Abstract
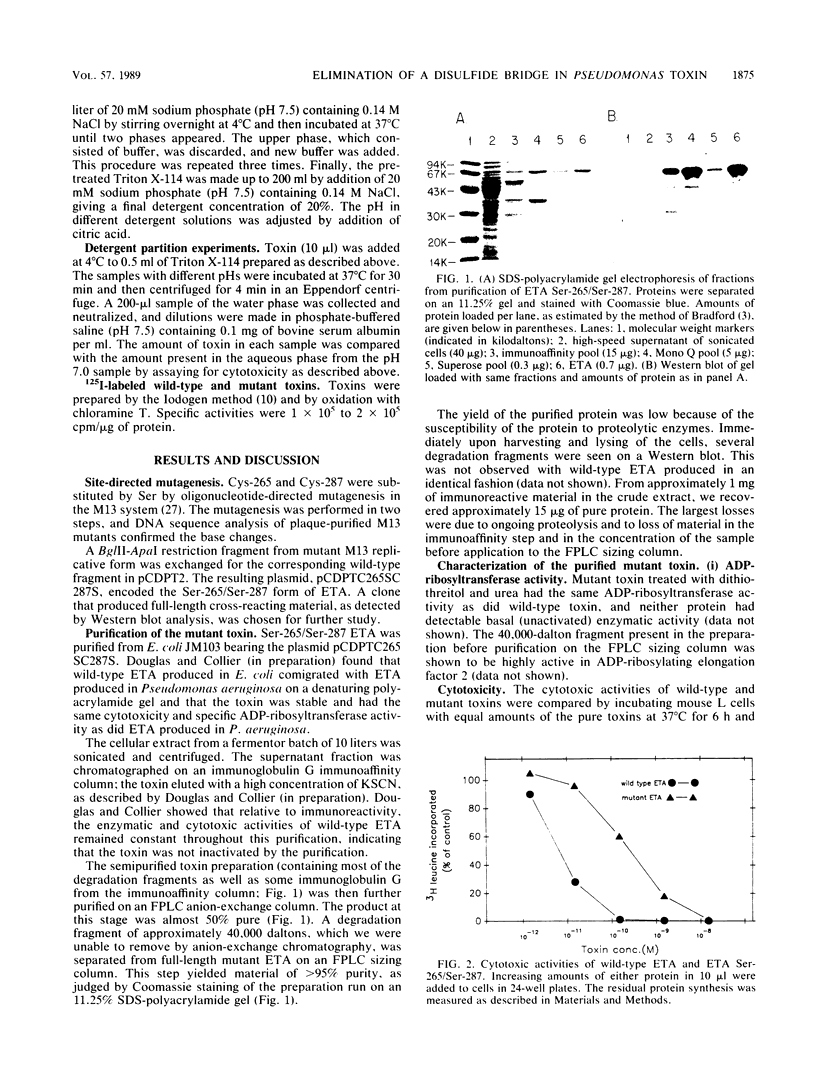
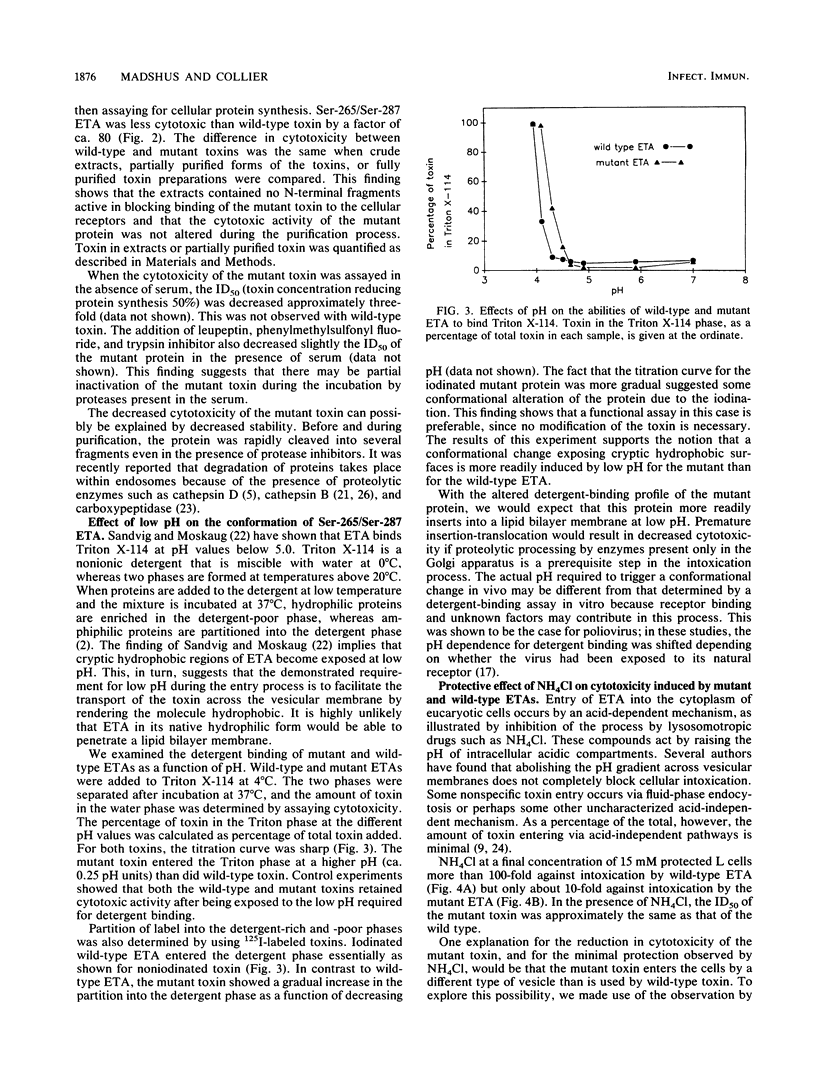
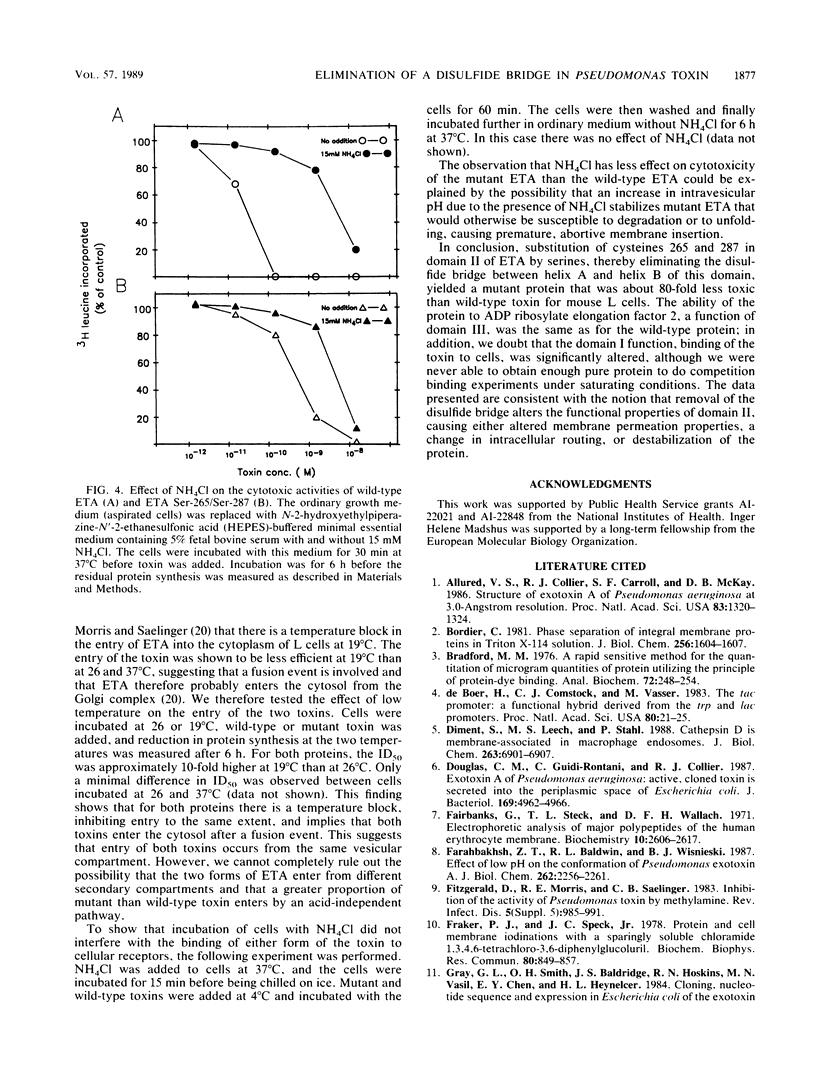
Cysteines 265 and 287 of Pseudomonas aeruginosa exotoxin A (ETA) were substituted by serine, thereby eliminating a disulfide bridge within domain II, the putative membrane insertion-translocation domain. Purified mutant toxin was 80-fold less toxic for mouse L cells than was wild-type ETA while retaining the same specific activity in the ADP-ribosyltransferase reaction as did wild-type toxin. Binding of the nonionic detergent Triton X-114 by mutant ETA occurred at a slightly higher pH than did binding by wild-type ETA, suggesting that the mutant protein more readily undergoes a conformational change exposing hydrophobic regions. Data are presented supporting the notion that the mutant and wild-type toxins enter from the same intracellular compartment. The lower cytotoxicity of the mutant protein could be due to accelerated intracellular degradation or abortive, premature membrane insertion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Douglas C. M., Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987 Nov;169(11):4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh Z. T., Baldwin R. L., Wisnieski B. J. Effect of low pH on the conformation of Pseudomonas exotoxin A. J Biol Chem. 1987 Feb 15;262(5):2256–2261. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: evidence that domain I functions in receptor binding. Mol Microbiol. 1987 Jul;1(1):67–72. doi: 10.1111/j.1365-2958.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lory S., Collier R. J. Expression of enzymic activity by exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1980 May;28(2):494–501. doi: 10.1128/iai.28.2.494-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Requirements for entry of poliovirus RNA into cells at low pH. EMBO J. 1984 Sep;3(9):1945–1950. doi: 10.1002/j.1460-2075.1984.tb02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart M. D., Morris R. E., Bonventre P. F., Leppla S., Saelinger C. B. Evidence for pseudomonas exotoxin A receptors on plasma membrane of toxin-sensitive lm fibroblasts. Infect Immun. 1984 Sep;45(3):596–603. doi: 10.1128/iai.45.3.596-603.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Saelinger C. B. Reduced temperature alters Pseudomonas exotoxin A entry into the mouse LM cell. Infect Immun. 1986 May;52(2):445–453. doi: 10.1128/iai.52.2.445-453.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M., Bowser R., Murphy R. F. Kinetics and temperature dependence of exposure of endocytosed material to proteolytic enzymes and low pH: evidence for a maturation model for the formation of lysosomes. J Cell Physiol. 1987 May;131(2):200–209. doi: 10.1002/jcp.1041310209. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Moskaug J. O. Pseudomonas toxin binds triton X-114 at low pH. Biochem J. 1987 Aug 1;245(3):899–901. doi: 10.1042/bj2450899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaudies R. P., Gorman R. M., Savage C. R., Jr, Poretz R. D. Proteolytic processing of epidermal growth factor within endosomes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):710–715. doi: 10.1016/0006-291x(87)91412-4. [DOI] [PubMed] [Google Scholar]
- Sundan A., Sandvig K., Olsnes S. Calmodulin antagonists sensitize cells to pseudomonas toxin. J Cell Physiol. 1984 Apr;119(1):15–22. doi: 10.1002/jcp.1041190104. [DOI] [PubMed] [Google Scholar]
- Tweten R. K., Collier R. J. Molecular cloning and expression of gene fragments from corynebacteriophage beta encoding enzymatically active peptides of diphtheria toxin. J Bacteriol. 1983 Nov;156(2):680–685. doi: 10.1128/jb.156.2.680-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S., VanNostrand W., McKinley D. N., Cunningham D. D. Intracellular processing of epidermal growth factor and its effect on ligand-receptor interactions. J Biol Chem. 1985 May 10;260(9):5290–5295. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]




