. 1993;1:209–18. doi: 10.1016/s1063-4584(05)80327-9. [DOI] [PubMed] [Google Scholar]
- 15.Ravaud P, Chastang C, Auleley GR, Giraudeau B, Royant V, Amor B, et al. Assessment of joint space width in patients with osteoarthritis of the knee: a comparison of 4 measuring instruments. J Rheumatol. 1996;23(10):1749–55. [PubMed] [Google Scholar]
- 16.Duryea J, Li J, Peterfy CG, Gordon C, Genant HK. Trainable rule-based algorithm for the measurement of joint space width in digital radiographic images of the knee. Med Phys. 2000;27(3):580–91. doi: 10.1118/1.598897. [DOI] [PubMed] [Google Scholar]
- 17.Peterfy C, Li J, Zaim S, Duryea J, Lynch JA, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;3:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 18.Vignon E, Piperno M, Le Graverand MP, Mazzuca SA, Brandt KD, Mathieu P, et al. Measurement of radiographic joint space width in the tibiofemoral compartment of the osteoarthritic knee: comparison of standing anteroposterior and Lyon schuss views. Arthritis Rheum. 2003;48(2):378–84. doi: 10.1002/art.10773. [DOI] [PubMed] [Google Scholar]
- 19.Duryea J, Zaim S, Genant HK. New radiographic-based outcome measures for osteoarthritis of the knee. Osteoarthritis Cartilage. 2003;11(2):102–10. doi: 10.1053/joca.2002.0866. [DOI] [PubMed] [Google Scholar]
- 20.Nevitt MC, Peterfy C, Guermazi A, Felson DT, Duryea J, Woodworth T, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis Rheum. 2007;56(5):1512–20. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 21.Vignon E, Conrozier T, Hellio Le Graverand MP. Advances in radiographic imaging of progression of hip and knee osteoarthritis. J Rheumatol. 2005;32(6):1143–5. [PubMed] [Google Scholar]
- 22.Vignon E. Radiographic issues in imaging the progression of hip and knee osteoarthritis. J Rheumatol Suppl. 2004;70:36–44. [PubMed] [Google Scholar]
- 23.Mazzuca SA, Brandt KD, Buckwalter KA, Lequesne M. Pitfalls in the accurate measurement of joint space narrowing in semiflexed, anteroposterior radiographic imaging of the knee. Arthritis Rheum. 2004;50(8):2508–15. doi: 10.1002/art.20363. [DOI] [PubMed] [Google Scholar]
- 24.Conrozier T, Favret H, Mathieu P, Piperno M, Provvedini D, Taccoen A, et al. Influence of the quality of tibial plateau alignment on the reproducibility of computer joint space measurement from Lyon schuss radiographic views of the knee in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2004;12(10):765–70. doi: 10.1016/j.joca.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Le Graverand MP, Mazzuca S, Lassere M, Guermazi A, Pickering E, Brandt K, et al. Assessment of the radioanatomic positioning of the osteoarthritic knee in serial radiographs: comparison of three acquisition techniques. Osteoarthritis Cartilage. 2006;14(Suppl A):A37–43. doi: 10.1016/j.joca.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Hellio Le Graverand MP, Vignon EP, Brandt KD, Mazzuca SA, Piperno M, Buck R, et al. Head-to-head comparison of the Lyon schuss and fixed flexion radiographic techniques. Long-term reproducibility in normal knees and sensitivity to change in osteoarthritic knees. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.077834. [DOI] [PubMed] [Google Scholar]


