Abstract
Trigeminal nerve activation in response to inflammatory stimuli has been shown to increase neuron–glia communication via gap junctions in trigeminal ganglion. The goal of this study was to identify changes in the expression of gap junction proteins, connexins (Cxs), in trigeminal ganglia in response to acute or chronic joint inflammation. Although mRNA for Cxs 26, 36, 40 and 43 was detected under basal conditions, protein expression of only Cxs 26, 36 and 40 increased following capsaicin or complete Freund’s adjuvant (CFA) injection into the temporomandibular joint (TMJ). While Cx26 plaque formation between neurons and satellite glia was transiently increased following capsaicin injections, Cx26 plaque formation between neurons and satellite glia was sustained in response to CFA. Interestingly, levels of Cx36 and Cx40 were only elevated in neurons following capsaicin or CFA injections, but the temporal response was similar to that observed for Cx26. In contrast, Cx43 expression was not increased in neurons or satellite glial cells in response to CFA or capsaicin. Thus, trigeminal ganglion neurons and satellite glia can differentially regulate Cx expression in response to the type and duration of inflammatory stimuli, which likely facilitates increased neuron–glia communication during acute and chronic inflammation and pain in the TMJ.
Keywords: Capsaicin, complete Freund’s adjuvant, gap junctions, hemichannels, temporomandibular joint disorders
INTRODUCTION
The head and face represent some of the most common pain sites in the body (Sessle, 1987; Lipton et al., 1993; Carlsson, 1995). These craniofacial symptoms can manifest as acute or transient conditions such as toothaches and headaches, or can transform into more chronic conditions such as temporomandibular joint (TMJ) disorder or trigeminal neuralgia. All of these conditions are directly related to activation of the fifth cranial nerve or trigeminal nerve. The trigeminal nerve is the largest and most complex of the 12 cranial nerves and is comprised of three major divisions: the ophthalmic (V1), the maxillary (V2) and the mandibular (V3) (Shankland, 2000). Activation of the mandibular division, which provides sensory innervation to the TMJ and associated muscles of mastication, is implicated in the underlying pathology of TMJ disorders. Based on a recent epidemiology study, it was reported that TMJ disorders affect >13 million people in the United States with an overall prevalence of 4.6%, affecting 6.3% of women and 2.8% of men (Isong et al., 2008).
The cell bodies of the mandibular nerves, which when stimulated release neuropeptides that promote peripheral inflammation and pain, are located in the trigeminal ganglion (Shankland, 2000). The trigeminal ganglion is comprised primarily of sensory neurons and two types of glial cells, satellite glial cells and Schwann cells (Hanani, 2005). Several satellite glial cells are located in close association with the neuronal cell bodies in the trigeminal ganglion and are thought to form distinct, functional units (Hanani, 2005). There is increasing evidence to support an important role of glial cells in pathological states by directly modulating the threshold of activation and excitability state of neurons, and thus their function (Watkins and Maier, 2002; Hanani, 2005; Takeda et al., 2007). In addition, interactions between neurons and glial cells have been shown to be involved in all stages of inflammation and pain associated with several central nervous system (CNS) diseases (Watkins et al., 2001a,b; Wieseler-Frank et al., 2004). Under both normal and pathological conditions, communication between neurons and glia can occur by paracrine signaling as well as through the formation of gap junctions (Haydon, 2001).
Gap junctions are specialized intercellular membrane channels that allow molecules <1 kDa (i.e. secondary messengers, ions, siRNA and metabolic precursors) to pass directly from one cell to another (Goldberg, 1999, 2002). Gap junctions are thought to be present in nearly all mammalian cell types (Beyer et al., 1995; White, 1996). They are dynamic structures that appear to be preprogrammed to be continuously biosynthesized and degraded in the cell, and typically exhibit a short in vivo half-life between 1–5 h (Fallon and Goodenough, 1981; Beardslee et al., 1998; Laird, 2006). Gap junctions are not thought to function as a single unit, but rather, they cluster together to form tightly packed arrays known as gap junction plaques that facilitate ionic and metabolic coupling between two cells. Importantly, evidence suggests that gap junction channels may not open until they cluster into plaques on the cell membranes (Bukauskas et al., 2000).
Gap junctions are composed of polytopic membrane proteins known as connexins (Cxs). Currently the Cx family has 21 human members, ten of which have been identified in the nervous system (Laird, 2006). Six Cxs from one cell oligomerize into hexamers that are commonly referred to as either ‘connexons’ or ‘hemichannels’. Therefore, two adjacent hemi-channels from opposing plasma membranes come together to form the gap junction channel (Goodenough et al., 1996). In the nervous system, gap junction channels have been shown to facilitate neuron–neuron, glia–glia and neuron–glia communication (Rouach et al., 2002). Under normal neurological conditions, gap junction intercellular communication (GJIC) helps to maintain a homeostatic environment by facilitating spatial buffering of important cellular ions such as K+, Na+ and Ca2+ (Rose and Ransom, 1997; Mobbs et al., 1998; Venance et al., 1998; Holthoff and Witte, 2000). Consequently, changes in the expression of Cxs and, hence, disruption of the GJIC have been implicated in a number of CNS pathologies including Alzheimer’s disease, Parkinson’s disease, epilepsy and cortical spreading depression (Rouach et al., 2002).
OBJECTIVE
Data from recent studies have demonstrated that there is increased communication via gap junctions between trigeminal ganglion neurons and the surrounding satellite glia in response to acute inflammatory stimuli (Thalakoti et al., 2007; Damodaram et al., 2009) and between glia in a neuropathic pain model (Vit et al., 2006, 2008; Ohara et al., 2008). The goal of this study was to investigate temporal and spatial changes in Cx expression in both trigeminal ganglion neurons and satellite glial cells in a chronic as well as acute model of TMJ inflammation. Initially, we used quantitative PCR (qPCR) to determine which Cx mRNA are expressed in trigeminal ganglion under basal conditions. We then utilized immunohistochemistry to investigate the spatial and temporal changes in the expression of Cxs in neuronal and glial cells within trigeminal ganglia in response to a stimulus known to cause either chronic or acute inflammation in the TMJ.
METHODS
Animal subjects
The animal studies were approved by the Institutional Animal Care and Use Committee at Missouri State University in accordance with the guidelines established in the Animal Welfare Act and National Institutes of Health. Every effort was made to minimize animal suffering and to reduce the number of animals used in our study. Adult male Sprague–Dawley rats (250–300 g) were housed in structurally sound, clean plastic cages on a 12-h light/dark cycle and with unrestricted access to food and water for the duration of the experiment.
Quantitative PCR
In initial experiments, trigeminal ganglia were removed from unstimulated control animals (N = 5) and total RNA isolated using the SV total RNA isolation system (Promega, Madison, WI, USA) following the manufacturer’s instructions. The quality and quantity of the RNA were assessed by spectrophotometry and electrophoresis in a formaldehyde-agarose gel. The O.D.260 and O.D.280 were determined for each RNA sample using a Spectra Max Plus plate reader (Molecular Devices, Sunnyvale, CA, USA). An O.D.260/O.D.280 ratio of 1.7–2.1 was within an acceptable range. Following electrophoresis, RNA samples were visualized using a Kodak Image Station and Kodak Molecular Imaging Software (Rochester, NY, USA). Only RNA samples that had sharp 28 s and 18 s rRNA bands with the 28 s rRNA appearing approximately twice as intense as the 18 s rRNA band were used for further analysis. cDNA was generated from RNA samples (100 ng) using a high capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s protocol and a Mx3005P thermocycler (Stratagene, La Jolla, CA, USA) programmed for 10 min at 25°C followed by 120 min at 37°C and 5 s at 85°C. The resultant cDNA was stored at −20°C. The qPCR was based on the TaqMan fluorogenic detection system (Taqman®, PE Applied Biosystems, USA), using commercially available fluorogenic oligonucleotide probes designed to hybridize to the specific target sequence of Cxs 26 (Rn02376786_s1), 36 (Rn00439121_m1), 40 (Rn00570632_m1) and 43 (Rn01433957_m1) (Applied Biosystems). As a positive control, commercial primers designed to amplify glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used (Applied Biosystems). The thermocycler was programmed as follows: pre-denaturation at 95°C for 10 min followed by 40 cycles of denaturation for 15 s at 95°C and annealing/extending for 1 min at 60°C. Following the annealing/extending cycle, the cycle threshold values (Ct) were determined by the Mx Pro software (Stratagene). The data are reported as average Ct value ± SEM as well as reported as ΔCt values normalized to GAPDH levels for five independent experiments performed in duplicate.
Chronic and acute inflammation models
Rats were anesthetized by injecting 0.3 ml of a mixture of ketamine (45 mg/kg) and xylazine (5 mg/kg) solution intra-peritonealy (i.p.). Rats were observed and assessed for effects of anesthesia using a writhing reflex and tonicity of the tail (tail flick reflex). Bilateral injections into the TMJ were performed using a 50 μl Hamilton syringe (Hamilton Company, Reno, NV, USA) and a 261/2 G needle (Becton Dickinson, Franklin Lakes, NJ, USA). Young adult male rats were left untreated (control), injected bilaterally with 50 μl of complete Freund’s adjuvant (1:1 CFA/saline; Sigma-Aldrich, St. Louis, MO, USA), 25 μl of capsaicin (10 μM in 100% DMSO; Sigma-Aldrich) or corresponding vehicle into each TMJ. Prior to injection, a 1:1 CFA/physiological saline emulsion was formed by sonication (3×, each for 3 s). For the chronic inflammation model, rats were sacrificed and ganglia collected 3, 5 or 7 days following bilateral CFA injections. For the acute inflammation model, animals were sacrificed and ganglia collected 15 min, 30 min, 1, 2 or 24 h following capsaicin stimulation.
Trigeminal tissue isolation and mounting
Trigeminal ganglia were removed from control rats as well as treated animals after CO2 asphyxiation. Ganglia were mounted in Neg-50 Frozen Section Medium (Richard Allan Scientific, Kalamazoo, MI, USA), quickly frozen and stored at −25°C. Dorsal to ventral 20-μm serial longitudinal sections of the entire ganglion were prepared using a cryostat (Microm HM 525, Richard Allan Scientific). On average, five individual ganglion sections, which included one control and several experimental conditions, were mounted on Superfrost Plus microscope slides (Fischer Scientific, Pittsburgh, PA, USA).
Immunohistochemistry
For immunohistochemical studies, 20-μm cryostat sections of control and treated ganglia were incubated in 4% paraformal-dehyde for 60 min to fix the tissue. The tissue was then permeabilized with 0.3% triton X-100 in PBS for 60 min. Fixed and permeabilized tissues were then incubated in PBS containing 5% donkey serum for 60 min to reduce non-specific binding of antibodies. The tissue sections were incubated overnight at 4°C with rabbit polyclonal antibodies directed against Cx26 (diluted 1:500 in PBS, Millipore, Billerica, MA, USA), Cx40 (diluted 1:100 in PBS, Millipore), Kir 4.1 (diluted 1:1000 in PBS, Alomone Labs, Jerusalem, Israel) or goat polyclonal antibodies against Cx36 (diluted 1:100 in PBS, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and Cx43 (diluted 1:100 in PBS, Santa Cruz). Sections were then incubated for 1 h at room temperature in Rhodamine red X-conjugated donkey anti-rabbit or anti-goat IgG antibodies (diluted 1:100 in PBS, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) to detect immuno-reactive proteins at 555 nm using UV-fluorescence microscopy. Sections were mounted using Vectashield medium (H-1200) containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA) and viewed at wavelength 350 nm to identify neuronal and glial cell nuclei. Images (40×, 100× and 400×) were collected using an Olympus DP70 camera mounted on an Olympus BX41 fluorescent microscope.
Quantification of staining intensity
Each captured image was converted to grayscale prior to analysis. Only slides with similar levels of background fluorescent intensities were used. A total of six images from each experimental condition were used to determine the intensity level of Cx staining. Initially, the average gray value was measured in three regions of interest in the mandibular or V3 region that contained clearly delineated bands of neurons and satellite glia. This average was normalized to the average gray value in three regions of interest that contained only glial Schwann cells and fibers, which was used as a background intensity level of staining since no Cx staining was observed in these regions. The data are reported as a ratio of average grayscale intensity of neurons and satellite glial cells versus Schwann cells ± SEM. Each experimental condition was repeated in at least three independent experiments performed in duplicate in which laboratory personnel were blinded to the experimental design.
Statistical analysis
Statistical analysis was performed using the parametric two-sample t-test. Differences were considered statistically significant at P < 0.05. All statistical tests were performed using Minitab Statistical Software, Release 14.
RESULTS
Relative Cx mRNA levels detected using qPCR
Initially, to determine which Cxs might be expressed in the trigeminal ganglia under basal conditions, total RNA was extracted from trigeminal ganglia obtained from untreated control rats and used for analysis by qPCR. Commercially available oligonucleotide probes for Cxs 26, 36, 40 and 43 as well as GAPDH were used to determine mRNA levels. The data are reported as cycle threshold value (Ct), which represents the time at which fluorescence intensity is greater than background fluorescence and as ΔCt values, which represents the relative change when normalized to GAPDH levels. GAPDH was included in each experimental qPCR and served as an abundant standard control value (Ct = 20.5 ± 0.92). Cx43 was found most abundantly in the control ganglia and had a Ct value of 28.5 ± 0.44 and a ΔCt value of 8.0 (Table 1). A similar Ct value was found for Cx26, which had a Ct value of 28.8 ± 1.19 and a ΔCt value of 8.3. The mRNA levels for the other Cxs were less abundant than those for Cxs 43 and 26. For Cx40, the Ct value was 32.5 ± 0.38 with a ΔCt value of 12.0, while for Cx36, the Ct value was 32.6 ± 0.54 with a ΔCt value of 12.1. These data provide evidence that mRNA for several Cxs are expressed in trigeminal ganglion obtained from unstimulated control animals.
Table 1.
Relative connexin mRNA levels detected under basal conditions using quantitative PCR. Average cycle threshold (Ct) values ± SEM for Cxs 26, 36, 40 and 43 normalized to levels of GAPDH are reported ΔCt.
| Avg. Ct value | ΔCt | |
|---|---|---|
| Cx26 | 28.8 ± 1.19 | 8.3 |
| Cx36 | 32.6 ± 0.54 | 12.1 |
| Cx40 | 32.5 ± 0.38 | 12.0 |
| Cx43 | 28.5 ± 0.44 | 8.0 |
| GAPDH | 20.5 ± 0.92 | — |
Expression of Cxs: 26, 36 and 40 in trigeminal ganglion in response to CFA
Based on the qPCR data, antibodies directed against the four Cxs were used to determine their cellular expression in trigeminal ganglia obtained from untreated animals as well as animals injected with agents known to cause chronic or acute joint inflammation. The chronic inflammation model used in our studies is a well-established model for studying signaling pathways involved in mediating inflammation and nociception in the TMJ (Suzuki et al., 2007; Thut et al., 2007; Wang et al., 2008; Xu et al., 2008). To create a chronic inflammatory state, 50 μL of a saline emulsion of CFA, which contains heat-killed mycobacteria, was injected directly into each TMJ capsule. Animals were sacrificed 3, 5 and 7 days following CFA injections. To verify the exact location of the CFA injections into the capsule and the amount of inflammation within the TMJ, the joint was routinely exposed before the trigeminal ganglion was dissected. As expected, a large amount of pus was observed in the CFA-injected TMJ capsule 3, 5 and 7 days after injection (data not shown). However, no pus or other signs of inflammation were observed in the capsules of untreated animals or 3, 5 or 7 days after injection of vehicle (data not shown).
Cx26
Cx26 levels were determined in trigeminal ganglia by immunohistochemistry 3, 5 and 7 days after CFA injection. Each ganglion tissue section was costained with the fluorescent dye DAPI, which stains nuclear DNA, to facilitate identification of neuronal cells (large round nucleus) and satellite glial cells (smaller, elliptical nuclei). In ganglia obtained from control, untreated animals, faint Cx26 staining was observed in the neuron–satellite glia clusters (Fig. 1A–C). In contrast, elevated levels of Cx26 were readily detected in both neurons and the surrounding satellite glial cells in the V3 region of the ganglia at each time point following CFA stimulation. The intensity of Cx26 immunostaining was significantly increased (P < 0.01) at day 3 and the staining intensity remained significantly higher than control levels on days 5 and 7. Similarly, the level of Cx26 was increased in neuron–satellite glia clusters located in the V2 and V1 regions of the ganglia in response to CFA at all three time points when compared to control levels (data not shown). However, the intensity of Cx26 staining in response to vehicle injections was similar to that observed for the untreated animals (data not shown). As another control, some sections were incubated with only secondary antibodies and immunohistochemistry performed on entire trigeminal ganglion sections. No detectable staining was observed in neuronal or glial cells in the absence of the Cx26 primary antibodies although the nuclei of these cells were clearly visible with DAPI staining of the same sections (data not shown).
Fig. 1. Expression of Cx26 in trigeminal ganglion neurons and satellite glia is increased in response to CFA.
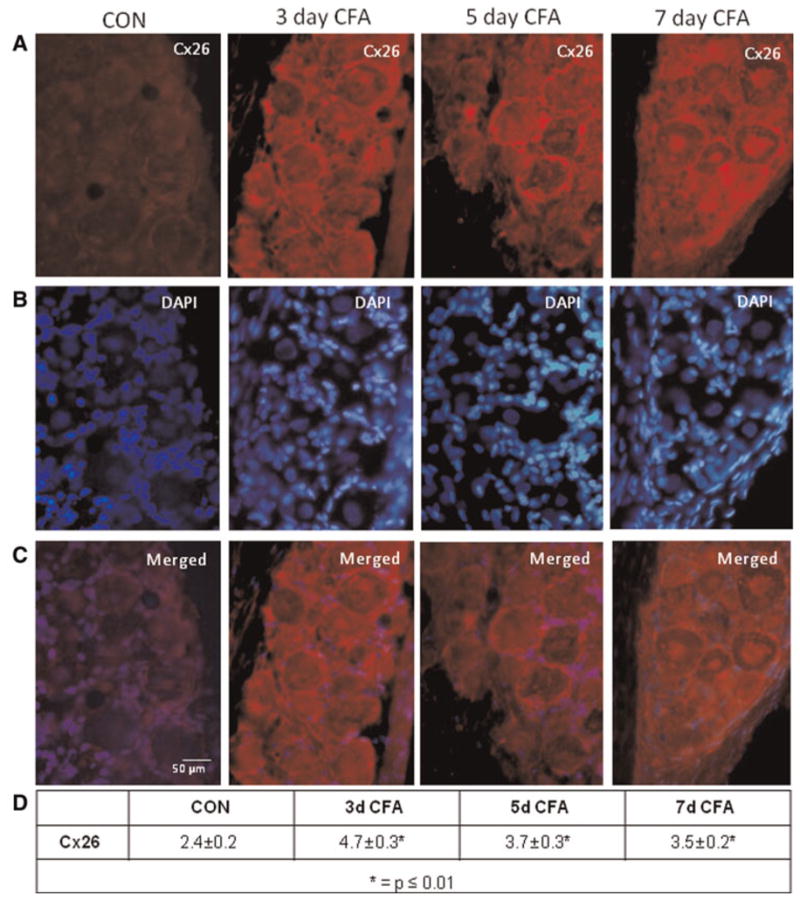
(A) Images (400×) of a representative area in the V3 region of ganglia obtained from control animals (CON) as well as 3, 5 and 7 days after CFA injection in the TMJ capsule are shown. (B) The same tissue sections seen in panel A were costained with the nuclear dye DAPI. (C) A merged image of panels A and B is shown. (D) The average relative staining levels for Cx26 are reported. The normalized values, which are the ratio of the staining intensity of neuron and satellite glial cells to the intensity of Schwann cells (non-staining regions) in six independent fields, are reported as the average staining intensity ± SEM (n = 3 independent experiments).
Cx36
In untreated control ganglia, a low level of Cx36 was observed within the neuron–glia clusters in the V3 region (Fig. 2). However, the level of Cx36 staining was significantly increased 3, 5 and 7 days following CFA injection (P < 0.01) in the TMJ capsule when compared to control levels. In contrast to the staining pattern observed for Cx26, Cx36 staining was observed primarily in neuronal cell bodies, but not satellite glial cells in response to CFA. This pattern and increased level of Cx36 staining were also observed in the V2 and V1 regions of the ganglia in response to CFA at all three time points when compared to control levels (data not shown). No detectable staining was observed in neuronal or glial cells in the absence of the Cx36 primary antibodies in ganglia from control or CFA-injected animals (data not shown).
Fig. 2. Expression of Cx36 in trigeminal ganglion neurons is increased in response to CFA.
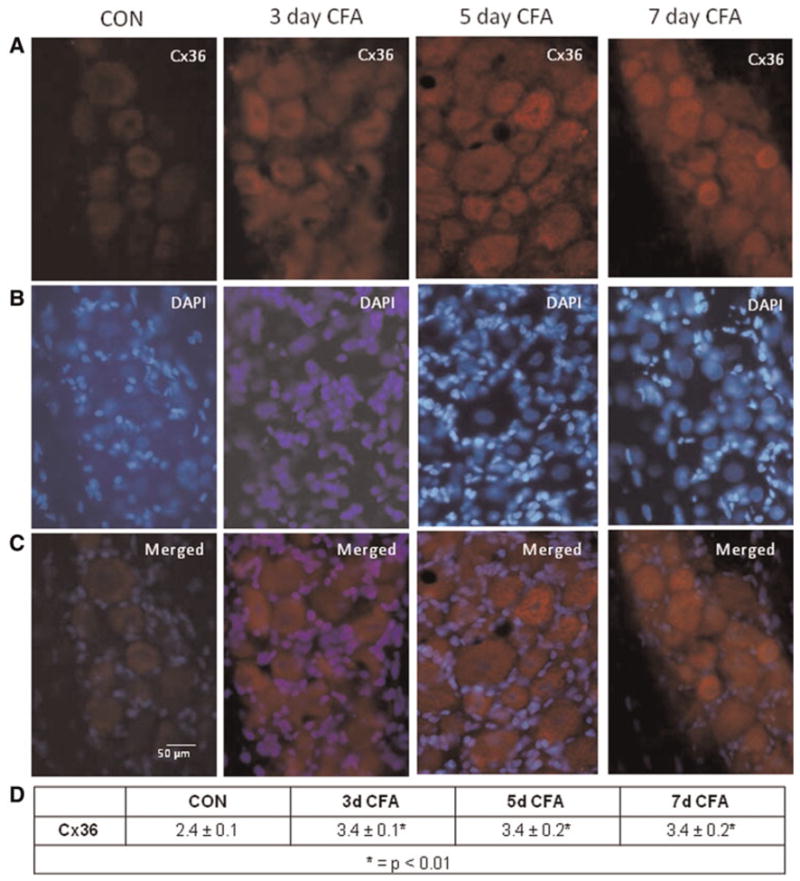
(A) Images (400×) of a representative area in the V3 region of ganglia obtained from control animals (CON) as well as 3, 5 and 7 days after CFA injection in the TMJ capsule are shown. (B) The same tissue sections shown in panel A were costained with the nuclear dye DAPI. (C) A merged image of panels A and B is shown. (D) The average relative staining levels for Cx36 are reported. The normalized values are reported as the average staining intensity ± SEM (n = 3).
Cx40
The overall staining pattern observed for Cx40 expression in trigeminal ganglia was similar to that seen for Cx36. Again, a low level of Cx40 was detected in neuron–satellite glia clusters in the V3 region of the ganglia (Fig. 3). At each time point, 3, 5 and 7 days following CFA injection, the intensity of Cx40 staining in neuronal cells was significantly elevated (P < 0.01) compared to control levels. The intensity and staining pattern for Cx40 expression observed in the V2 and V1 regions of trigeminal ganglia in response to CFA injections were similar to that observed in the V3 region (data not shown). No detectable staining was observed in neuronal or glial cells in the absence of the Cx40 primary antibodies in any of the ganglia from control or CFA animals (data not shown).
Fig. 3. Expression of Cx40 in trigeminal ganglion neurons is increased in response to CFA.
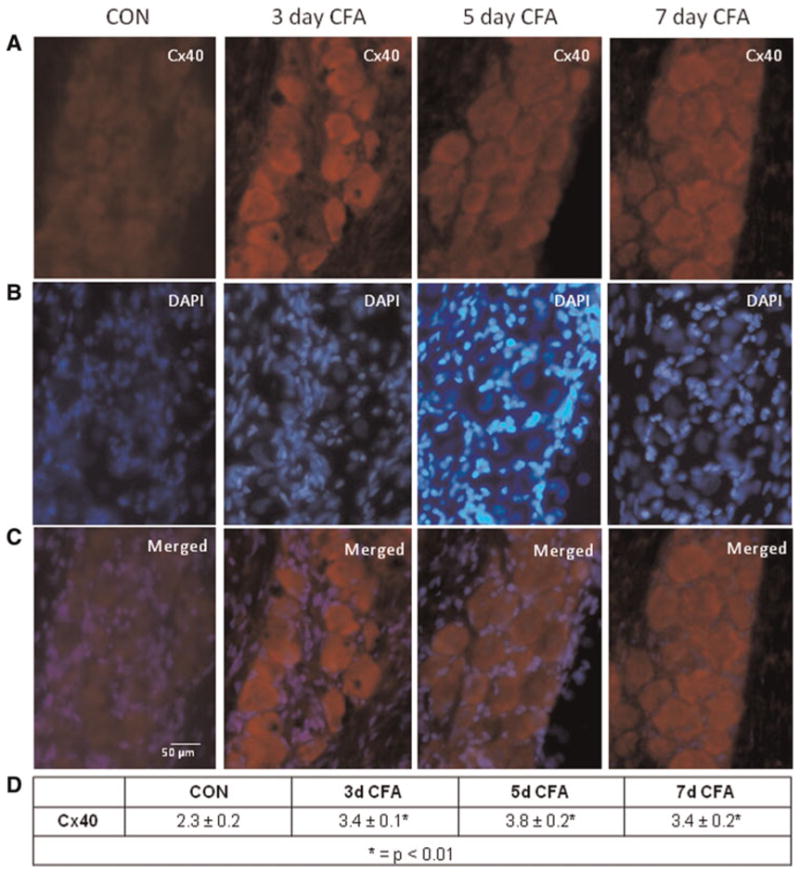
(A) Images (400×) of a representative area in the V3 region of ganglia obtained from control animals (CON) as well as 3, 5 and 7 days after CFA injection in the TMJ capsule are shown. (B) The same tissue sections seen in panel A were costained with the nuclear dye DAPI. (C) A merged image of panels A and B is shown. (D) The average relative staining levels for Cx40 are reported. The normalized values are reported as the average staining intensity ± SEM (n = 3).
At a higher magnification, the cellular localization of the Cxs within a single functional unit, which consists of a single neuronal cell body surrounded by many satellite glial cells, is more readily discerned. As seen in Fig. 4, intense Cx26 staining was observed in the periphery of a neuronal cell body and most surrounding satellite glial cells in ganglia 5 days after CFA injection. In contrast, increased Cx36 and Cx40 expression was primarily observed in trigeminal ganglion neuronal cell bodies. Interestingly, evidence of intense peripheral staining in neurons and satellite glial cells was not seen with Cx36 or Cx40. Rather, the expression of Cx36 and Cx40 appears to be increased in the cytosol of mostly neuronal cells. To aid in the identification of satellite glial cells, some sections were costained with antibodies directed against Kir 4.1. Staining for Kir 4.1 was only observed in the cytosol of satellite glial cells.
Fig. 4. Localization of Cxs26, 36 and 40 within a functional unit in trigeminal ganglion 5 days after CFA injection in the TMJ capsule.
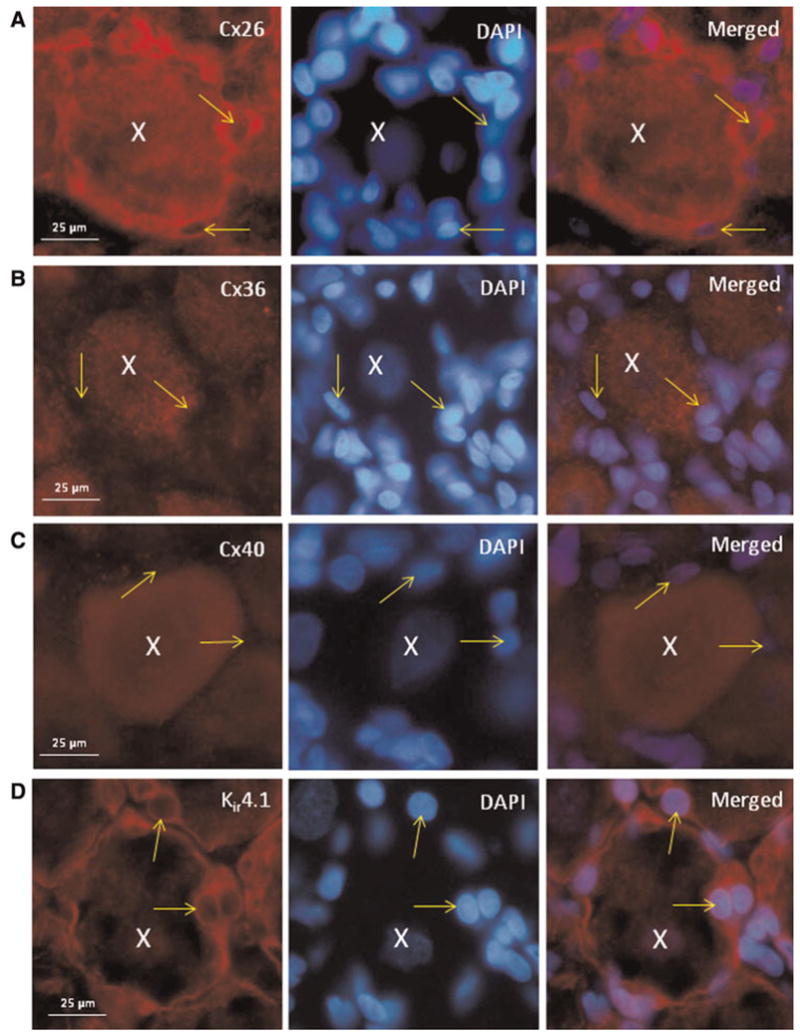
A typical functional unit contains a neuronal cell body (nucleus identified by an X) surrounded by satellite glial cells (nuclei identified by arrows) is shown in each image. (A) Staining for Cx26 is shown in the left panel, while staining of the same image is shown in the middle panel and a merged image shown in the right panel. Increased Cx26 expression in both neurons and satellite glial cells is seen. In contrast, Cx36 expression is localized primarily to neuronal cell bodies in trigeminal ganglion in response to CFA (B). Similar to Cx36, the expression of Cx40 is primarily localized to neuronal cell bodies (C). Some sections were incubated with antibodies against the satellite glial cell marker Kir 4.1 and costained with DAPI (D).
Cxs 26, 36 and 40 expression in trigeminal ganglia in response to capsaicin
To determine whether an acute inflammatory stimulus injected into the TMJ would result in a different temporal or spatial response in the rat trigeminal ganglion, capsaicin was injected bilaterally in the TMJ capsules. Levels of Cxs were determined by immunohistochemistry 15 min, 30 min, 1, 2 and 24 h after injection. While the level of expression for Cx26, Cx36 and Cx40 was low in the control tissues, the intensity of immunostaining for each of these Cxs was significantly increased (P < 0.01) within 15 min after capsaicin injection and remained significantly elevated 30, 60 and 120 min post-injection (Fig. 5). The cellular pattern of staining for each Cx was similar to that observed following CFA injections. For example, increased Cx26 expression was seen in both neurons and satellite glial cells, and in some instances was localized to regions between neuronal cell bodies and satellite glial cells. In contrast, expression of Cx36 and Cx40 was primarily observed in neuronal cells. A somewhat surprising finding was that the levels of Cx26, Cx36 and Cx40 all returned to basal levels within 24 h following capsaicin injection. Thus, our results provide evidence that Cxs are transiently upregulated in response to an acute inflammatory stimulus (capsaicin) but are more stably expressed in response to a chronic inflammatory stimulus (CFA).
Fig. 5. Expression of Cxs26, 36 and 40 in trigeminal ganglion is transiently increased in response to capsaicin (CAP).
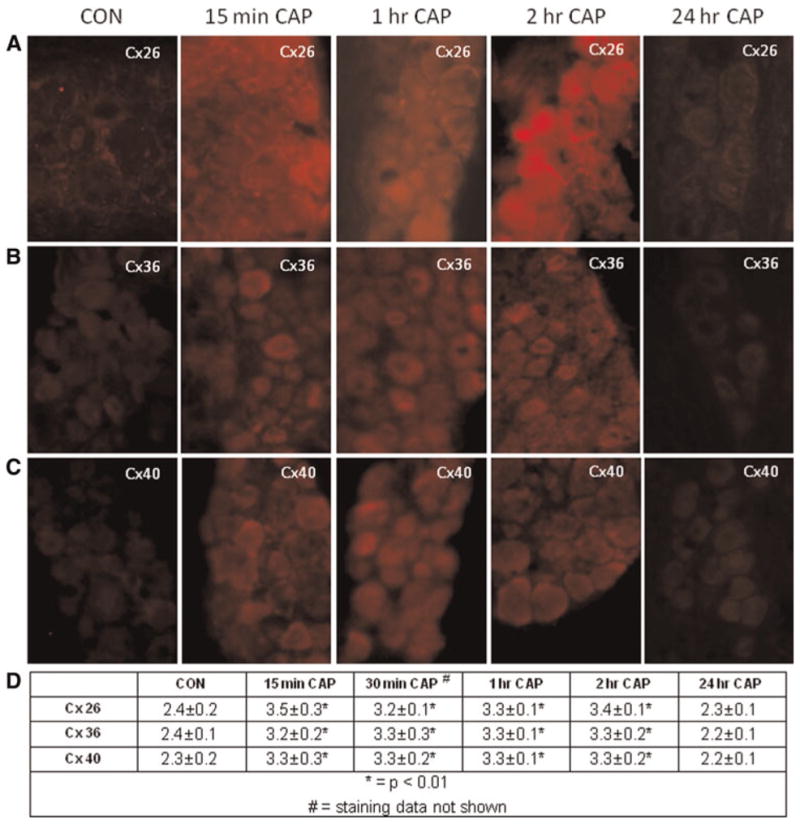
Images (400×) of a representative area in the V3 region of ganglia are shown. Staining for Cx26 (A), Cx36 (B) and Cx40 (C) from ganglia obtained from control animals (CON) as well 15 min, 1, 2 and 24 h following CAP injection into TMJ capsule are shown. (D) The average relative staining levels for Cx26, Cx36 and Cx40 are reported. The normalized values are reported as the average staining intensity ± SEM (n = 3).
Cx43 expression in response to CFA and capsaicin
It has previously been reported that Cx43 expression in trigeminal ganglia increases in a neuropathic pain model (Vit et al., 2006, 2008; Ohara et al., 2008). However, it is not known if Cx43 expression in trigeminal ganglia would be changed in response to either an acute or chronic inflammatory stimulus. As seen in Fig. 6, Cx43 was expressed at barely detectable levels in neurons and was expressed at slightly higher levels in satellite glial cells in ganglia from control animals. However, the intensity and cellular pattern of Cx43 staining did not change in response to CFA (day 3) or capsaicin (2 h) injection in the TMJ. This finding is in stark contrast to the response seen following acute or chronic inflammation of the TMJ. Taken together, it appears that regulation of Cx43 expression in trigeminal ganglion neurons and satellite glial cells is dependent on the type of stimulus, inflammatory versus neuropathic, that causes trigeminal neuron activation.
Fig. 6. Expression of Cx43 in trigeminal ganglion is not increased in response to CFA or capsaicin.
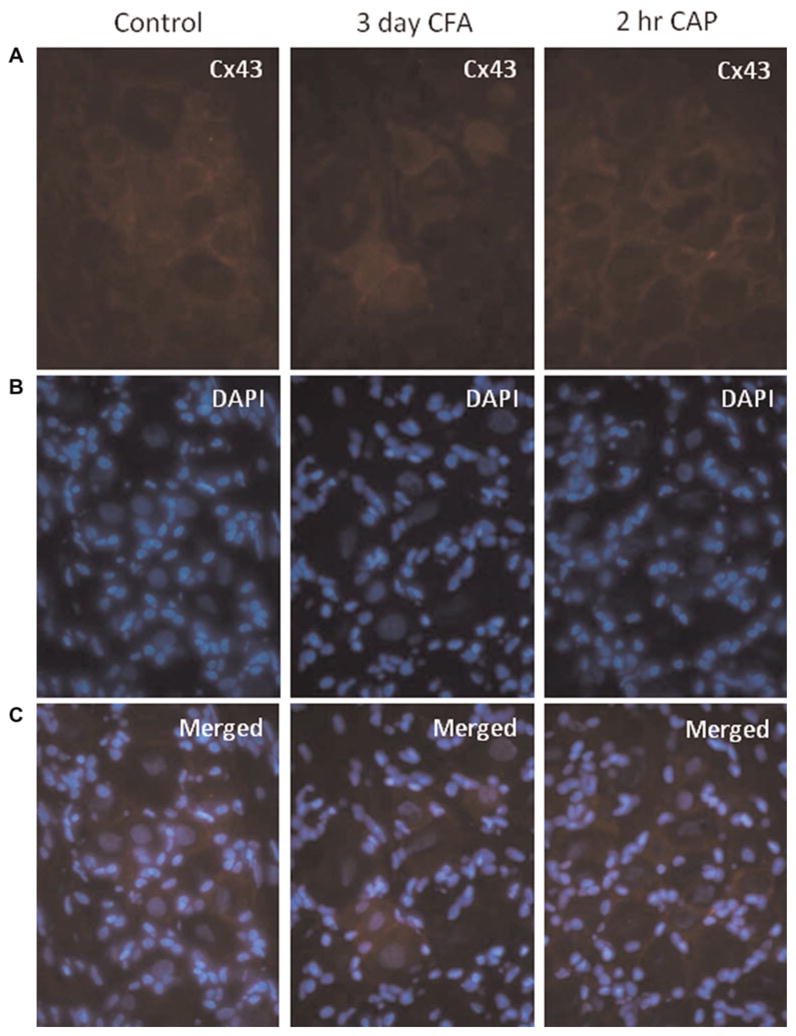
(A) Images (400×) of Cx43 expression in a representative area in the V3 region of ganglia obtained from control animals (CON) as well as 3 days after CFA injection or 2 h after capsaicin (CAP) injection in the TMJ capsule are shown. (B) The same tissue sections shown in panel A were costained with the nuclear dye DAPI. (C) A merged image of panels A and B is shown.
CONCLUSIONS
Under basal conditions, mRNA for Cxs 26, 36, 40 and 43 were detected in trigeminal ganglia.
In response to chronic joint inflammation following CFA injection, a sustained elevation in the level of Cx26 expression was observed in both neurons and satellite glial cells.
The levels of Cx36 and Cx40 were also significantly elevated in a sustained manner in response to CFA, but the increase was primarily observed in the neuronal cells and, thus, may function as hemichannels.
The expression of Cx26, Cx36 and Cx40 was transiently increased in a similar cellular pattern to that observed for CFA in response to acute TMJ inflammation following capsaicin injection.
Cx43 expression was not increased in either trigeminal ganglion neurons or satellite glial cells in response to acute or chronic inflammation of the TMJ.
DISCUSSION
In this study, chronic and acute inflammation of the TMJ was used to investigate changes in the spatial and temporal expression of several Cxs in trigeminal ganglia when compared to untreated control ganglia. In our initial studies, qPCR and immunohistochemistry were used to determine which Cxs were expressed in trigeminal ganglion under basal conditions. Detectable levels of mRNA and protein for Cxs 26, 36, 40 and 43 were present in trigeminal ganglia isolated from untreated animals. Our findings are similar to the reported cellular expression of these particular Cxs in the CNS. For example, the expression of Cx26 has been demonstrated in neurons and astrocytes, a type of glial cell found in association with neuronal cells within the CNS (Venance et al., 2000; Nagy et al., 2001). In addition, Cx36 expression was reported to be restricted to CNS neurons (Condorelli, 1998; Rash, 2000), while Cx40 expression has previously been reported in neurons in the CNS under basal conditions (Chang et al., 1999; Dermietzel et al., 2000). Our finding that Cx43 is expressed in trigeminal ganglia is also in agreement with previous studies (Vit et al., 2006, 2008; Ohara et al., 2008). However, to our knowledge, this is the first evidence of identification of Cxs 26, 36 and 40 protein expression in trigeminal ganglion obtained from untreated animals. Furthermore, this is the first study to demonstrate increased Cx expression in neurons and satellite glial cells within the trigeminal ganglion under chronic and acute inflammatory conditions of the TMJ.
A novel finding of our study was that the duration of Cx expression in trigeminal ganglion neurons and satellite glial cells temporally correlated with the type of inflammatory stimulus injected into the TMJ capsule. For example, when CFA, which is known to cause a strong and long-lasting inflammatory response in the joint (Suzuki et al., 2007; Thut et al., 2007; Wang et al., 2008; Xu et al., 2008), was injected into the TMJ, we observed a sustained increase in the expression of Cx26 in neurons and satellite glial cells as well as sustained Cx36 and Cx40 expression in neurons when compared to basal levels. These findings are somewhat surprising since typically Cxs are reported to exhibit a short half-life in the range of several hours (Laird, 2006). We are not aware of any study that has provided evidence of stable Cx expression in the trigeminal ganglia or other neuronal tissue over several days. A sustained increase in Cx26 expression in both neurons and satellite glial cells that result in the formation of gap junction plaques may allow molecules such as cAMP, glutamate, as well as K+, Na+ and Ca2+ ions to pass directly between these cells in the ganglia for a prolonged period of time. Importantly, increased neuronal–glial cell signaling via gap junctions has been documented in CNS inflammatory diseases and is considered an important factor in the underlying pathology by increasing neuronal excitability (Nakase and Naus, 2004). Thus, it is likely that increased neuron–satellite glia gap junction communication may lead to increased neuronal excitability and a lower activation threshold. Similarly, given that Cx36 and Cx40 expression was increased primarily in neurons and the fact that these Cx are known to form hemichannels (Schock et al., 2008; Toma et al., 2008), their increased expression in response to inflammatory stimuli could result in enhanced autocrine and paracrine signaling within the ganglion that is likely to increase the excitability state of the neurons and satellite glial cells. In support of this notion, hemichannels have been found to act through paracrine signaling by releasing molecules such as ATP, NAD+, glutamate and prostaglandins, which can modulate neuronal and glial cell activity (Goodenough et al., 1996; Bruzzone, 2001; Bennett, 2003; Ebihara, 2003; Ye, 2003; Cheriann et al., 2005).
In contrast to the sustained increase in Cx expression observed in response to CFA, injection of capsaicin caused a transient elevation in Cxs 26, 36 and 40 levels. Capsaicin binds to the transient receptor potential vanilloid subfamily member 1 (TRPV1) receptor (Caterina et al., 1997), which are expressed by neurons found in all regions of the trigeminal ganglion (Thalakoti et al., 2007). Binding to the TRPV1 receptor causes excitation of sensory neuronal C fibers that are involved in pain transmission. As shown in our study, the capsaicin-mediated increase in Cx expression was observed within 15 min and remained elevated for at least 2 h post-injection, but returned to basal levels within 24 h. Despite the temporal differences in response to CFA and capsaicin injections observed in our study, it is interesting that the cellular changes within the ganglia were quite similar. We found that Cx26 expression was markedly increased in both neurons and satellite glial cells in response to capsaicin. It is likely that the formation of Cx26 gap junction plaques is responsible for the increased dye coupling that we previously reported between trigeminal ganglion neurons and satellite glial cells in response to capsaicin injection into the TMJ (Thalakoti et al., 2007). In contrast to Cx26, increased Cx36 and Cx40 expression was primarily localized to neuronal cell bodies as seen following CFA injections. Thus, it appears that while spatial changes in response to either chronic or acute inflammatory stimuli are similar within trigeminal ganglia, temporal changes are dependent on the duration and type of inflammatory stimulus. Although not known, differential regulation of Cx expression may have important implications in the underlying pathology of acute versus chronic TMJ inflammation and pain.
We have provided evidence that peripheral stimulation of trigeminal neurons in response to CFA or capsaicin injection into the TMJ capsule results in increased expression of Cx26 in neurons and satellite glial cells and increased expression of Cx36 and Cx40 in trigeminal neurons. Based on previous studies (Thalakoti et al., 2007; Damodaram et al., 2009), the changes in Cx expression seen in our study likely facilitate direct communication of neurons and satellite glial cells via gap junctions and possibly enhanced autocrine and paracrine signaling in the ganglion that contributes to increased neuronal and glial excitability. Of relevance to TMJ pathology, an increase in neuronal excitability is characteristic of both peripheral sensitization, which occurs in response to an acute inflammatory stimulus, and priming, which is thought to be involved in long-term changes in the excitability state of sensory nociceptive neurons (Hucho and Levine, 2007). Peripheral sensitization, which can last from minutes to hours, is characterized by increased neuronal excitability and a lowering of the threshold stimulus for increasing gene expression, ion channel activities and release of inflammatory molecules (Dodick and Silberstein, 2006). More recently, a primed state of nociceptors has been described that is characterized by changes in nociceptors that significantly lower the concentrations of inflammatory mediators required to elicit a heightened state of pain (hyperalgesia) that can persist for several weeks (Hucho and Levine, 2007). Given the importance of glial cells in regulation of neuronal excitability and activation thresholds (Watkins and Maier, 2002; Hanani, 2005; Takeda et al., 2007), it is likely that the transient increased Cx expression observed in response to capsaicin contributes to peripheral sensitization, while the more stable expression of Cxs in response to CFA is involved in the generation and/or maintenance of the primed state. Furthermore, it is possible that the sustained increase in Cx expression may play a role in the transition from acute episodic pain in the TMJ to a more chronic pain state.
Cx43 is the most ubiquitous Cx protein and is found in most mammalian tissues (Saez et al., 2003). Although Cx43 mRNA was detected in trigeminal ganglia from untreated animals and a low level of Cx43 immunoreactivity was detected in satellite glial cells as previously reported (Vit et al., 2006; Ohara et al., 2008), we did not observe increased Cx43 expression in response to CFA or capsaicin injection into the TMJ. The lack of effect of inflammatory stimuli on Cx43 expression in the trigeminal ganglion was somewhat surprising since Cx43 expression was shown to increase in trigeminal ganglion satellite glia in response to trigeminal nerve injury (Vit et al., 2006; Ohara et al., 2008). Thus, it appears changes in Cx expression within the trigeminal ganglion are dependent on whether the trigeminal nerve was injured (neuropathic) or on whether a peripheral inflammatory stimulus causes acute or chronic excitation of trigeminal nerves. This difference in the underlying pathologies is likely to have important therapeutic implications.
In summary, we have provided the first evidence of increased expression of Cxs within trigeminal ganglion neurons and satellite glial cells following TMJ injection of CFA or capsaicin, agents known to promote joint inflammation and pain (Carleson et al., 1997; Spears et al., 1998; Suzuki et al., 2007; Thut et al., 2007; Wang et al., 2008; Xu et al., 2008). While increased Cx26 expression was observed in both neurons and satellite glia and, thus, may form gap junctions, increased expression of Cx36 and Cx40 was primarily localized to only neuronal cells where they may function in the formation of hemichannels. Furthermore, we found that while Cx26, Cx36 and Cx40 expression was transiently increased in response to capsaicin, their level of cellular expression was sustained for at least 7 days after injection of CFA. In conclusion, we propose that increased neuron–glia communication within trigeminal ganglia mediated by Cxs is involved in regulating the response to acute or chronic stimulation of trigeminal neurons and, thus, plays an important role in TMJ pathology.
Acknowledgments
We would like to thank Jing Li for her technical assistance. This work was supported by research grants from NIH (DE017805) and Minster Pharmaceuticals plc.
References
- Beardslee M, Laing J, Beyer E, Saffitz J. Rapid turnover of connexin43 in the adult rat heart. Circulation Research. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- Bennett M, Contreras J, Bukauskas F, Sáez J. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends in Neuroscience. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E, Davis L, Saffitz J, Veenstra R. Cardiac intercellular communication: consequences of connexin distribution and diversity. Brazilian Journal of Medical and Biological Research. 1995;4:415–425. [PubMed] [Google Scholar]
- Bruzzone S, Guida L, Zocchi E, Franco L, DeFlora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB Journal. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- Bukauskas F, Jordan K, Bukauskiene A, Bennett M, Lampe P, Laird D, et al. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proceedings of the National Academy of Sciences. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleson J, Kogner P, Bileviciute I, Theodorsson E, Appelgren A, Appelgren B, et al. Effects of capsaicin in temporomandibular joint arthritis in rats. Archives of Oral Biology. 1997;42:869–876. doi: 10.1016/s0003-9969(97)00005-8. [DOI] [PubMed] [Google Scholar]
- Carlsson G, LeResche L. Epidemiology of temporomandibular disorders. In: Sessle B, Bryant P, Dionne R, editors. Temporomandibular disorders and related pain conditions. Progress in pain research and management. IASP Press; 1995. pp. 211–226. [Google Scholar]
- Caterina M, Schumacher M, Tominaga M, Rosen T, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gonzales M, Pinter M, Balice-Gordon R. Gap junctional coupling and petterns of connexin expression among neonatal rat lumbar spinal motor neurons. Journal of Neuroscience. 1999;19:10813–10828. doi: 10.1523/JNEUROSCI.19-24-10813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriann P, Siller-Jackson A, Gu S, Wang X, Bonewald L, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Molecular Biology of the Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli D, Parenti R, Spinella F, Trovato S, Belluardo N, Cardile V, et al. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. European Journal of Neuroscience. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- Damodaram S, Thalakoti S, Freeman SE, Garrett FG, Durham PL. Tonabersat inhibits trigeminal ganglion neuronal–satellite glial cell signaling. Headache. 2009;49:5–20. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, et al. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Research Brain Research Reviews. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46:S182–S191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Ebihara L. New roles for connexons. News in Physiological Sciences. 2003;18:100–103. doi: 10.1152/nips.01431.2002. [DOI] [PubMed] [Google Scholar]
- Fallon R, Goodenough D. Five-hour half-life of mouse liver gap-junction protein. Journal of Cell Biology. 1981;90:521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nature Cell Biology. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- Goldberg G, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. Journal of Biological Chemistry. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Goodenough D, Goliger J, Paul D. Connexins, connexons, and intercellular communication. Annual Review of Biochemistry. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Research Reviews. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Haydon P. Glia: listening and talking to the synapse. Nature. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Witte O. Directed spatial potassium redistribution in rat neocortex. Glia. 2000;29:288–292. doi: 10.1002/(sici)1098-1136(20000201)29:3<288::aid-glia10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Isong U, Gansky S, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. Journal of Orofacial Pain. 2008;22:317–322. [PMC free article] [PubMed] [Google Scholar]
- Laird D. Life cycle of connexins in health and disease. Biochemical Journal. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton J, Ship J, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. Journal of the American Dental Association. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- Mobbs P, Brew H, Attwell D. A quantitative analysis of glial cell coupling in the retina of the axolotl (Ambystoma mexicanum) Brain Research. 1998;460:235–245. doi: 10.1016/0006-8993(88)90368-x. [DOI] [PubMed] [Google Scholar]
- Nagy J, Li X, Rempel J, Stelmack G, Patel D, Staines W, et al. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. Journal of Comparative Neurology. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- Nakase T, Naus C. Gap junctions and neurological disorders of the central nervous system. Biochimica et Biophysica Acta. 2004;1662:149–158. doi: 10.1016/j.bbamem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Ohara P, Vit J, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. Journal of Neurophysiology. 2008;100:3064–3073. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash J, Staines W, Yasunura T, Patel D, Furman C, Stelmack G, et al. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proceedings of the National Academy of Sciences of the USA. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C, Ransom B. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia. 1997;20:299–307. doi: 10.1002/(sici)1098-1136(199708)20:4<299::aid-glia3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biology of the Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Saez J, Berthoud V, Branes M, Martinez A, Byer E. Plasma membrane channels formed by connexins: their regulation and function. Physiological Reviews. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Schock S, Leblanc D, Hakim A, Thompson C. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochemical and Biophysical Research Communications. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- Sessle B. The neurobiology of facial and dental pain: present knowledge, future directions. Journal of Dental Research. 1987;66:962–981. doi: 10.1177/00220345870660052201. [DOI] [PubMed] [Google Scholar]
- Shankland W. The trigeminal nerve. Part I: An over-view. Cranio. 2000;18:238–248. doi: 10.1080/08869634.2000.11746137. [DOI] [PubMed] [Google Scholar]
- Spears R, Hutchins B, Hinton R. Capsaicin application to the temporomandibular joint alters calcitonin gene-related peptide levels in the trigeminal ganglion of the rat. Journal of Orofacial Pain. 1998;12:108–115. [PubMed] [Google Scholar]
- Suzuki I, Harada T, Asano M, Tsuboi Y, Kondo M, Gionhaku N, et al. Phosphorylation of ERK in trigeminal spinal nucleus neurons following passive jaw movement in rats with chronic temporomandibular joint inflammation. Journal of Orofacial Pain. 2007;21:225–231. [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, et al. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil V, Damodaram S, Vause C, Langford L, Freeman S, et al. Neuron–glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut P, Hermanstyne T, Flake N, Gold M. An operant conditioning model to assess changes in feeding behavior associated with temporomandibular joint inflammation in the rat. Journal of Orofacial Pain. 2007;21:7–18. [PubMed] [Google Scholar]
- Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2008;294:R1769–R1776. doi: 10.1152/ajpregu.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Prémont J, Glowinski J, Giaume C. Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum. Journal of Physiology. 1998;510:429–440. doi: 10.1111/j.1469-7793.1998.429bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proceedings of the National Academy of Sciences of the USA. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vit J, Jasmin L, Bhargava A, Ohara P. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biology. 2006;2:247–257. doi: 10.1017/s1740925x07000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Bhargava A, Kelley K, Jasmin L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. Journal of Neuroscience. 2008;28:4161–4171. doi: 10.1523/JNEUROSCI.5053-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lim G, Mao J, Backil S, Jianren M. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain. 2008;10:1–7. doi: 10.1016/j.pain.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L, Maier S. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiological Reviews. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Watkins L, Milligan E, Maier S. Glial activation: a driving force for pathological pain. Trends in Neurosciences. 2001a;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Watkins L, Milligan E, Maier S. Spinal cord glia: new players in pain. Pain. 2001b;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- White T, Bruzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. Journal of Bioenergetics and Biomembranes. 1996;28:339–350. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier S, Watkins L. Glial activation and pathological pain. Neurochemistry International. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Xu Q, Garraway S, Weyerbacher A, Shin S, Inturrisi C. Activation of the neuronal extracellular signal-regulated kinase 2 in the spinal cord dorsal horn is required for complete Freund’s adjuvant-induced pain hypersensitivity. Journal of Neuroscience. 2008;28:14087–14096. doi: 10.1523/JNEUROSCI.2406-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Wyeth M, Baltan-Tekkok S, Ransom B. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. Journal of Neuroscience. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


