Abstract
Vibro-acoustography is an ultrasound-based imaging modality that uses two ultrasound beams of slightly different frequencies to produce images based on the acoustic response due to harmonic ultrasound radiation force excitation at the difference frequency between the two ultrasound frequencies. Vibro-acoustography has demonstrated feasibility and usefulness in imaging of breast and prostate tissue. However, previous studies have been performed either in controlled water tank settings or a prototype breast scanner equipped with a water tank. In order to make vibro-acoustography more accessible and relevant to clinical use, we report here on the implementation of vibro-acoustography on a General Electric Vivid 7 ultrasound scanner. In this paper, we will describe software and hardware modifications that were performed to make vibro-acoustography functional on this system. We will discuss aperture definition for the two ultrasound beams and beamforming using a linear array transducer. Experimental results from beam measurements and phantom imaging studies will be shown. The implementation of vibro-acoustography provides a step towards clinical translation of this imaging modality for applications in various organs including breast, prostate, thyroid, kidney, and liver.
Introduction
Vibro-acoustography (VA) is an imaging modality that uses the dynamic acoustic radiation force to push on tissue and measure the acoustic response of that stimulation or acoustic emission [1, 2]. The formation of this dynamic acoustic radiation force is accomplished by using two ultrasound beams that are focused to the same spatial location. The ultrasound frequencies used are in the range of 1–10 MHz and the frequencies used for the separation of beams typically range from 10–100 kHz. The images produced are free of the speckle usually associated with traditional ultrasound images. Previous studies have shown contrast between soft tissue and objects such as calcifications and other abnormalities [1, 3, 4].
Beamforming for vibro-acoustography has been studied in a number of different configurations including amplitude modulation (AM), confocal, X-focal, and with different arrays such as sector and linear arrays [2, 5, 6]. Silva, et al., specifically studied linear array beamforming for vibro-acoustography in three different configurations [6]. Heikkila and Hynynen performed a numerical study using linear arrays and beamforming to create a stress field for producing harmonic motion with two ultrasound beams of slightly different frequencies [7]. The studies performed with these dual beam configurations were done numerically and have not been validated experimentally.
Vibro-acoustography has been studied in a number of different organs [4, 8] including intensive studies in arteries [1, 9, 10], breast [11–17], and prostate [18–22]. Only a few of the studies mentioned above involve in vivo measurements in animals or humans [10, 13, 15]. The in vivo human breast vibro-acoustography images were acquired using a stereotactic mammography system combined with a custom-made water tank that housed a two-element transducer [13, 15]. The transducer was mechanically raster-scanned over a 5.0 × 5.0 cm region to create an image in a C-scan orientation. That type of scan took about 7 minutes. The in vivo arterial images acquired in sedated pigs were obtained using a water bath on the hind leg of the pig and a two-element transducer and the scanning procedure was similar to the human breast studies [10]. Considering a clinical setting and the need to acquire multiple planes necessitates a faster system.
The aforementioned breast imaging system with the water tank is a prototype that would probably not have wide applicability in a clinical setting. An ideal system would utilize standard clinical ultrasound scanners and transducers. This would include forming vibro-acoustography beams with linear array transducers. Such a system offers several clear advantages. First, VA and B-mode ultrasound images could be acquired from the same tissue volume and the images could be compared by a physician. Secondly, there would be no need for a water tank as in the studies by Alizad and colleagues [13, 15]. Simple contact transducers could be used either on the skin or with a stand-off pad. Third, implementation in a linear array system provides significant time savings due to electronic scanning of the two ultrasound beams along the azimuthal direction of the transducer, as well as the ability to electronically focus the beams at different depths. The transducer would still have to be mechanically translated in the elevation direction to obtain C-scan images at each focal depth. Implementing VA on an ultrasound system can be an economical way to add a new imaging modality to the widely established available ultrasound technology. Lastly, implementation on a clinical system would allow for a number of clinical applications including breast, thyroid, prostate, and kidney imaging.
We have implemented vibro-acoustography on a General Electric Vivid 7 ultrasound scanner (GE HealthCare Ultrasound Cardiology, Horton, Norway) using software and hardware modifications. This system electronically scans the focus of the two ultrasound beams across the azimuthal dimension of the transducer. The scanner is controlled with an external computer which also controls mechanical translation of the transducer, timing of the ultrasound transmission, and data acquisition. An illustration of the implementation is shown in Figure 1 where two ultrasound beams are transmitted from the transducer. The intersection of the beams is electronically swept in the azimuthal direction of the transducer and then mechanically scanned in the elevation direction of the transducer. Different planes can be imaged by changing the electronic focal depth. The interaction of the two ultrasound beams in the tissue produces an acoustic signal at the difference frequency, Δf, between the two ultrasound beams. The acoustic signal is detected by a nearby hydrophone and the signal is filtered, amplified, digitized, and processed for image formation and display.
Figure 1.
Block diagram of vibro-acoustography performed with a linear array transducer. The intersection of the two ultrasound beams is electronically swept in the azimuthal direction of the transducer and then mechanically scanned in the elevation direction of the transducer. Different planes can be imaged by changing the electronic focal depth. The interaction of the two ultrasound beams in the tissue produces an acoustic signal at the difference frequency, Δf, between the two ultrasound beams. The acoustic signal is detected by a nearby hydrophone and the signal is filtered, amplified, digitized, and processed for image formation and display.
In this paper, we describe the system overview and the control of the ultrasound scanner for acquiring vibro-acoustic data. We will describe the software design for producing the long ultrasound tonebursts at two different frequencies and assignment of those signals to different apertures. Furthermore, we will describe other parameters related to beamforming and electronic scanning. We will also describe hardware modifications made to the Vivid 7 system as well as the mechanical slider used for translation of the transducer.
We will provide VA beamforming results with linear arrays, and we will show images of phantoms as examples of the types of images acquired using this system.
Methods
System Overview
The four main components of the system are the control computer (PC), ultrasound scanner and transducer, mechanical slider, and hydrophone and associated electronics. The PC, which is external to the ultrasound scanner, uses an interface built using LabVIEW (National Instruments, Austin, TX). Through this LabVIEW interface, which was created by one of our team members at the Mayo Clinic, the control computer communicates with the Vivid 7 scanner using a TCP/IP socket-based client-server protocol. The TCP/IP protocol is supported by the Vivid 7, but the specific interface that was used was proprietary to GE. Instructions are sent to the scanner to set and check parameters and initiate scanning. The parameters include the ultrasound frequencies, the difference frequency, the beam repetition rate, and the transmit focus depth. Once all the parameters are set for the scan, the scan is started. An instruction is sent to the scanner to transmit the beams that make up one raster line of a C-scan image. To compare with conventional B-mode imaging, transmitting the beams for one line in a VA C-scan is analogous to transmitting one frame for B-mode imaging. The scanner emits a trigger signal when each beam is transmitted, and that trigger signal is used to synchronize the digitizer in the PC for collecting the acoustic emission data from the hydrophone. After all the beams have been transmitted, the PC sends an instruction to the motor controller for the mechanical slider via an RS-232 connection, and the motor moves in the elevation direction of the transducer a specified distance. This process repeats until the end of the scan. The acoustic emission data are collected, processed, and an image is formed for display. A block diagram of the system is shown in Figure 2.
Figure 2.
Block diagram of vibro-acoustography implementation with the General Electric Vivid 7. The control and analysis computer communicates with the Vivid 7 via TCP/IP protocol. Once an instruction from the computer is sent to the Vivid 7, a synchronization trigger (Sync) was sent to the digitizer and a waveform generator used to shade the power amplifier’s output. The power amplifier’s output served as a power supply for the transmit board in the Vivid 7. The control and analysis computer also controlled the motion control system which operated the mechanical slider with the ultrasound transducer.
Software Design
Signal Generation
The transmit signals used to produce B-mode images in a digital ultrasound system such as the GE Vivid 7 typically consist of a short burst of square wave pulses with voltages on the order of ±100 V. These pulses, appropriately delayed, are applied to the transducer elements where they are converted to sound at the desired ultrasound frequencies. Generally, all of the elements transmit identical waveforms. For vibro-acoustography, we need to use two slightly different waveforms to produce the desired difference frequency. Additionally, VA necessitates using long tonebursts on the order of several hundred microseconds, much longer than conventional B-mode pulses. Software modifications were required and made to the Vivid 7 by engineers at GE to make this possible. These modifications could only be performed by GE engineers because they relate to the GE proprietary software that runs the normal function of this machine as an ultrasound scanner.
The transmit circuitry of the Vivid 7 can create somewhat arbitrary pulse trains. This is accomplished by specifying the duration of each half cycle of the waveform in terms of periods of a master clock at frequency Fc. An appropriate number of clock cycles is chosen to produce the desired ultrasound frequency emitted by the transducer. We will refer to this number of clock cycles as N. For a normal B-mode waveform, the number of half-cycles to be transmitted is chosen to optimize the axial resolution or other imaging parameter(s), and that number is typically very small. For vibro-acoustography, however, we need to transmit two much longer tonebursts at different frequencies to produce the low frequency difference signal. The two tonebursts at different frequencies are produced by occasionally lengthening one or more of the half cycles of one or both of the waveforms relative to the other waveform. Doing this causes the frequency spectra of the two signals to shift with respect to each other, resulting in the low frequency beat signal. On the Vivid 7 we can basically instruct the transmitter circuitry to generate repeating “packets” of tone bursts. As in the B-mode case, the fundamental ultrasound frequency is specified by N (the number of clock cycles in a cycle). The number of cycles in a “packet” is referred to as K. Finally, the number of extra clock cycles to be added to each packet is referred to as L. Each of the two waveforms is specified by a (N, K, L) tuple. In VA mode, the transmitter circuitry is set up to continuously output these packets for each beam for 800 μs, but the final output is amplitude modulated (shaded) by a single cycle of a 3 kHz raised sine wave, as will be discussed later. The 800 μs value was implemented to provide flexibility for long tonebursts. Figure 3(a) illustrates the definitions of the parameters. The parameter (N1, K1, L1) describes one of the waveforms, and (N2, K2, L2) describes the other waveform.
Figure 3.
Waveform generation for vibro-acoustography. (a) The waveforms are defined by the parameters (N1, K1, L1) and (N2, K2, L2). FC represents the transmitter generator system clock. (b) Simulated signals for (N1, K1, L1) = (16, 6, 1) and (N2, K2, L2) = (16, 6, 0). Each dot represents one cycle of the 80 MHz clock.
The nominal ultrasound frequencies of the two waveforms are given as F1 = Fc/N1 and F2 = Fc/N2, where Fc is given in megahertz. The total number of clock cycles in a “packet” defined by (N, K, L) is KN + L, and the average number of clock cycles in a “packet” is given by (KN + L)/K. Thus the average periods of the two waveforms are given in units of microseconds by
| (1) |
| (2) |
The center frequency of beams 1 and 2, given in megahertz, are f1 = 1/T1,avg and f2 = 1/T2,avg, respectively. The resulting beat frequency, Δf, is given in units of megahertz as
| (3) |
For example, if we assume Fc = 80 (in MHz) and set (N1, K1, L1) = (16, 6, 1) and (N2, K2, L2) = (16, 6, 0), then using these parameters we find that F1 and F2 are 5 MHz and T1,avg = 0.2021 and T2,avg = 0.2000, which are expressed in microseconds, and f1 = 4.9480 MHz and f2 = 5.000 MHz. Using (3) yields Δf = 0.0515464 MHz = 51.5464 kHz. To illustrate the signals for this case, we simulated the signals, and they are shown in Fig. 3(b) where each dot represents one cycle of the 80 MHz clock.
The system-calculated beamforming delays are (essentially) independent of frequency, and thus there is no need to modify these calculations for vibro-acoustography. We simply choose the transducer and an application setup appropriate for the anatomy to be studied, allow the system to scan in normal B-mode, and then issue commands to the system to configure itself for VA mode. These commands include the values for (N1, K1, L1), (N2, K2, L2), pulse repetition period, and frequency assignments for the elements. In VA mode the system is essentially used as a transmitter only, and the received image displayed on the screen of the Vivid 7 is ignored.
Beamforming
Vibro-acoustography relies on the intersection of two beams. With linear array transducers this is accomplished by dividing the active aperture into two subapertures. The VA software allows arbitrary assignment of the two frequencies to the available 128 system channels. We can write the pressure of these two apertures as
| (4) |
| (5) |
where Pi(r) and φi(r) (i = 1,2) are the amplitude and phase, respectively, at location r. Vibro-acoustography is based on the radiation force of ultrasound and the force can be written as [2, 23]
| (6) |
where F is the radiation force, dr is the drag coefficient, S is the surface area of an object, E is the energy density and 〈·〉 is the short-term time average, p(r,t) is the total pressure at location r and time t (p(r,t) = p1(r,t) + p2(r,t)), ρ is the density of the medium and c is the sound speed of the medium. The radiation force is proportional to the short-term time average of the squared total pressure which can be written as
| (7) |
where Δf = f1 − f2.
We will consider one configuration for frequency assignment in this paper. This configuration splits the active aperture into two equal subapertures that are adjacent, which we denote the Split (Spl) configuration. If we assume 128 channels, then 64 are assigned f1 and the other 64 are assigned f2.
There are two methods by which we can move the common focal region of the two beams to different locations along the azimuthal dimension of the transducer. We can either use a static active aperture and steer the two ultrasound beams to different locations, or we can fix the focusing time delay law and translate the active aperture along the full aperture of the transducer. In most cases during a scan, we perform both of these actions. For instance, the General Electric 7L and 10L transducers have 192 elements, but we only have 128 channels available so we use the multiplexer in the probes to switch the channels that are connected to elements in the transducer. So, in the case that we have 192 beams and 128 active channels or elements we can translate the active aperture for 64 of these beams, maintaining a constant focusing time delay law.
In Figure 4 we show a diagram of active aperture for each beam assuming 128 active elements out of 192 total elements transmitting 192 beams. Therefore, the distance between beams is the same as the pitch of the transducer. For the first 64 beams, the active aperture is static and the beams are steered. Then for beams 65–128, the active aperture is translated across the transducer one element per beam. The last 64 beams then have a static aperture and the beams are steered. The implication of using steering is that the amplitude of Δf component may be different from beam to beam, leading to nonuniformity in the image. By translating the active aperture, the variation in beam-to-beam amplitude should be minimized. It would be ideal to use a translating aperture for the entire scan line, but that would limit the field of view that we could interrogate, so steering is employed to obtain data near the edges of the transducer.
Figure 4.
Aperture assignment for 128 active elements in a 192 element transducer. The number of beams transmitted is 192. Gray denotes elements assigned a signal with f1, white are elements assigned a signal with f2 and black are not used. For beams 1–64 and 129–192 both the subapertures are steered and for beams 65–128, the subapertures are translated.
Scanning Parameters
There are parameters related to the scanning and beamforming that are selected by the user. The ultrasound transducer used is designated and information related to the transducer is used for beamforming, particularly the pitch of the elements for focusing time delay calculation. The practical ultrasound frequencies used are fundamentally limited by the bandwidth of the transducer as well as the N parameter discussed in the section on signal generation. The difference frequency is set by the (N, K, L) parameters as detailed above. The focal depth for the C-scan plane can be set by the user. The pulse repetition period can also be set by the user. This parameter is important for a number of reasons. It is directly related to maintaining time-averaged intensity values that are safe for use in humans. It is also important in limiting heating to the transmit board and the transducer. Lastly, the pulse repetition period must be long enough for the acoustic emission to propagate to the hydrophone, and to allow any residual reverberating sound to dissipate. This time is typically on the order of a few milliseconds. The total time of a scan is proportional to the product of the number of beams, Nb, the number of elevation planes or lines in the image, Nl, and the pulse repetition period, Trep, or T = Nb·Nl·Trep. A typical scan with Nb = 192, Nl = 200, and Trep = 2 ms takes 76.8 seconds.
Signal Analysis and Image Display
The Vivid 7, mechanical slider, and digitizer are controlled by a LabVIEW (National Instruments, Austin, Texas) program running on a PC. More detail about the hardware components are described in the next section. The front panel of a “virtual instrument” provides the interface for the user to specify scanning parameters for the beam formation and subsequent digitization. Once everything is set up, the Vivid 7 is instructed to generate a sequence of beams for an azimuthal scan. With each beam, a pulse signal is provided by the Vivid 7 to synchronize and trigger the digitizer for data collection from the hydrophone. The data generated by this set of beams is a set of time series, which is transferred to the computer and stored on the hard drive. This two-dimensional (2D) set is processed to produce one line of the output display image by calculating a root-mean-square (RMS) value for each beam. This value can be calculated from the whole time-series or from a gated segment that represents an area of interest. This one-dimensional line, whose values are represented as intensity, is added to the display image. Then the probe motor is instructed to move to the next elevation imaging plane. As the process is repeated, the processing steps can be performed in real-time, and a 2D C-scan display is built up for display to the user as it is acquired.
Hardware Design
Transmit Signal
Typically the transmitted vibro-acoustic signal is a relatively long, shaded toneburst of greater than 100 μs. Shading of the toneburst is used to prevent transient turn-on/turn-off effects in the transducer from producing acoustic artifacts in the image. Since such a shaded toneburst signal could not be produced internally by the Vivid 7 system, external hardware was added to provide the necessary waveform. Additionally, to obtain adequate power levels for vibro-acoustography to be feasible in humans, an external power supply was added to the system to replace the internal transmit power supply. Figure 5 shows how the supplementary power supply was added. This was accomplished using a function generator (Model 33120A, Agilent, Santa Clara, CA) driving a two-channel linear power amplifier (Model LVC-623, AE Techron, Elkhart, IN). The raised sine wave output signal from the function generator is first split into inverted and non-inverted forms, which then drive the power amplifier inputs. The power amplifier outputs become the positive (+Vps) and negative (−Vps) power supply inputs to the transmit board in the Vivid 7 system. The power amplifier outputs typically range up to a peak voltage of 30 V. To accommodate the dynamic power supply signals, it was necessary to modify the transmit board by removing much of the power supply lines’ on-board capacitance. Because the slew rate for the transmit board power supply voltages was limited to 0.2 V/μs, due to circuit board heating effects, the transmit toneburst was shaded using a single cycle of a raised sine wave at frequency 3 kHz, resulting in a toneburst duration of 333 μs.
Figure 5.
Transmit signal chain. A function generator output is split into inverted and noninverted signals and amplified by a two-channel amplifier which provides the power supply voltages for the transmit board for the GE Vivid 7 scanner.
Acoustic Emission Acquisition
The acoustic emission arising from the ultrasound stimulation in a scanned object is detected by a sensitive hydrophone (Model 6050C, ITC, Santa Barbara, CA). The hydrophone’s signal is amplified and bandpass filtered (Model SR650, Stanford Research Systems, Sunnyvale, CA) using a 10 kHz bandwidth and then digitized with a 12-bit PC card digitizer (Model ATS330, AlazarTech, Montreal, QC, Canada).
Motion Control
Vibro-acoustography is a C-scan modality and to obtain different lines in the elevation direction, we need to mechanically translate the transducer over a specified distance. A slider mechanism, shown in Figure 6, was therefore developed which could be computer-controlled and able to accommodate a variety of ultrasound probes. The mechanical slider consists of a miniature linear servo motor (Model MX80L, Parker Daedal, Irwin, PA) mounted to a plastic frame and driven by a servo controller (Model ViX250IH, Parker Daedal, Irwin, PA). The motor has a maximum travel of 50 mm in the elevation direction, peak force of 12 N and 5 μm positional resolution. The plastic frame construction effectively isolates all electrical connections and exposed metal surfaces from user and patient contact.
Figure 6.

Motorized translation stage for moving the linear array transducer. The probe is held in a fixture attached to the linear motor stage. The linear motor stage is mounted on a plastic support frame.
Experiments
Ultrasound Field Measurements
We simulated the pressure signal at the focus of a 10L transducer (GE HealthCare Ultrasound Cardiology, Horton, Norway) using the Spl configuration. The simulations were performed using custom written code in MATLAB (The Mathworks, Natick, MA). The ultrasound pressure for the simulated case was measured using a needle hydrophone (HGL-0200, Onda Corporation, Sunnyvale, CA) having an active element with 0.200 mm diameter and a 20 dB preamplifier (AH-2000, Onda Corporation, Sunnyvale, CA). The hydrophone was placed at the focus of the two ultrasound beams.
To assess the resolution of the system, we evaluated the radiation force component at Δf for the GE 10L transducer which has an azimuthal field of view of 39 mm and a bandwidth that ranges from 4–10 MHz. The pressure was measured using a needle hydrophone that was scanned in the azimuthal/axial plane of the transducer. The ultrasound frequencies used were 5.00 and 4.95 MHz and the difference frequency was 51.5 kHz. The transducer was focused at a depth of z = 25 mm. To extract the difference frequency component, the pressure that was measured was squared and low-pass filtered as described in (7). The low-pass filter was applied to perform the short-term time average. A fast Fourier transform was performed and the magnitude of the component at 51.5 kHz was extracted for each measurement point.
The need for high power necessitates an evaluation of the safety of this method for patients. We made measurements of the highest pressures and intensities that we could produce with the Vivid 7 system using the parameters detailed above.
Phantom and Tissue Imaging
A urethane breast phantom with distributed lesions (CIRS Model 013, Norfolk, CT) was placed in a large water tank and scanned with the 10L transducer. A picture of the phantom is shown in Figure 7(a). Front and side view diagrams of the breast phantom are shown in Figure 7(b) to depict the depth of each simulated lesion. The surface of the phantom was placed 10 mm from the transducer surface, where the front surface of the phantom is depicted as the left edge in the side view of Figure 7(b). The transducer was mechanically translated over the phantom. The data was recorded as discussed above in the section on acoustic emission acquisition. The hydrophone was placed underneath the phantom and out of the way of the ultrasound propagation. Images were acquired using the Spl configuration.
Figure 7.
Photographs of objects used for imaging. (a) Breast phantom, (b) Diagram of breast phantom to scale with front and side views. The front view is scaled to be 40 × 80 mm and the side view is scaled to be 40 × 43 mm. In the side view, the left edge is the front of the phantom and is closest to the transducer. The vertical dashed line in the side view illustration depicts the location of the focal plane, about 15 mm from the surface of the phantom. During imaging, the transducer was offset from the front edge by 10 mm. (c) Excised human prostate.
A human cadaveric prostate gland was excised and used for this study. This was in compliance with a protocol approved by the Mayo Clinic Institutional Review Board. The prostate was fixed in formalin for one hour and then embedded in a gelatin mixture made from 300 Bloom gelatin powder (Sigma-Aldrich, St. Louis, MO) and glycerol (Sigma-Aldrich, St. Louis, MO) with concentrations of 10% by volume. A preservative of potassium sorbate (Sigma-Aldrich, St. Louis, MO) was also added with a concentration of 10 g/L. The prostate was degassed with gelatin before being put into a mold for setting of the gelatin. A photograph of the prostate is shown in Figure 7(c). The prostate was placed in a water tank like the breast phantom. Images were acquired using the Spl configuration with the GE 7L transducer (GE HealthCare Ultrasound Cardiology, Horton, Norway). An x-ray image was also taken with a stereotactic mammography system (Mammotest™ System Fischer Imaging Inc., Broomfield, CO) with an imaging field of 50.0 × 50.0 mm.
In our work we have found that a deterministic variation in the magnitude of the acoustic emission signal along the azimuthal direction arises which we have not been able to definitively identify its source. When the transducer is mechanically scanned, these variations manifest as streaks in the horizontal direction. The streaks are related to the beamforming because when focal depth or configuration is changed, the streaks change in a deterministic way. We made detailed measurements of the ultrasound intensity field versus beam position across the azimuthal direction of the transducer, and determined that slight intensity variations versus beam position are causing the streaks in the image. This phenomenon has to do with the way the internal beam forming is constructed. We have devised a streak removal algorithm based on a moving window method that estimates the vertical amplitude variation profile and divides it from the image data. All the images shown have been corrected for this streak artifact. We removed the background gray level to improve the contrast, and gamma correction was applied to the images to improve the dynamic range for viewing.
Results
Beamforming Results
Figure 8(a) shows the simulated pressure signal using the signals in Fig. 3(b) over two cycles of Δf. The signal in Figure 8(a) is expanded over a longer time than that shown in Figure 3(b) so that phase offsets that were used create the modulated ultrasound pressure can be appreciated. Figure 8(b) shows the experimentally measured signal using the needle hydrophone. The waveforms in Figure 8 show the carrier signals and the modulation created by the interaction of the two ultrasound beams. The measured signal has been shaded by a single cycle of a 3 kHz raised sine wave, shown as the dashed line in Figure 8(b).
Figure 8.
Pressure signals from vibro-acoustography beamforming. (a) Simulated pressure using signals from Fig. 3(b). Two cycles of modulated ultrasound with Δf = 51.5464 kHz are shown. (b) Measured pressure with a needle hydrophone for a full toneburst which was shaded by the raised 3 kHz signal depicted as a dashed line. Note that the time scales are different for each plot.
To evaluate the resolution of the system, the measured azimuthal and axial profiles for field focused at x = 0 mm and z = 25 mm is shown in Figure 9. The values of the azimuthal, elevational, and axial resolutions at −6 dB for were 0.573, 1.44, and 2.20 mm, respectively. The azimuthal sidelobes were −25.26 dB with respect to the main lobe.
Figure 9.
Experimentally measured azimuthal and axial profiles for the Spl configuration. The field is focused at x = 0 mm and z = 25 mm. The magnitudes are independently normalized. (a) Azimuthal, (b) Axial.
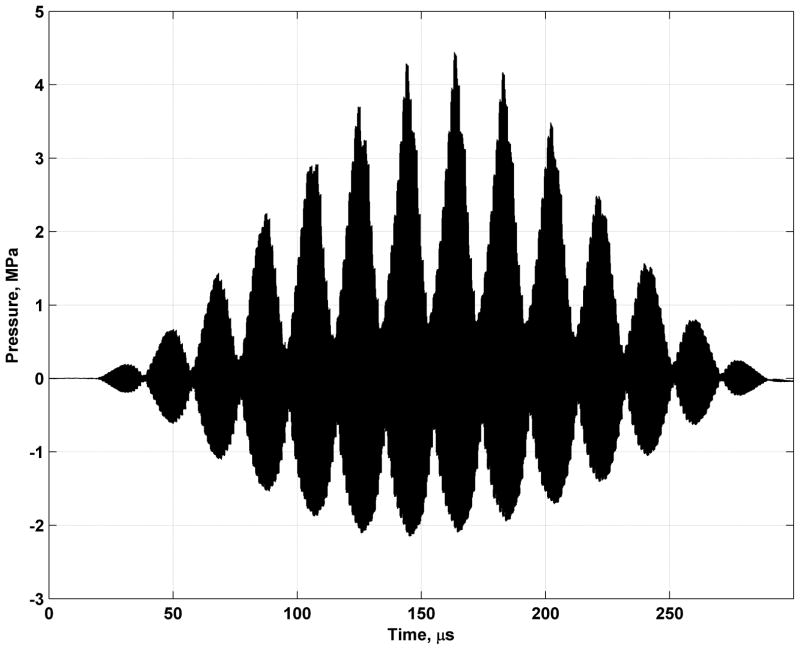
We measured the pressure at the location of the highest pressure, which is shown in Figure 10, using the 10L and the Onda hydrophone and found that the spatial-peak pulse-average intensity Isppa = 51.3 W/cm2 when focused at 25.0 mm focal depth. The spatial-peak temporal average for a line of VA imaging was Ispta = 0.173 W/cm2 assuming a beam repetition period of 2 ms, which is lower than the derated Ispta,0.3 Food and Drug Administration (FDA) regulatory limit of 0.72 W/cm2 [24]. The intensity values given above were not derated. The peak negative pressure was measured at 2.15 MPa at 5 MHz. If we do not derate by 0.3 dB/cm/MHz, the mechanical index (MI) is 0.96, and if derating is performed the MI decreases to 0.62. Both of these values are below the FDA regulatory limit of 1.9 [24]. Thermal index calculations could be performed with an established method based on the work of Chen and colleagues [25].
Figure 10.
Experimentally measured pressure at location of spatial peak pressure. The field is focused at x = 0 mm and z = 25 mm.
Phantom and Tissue Imaging Results
Figure 11 shows the result from imaging the urethane breast phantom. The transducer was mechanically translated in the horizontal direction (y-direction), and the azimuthal direction of the transducer corresponds to the vertical axis of the image (x-direction). The image has undergone streak correction. The two large lesions on the bottom of the image were in focus in these images. The brightness of the lesions and the presence of fine detailed edges are ways to differentiate which lesions are in focus and which are not. Other lesions can be detected such as those in the upper corners, which appear darker because they are deeper in the phantom.
Figure 11.

Breast phantom images. The azimuthal direction of the transducer corresponds to the vertical axis of the image and the mechanical translation of the transducer in the elevation direction corresponds to the horizontal axis. The image is 38.4 × 80 mm and streak correction has been applied.
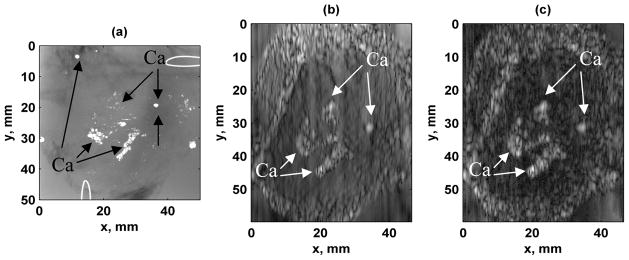
Figure 12 shows the images acquired from scanning the excised human prostate. The transducer was mechanically translated in the vertical direction (y-direction) and the azimuthal direction of the transducer corresponds to the horizontal axis of the image (x-direction). An x-ray image is shown in Figure 12(a). The original image and the image obtained after correction for the streaks are shown in Figure 12(b) and 12(c), respectively. The vibro-acoustic images depict a differentiation of the central region and a region around the edge of the prostate, where the central region appears smooth and the outer region appears to have a different texture. The clusters of calcifications that appear in the x-ray image are depicted very clearly in the VA images and the borders of those regions are very distinct.
Figure 12.
Excised human prostate images. (a) X-ray image of prostate gland. Calcium clusters are marked with the designation Ca in this panel and all other panels. Wire loops in upper right and lower left corners are fiducial markers. Image is 50.0 × 50.0 mm, (b) Original VA image, (b) VA image after streak correction. The azimuthal direction of the transducer corresponds to the horizontal axis of the image and the mechanical translation of the transducer in the elevation direction corresponds to the vertical axis. Images are 46.7 × 60 mm.
Discussion
We have shown that the Vivid 7 is able to form the ultrasound fields used as an excitation source for vibro-acoustography imaging and demonstrated good modulation of the ultrasound pressure. The azimuthal resolution was sub-millimeter and the axial resolution was 2.20 mm which is much less than the 10 mm axial resolution found with confocal VA imaging [23]. The acoustic output results showed that VA as implemented on the Vivid 7 is within FDA regulatory limits for the 10L transducer as well as other transducers although these data for other transducers were not shown.
Figure 11 shows the results of an imaging study of a urethane breast phantom. Two lower lesions in the breast phantom are easily detectable with good edge definition. The lesions in the top of the image do not appear the same as those in the lower portion of the image, mostly because they are out-of-plane in the axial direction.
Figure 12 shows results from imaging of an excised human prostate. The streaks, in the vertical direction, cause variations that reduce the contrast in the image. The streaks are best observed outside of the prostate gland in the surrounding gelatin. Figure 12 shows an excellent correlation between the VA images and the x-ray depicting calcifications. Also, the tissue detail in the different regions of the prostate is interesting. Though the prostate would not normally be imaged with a linear array transducer with a linear type of translation, these images were shown to exhibit the capabilities of the system to differentiate soft tissue from calcifications as well as different types of tissue within a given organ.
The source of the image contrast is a mixture of different factors including the mechanical response of the tissue or calcifications and factors that affect the radiation force such as the reflectivity/absorption of the tissue. The interplay between these factors and the distribution of the point-spread function is complicated, so at times, the calcifications can have similar image intensity compared to normal tissue. It should be noted that this prostate was fixed in formalin for one hour so that may also have an effect on the imaging results. The differing appearances of the tissue in different regions of the prostate arise from the interactions of the tissue structure and the asymmetric point-spread function in the elevation/azimuthal plane. Also, the interactions of tissue at different axial locations influence the appearance of the tissue in the images. The short-depth-of-field (2.20 mm) compared to previous work with a focused confocal transducer which has a long depth of field (~10 mm), may play a role in the appearance of the tissue as tissue at different axial depths influence the acoustic emission signal [26]. This is an active area of research by our group, and we are trying to better understand the appearance of different types of tissue using these linear array transducers.
The implementation of VA on a clinical scanner allows for integration of both B-mode and VA using the same transducer and that combination of data may provide complementary information. VA can also take advantage of other aspects of the Vivid 7 scanner such as transducer availability. The choice of probe technology is an important factor in the outcome of vibro-acoustography implementation. In this paper, we focused on linear array probes. Other probe types, such as multi-row probes with larger number elements, may have a better performance because such probes allow beam focusing in both the azimuthal and elevation directions. Further studies are needed to fully explore vibro-acoustography with such probe structures. To date, VA has been implemented with a phased array (M4S), several linear array transducers (7L, 10L, M12L), a transrectal linear array probe (ERBL), and a custom-made 1.75D transducer array. These various transducers allow for different ultrasound frequencies and provide optimized resolution and contrast as well as varied applications for scanning of the thyroid, breast, prostate, kidney, liver, and other organs. This implementation of VA on the Vivid 7 was accomplished primarily through software modifications. VA takes advantage of the transmit beamformer already implemented on the Vivid 7 for electronic focusing and scanning of the beam. This is important for saving time that would previously have been spent in mechanical translation of a transducer in one direction. It is also important to note that the assignment of the two subapertures for f1 and f2 is completely arbitrary. This flexibility of the system allows for implementation of different configurations, which may be useful when approaching different applications of this VA imaging method. In this paper, we mainly focused on split configuration. Alternative beamforming options include symmetric and interlaced configurations. The symmetric configuration consists of separating the aperture into three sections, where the group of elements in either end section is assigned to one frequency and the elements in the center section are assigned to the other frequency. Another possible configuration is to divide the elements on the array into M adjacent sections, with sections alternately assigned to one or the other frequency. One may also assign the two frequencies to elements in alternating or random fashion. We have studied the feasibility of these configurations and determined advantages and disadvantages of each method. However, the discussion on such comparisons is beyond the scope of this paper.
There are some limitations for implementation of VA on this clinical scanner. We did have to supplement the power supply with an amplifier to achieve power levels adequate for VA imaging. In our study of the GE Vivid 7 system, we determined that a supplementary power supply was needed to generate the appropriate output power without the drooping effect that we observed with original power supply. We evaluated a few different power amplifiers before settling on the one described above. This addition of an extra power supply could be avoided in the future either by implementation on a different scanner that has a more robust power supply or by redesigning the power supply of the existing scanner.
In theory, a 2D image could be acquired in the same plane as the B-mode image by focusing at all points in the VA image. However, VA is primarily a C-scan modality. We choose to keep a consistent focal plane and calculate the delays needed to focus at points along the azimuth of the transducer. So if a scan uses Nb = 192 beams, only 192 sets of delays need to be accessed during scanning. The delays are only recalculated if the focal depth plane is changed. If a 2D image were to be acquired in the B-mode imaging plane, with Nd levels in the axial direction, the number of sets of time delays that would need to be accessed would be Nb·Nd. This would potentially be too large a number for the Vivid 7 to store in memory and scanning may be slowed by accessing these delay values. Also, the excitation used to make each pixel could have a very different point-spread function, especially as the depth is increased. These variations may have detrimental effects on the image quality.
Because VA is inherently a C-scan method, we need to translate the transducer in the transducer’s elevation direction. This involves a full motorized assembly to accomplish this task. We also need the hydrophone to be in contact with the patient during scanning. This is not a significant issue but placement of the hydrophone may have to be optimized for each application.
In these studies an external PC was used for control, data acquisition, and display of the VA images. In an actual commercial realization of VA all of these functions could be accomplished using the scanner’s internal CPU. This would greatly simplify its use in a clinical setting, and allow, for example, simultaneous display of B-mode and VA images.
The implementation of VA on the Vivid 7 provides an avenue for a more transparent translation of VA into a clinical setting particularly for breast, prostate, thyroid, kidney, and liver imaging. Optimization of VA in these different applications depends on different parameters such as the ultrasound frequencies, difference frequency, transducer geometry and hydrophone position. We also have the opportunity to change the apertures for the two ultrasound beams to improve resolution and contrast in the images.
Conclusion
We have described the design and implementation of vibro-acoustography on a clinical GE Vivid 7 scanner. We modified the transmit signals to produce a difference frequency in the kilohertz range and demonstrated the beamforming design for VA using a linear array transducer. We evaluated the beamforming with experimental measurements with a needle hydrophone. Lastly, we showed imaging results using a breast phantom and excised human prostate. The implementation of VA on a clinical scanner provides a substantial opportunity for translation of this imaging modality for clinical imaging of various organs.
Acknowledgments
The authors wish to acknowledge Dr. Shigao Chen and the acoustic output measurements that he made. This work was supported in part by grants CA121579, CA127235, and CA091956 from the National Institutes of Health.
Footnotes
Disclosure of Conflict of Interest: Mayo Clinic and some of the authors have a potential financial interest related to the technology referenced in this paper.
References
- 1.Fatemi M, Greenleaf JF. Ultrasound-stimulated vibro-acoustic spectrography. Science. 1998 Apr 3;280:82–5. doi: 10.1126/science.280.5360.82. [DOI] [PubMed] [Google Scholar]
- 2.Fatemi M, Greenleaf JF. Vibro-acoustography: An imaging modality based on ultrasound-stimulated acoustic emission. Proc Natl Acad Sci U S A. 1999 Jun 8;96:6603–8. doi: 10.1073/pnas.96.12.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fatemi M, Manduca A, Greenleaf JF. Imaging elastic properties of biological tissues by low-frequency harmonic vibration. Proc IEEE. 2003 Oct;91:1503–1519. [Google Scholar]
- 4.Alizad A, Wold LE, Greenleaf JF, Fatemi M. Imaging mass lesions by vibro-acoustography: modeling and experiments. IEEE Trans Med Imaging. 2004 Sep;23:1087–93. doi: 10.1109/TMI.2004.828674. [DOI] [PubMed] [Google Scholar]
- 5.Silva GT, Chen S, Frery AC, Greenleaf JF, Fatemi M. Stress field forming of sector array transducers for vibro-acoustography. IEEE Trans Ultrason Ferroelectr Freq Control. 2005 Nov;52:1943–51. doi: 10.1109/tuffc.2005.1561663. [DOI] [PubMed] [Google Scholar]
- 6.Silva GT, Greenleaf JF, Fatemi M. Linear arrays for vibro-acoustography: a numerical simulation study. Ultrason Imaging. 2004 Jan;26:1–17. doi: 10.1177/016173460402600101. [DOI] [PubMed] [Google Scholar]
- 7.Heikkila J, Hynynen K. Investigation of optimal method for inducing harmonic motion in tissue using a linear ultrasound phased array - A simulation study. Ultrason Imaging. 2006 Apr;28:97–113. doi: 10.1177/016173460602800203. [DOI] [PubMed] [Google Scholar]
- 8.Alizad A, Fatemi M, Nishimura RA, Kinnick RR, Rambod E, Greenleaf JF. Detection of calcium deposits on heart valve leaflets by vibro-acoustography: an in vitro study. J Am Soc Echocardiogr. 2002 Nov;15:1391–5. doi: 10.1067/mje.2002.124985. [DOI] [PubMed] [Google Scholar]
- 9.Alizad A, Fatemi M, Whaley DH, Greenleaf JF. Application of vibro-acoustography for detection of calcified arteries in breast tissue. J Ultrasound Med. 2004 Feb;23:267–73. doi: 10.7863/jum.2004.23.2.267. [DOI] [PubMed] [Google Scholar]
- 10.Pislaru C, Kantor B, Kinnick RR, Anderson JL, Aubry MC, Urban MW, Fatemi M, Greenleaf JF. In vivo vibroacoustography of large peripheral arteries. Invest Radiol. 2008 Apr;43:243–252. doi: 10.1097/RLI.0b013e31816085fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alizad A, Fatemi M, Wold LE, Greenleaf JF. Performance of vibro-acoustography in detecting microcalcifications in excised human breast tissue: a study of 74 tissue samples. IEEE Trans Med Imaging. 2004 Mar;23:307–12. doi: 10.1109/TMI.2004.824241. [DOI] [PubMed] [Google Scholar]
- 12.Alizad A, Whaley DH, Greenleaf JF, Fatemi M. Potential applications of vibro-acoustography in breast imaging. Technol Cancer Res Treat. 2005 Apr;4:151–8. doi: 10.1177/153303460500400204. [DOI] [PubMed] [Google Scholar]
- 13.Alizad A, Whaley DH, Greenleaf JF, Fatemi M. Critical issues in breast imaging by vibro-acoustography. Ultrasonics. 2006 Dec 22;44(Suppl 1):e217–20. doi: 10.1016/j.ultras.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini HG, Alizad A, Fatemi M. Integration of vibro-acoustography imaging modality with the traditional mammography. Int J Biomed Imaging. 2007;2007:40980. doi: 10.1155/2007/40980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alizad A, Whaley DH, Greenleaf JF, Fatemi M. Image features in medical vibro-acoustography: In vitro and in vivo results. Ultrasonics. 2008;48:559–562. doi: 10.1016/j.ultras.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatemi M, Wold LE, Alizad A, Greenleaf JF. Vibro-acoustic tissue mammography. IEEE Trans Med Imaging. 2002 Jan;21:1–8. doi: 10.1109/42.981229. [DOI] [PubMed] [Google Scholar]
- 17.Urban MW, Silva GT, Fatemi M, Greenleaf JF. Multifrequency vibro-acoustography. IEEE Trans Med Imaging. 2006 Oct;25:1284–95. doi: 10.1109/tmi.2006.882142. [DOI] [PubMed] [Google Scholar]
- 18.Mitri FG, Trompette P, Chapelon JY. Improving the use of vibro-acoustography for brachytherapy metal seed imaging: a feasibility study. IEEE Trans Med Imaging. 2004 Jan;23:1–6. doi: 10.1109/TMI.2003.819934. [DOI] [PubMed] [Google Scholar]
- 19.Mitri FG, Davis BJ, Alizad A, Greenleaf JF, Wilson TM, Mynderse LA, Fatemi M. Prostate cryotherapy monitoring using vibroacoustography: preliminary results of an ex vivo study and technical feasibility. IEEE Trans Biomed Eng. 2008 Nov;55:2584–2592. doi: 10.1109/TBME.2008.2001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitri FG, Davis BJ, Urban MW, Alizad A, Greenleaf JF, Lischer GH, Wilson TM, Fatemi M. Vibro-acoustography imaging of permanent prostate brachytherapy seeds in an excised human prostate - Preliminary results and technical feasibility. Ultrasonics. 2009 Mar;49:389–394. doi: 10.1016/j.ultras.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitri FG, Davis BJ, Greenleaf JF, Fatemi M. In vitro comparative study of vibro-acoustography versus pulse-echo ultrasound in imaging permanent prostate brachytherapy seeds. Ultrasonics. 2009 Jan;49:31–38. doi: 10.1016/j.ultras.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alizad A, Mitri FG, Davis BJ, Sebo T, Kinnick R, Greenleaf JF, Fatemi M. Prostate tissue imaging by vibro-acoustography. IEEE Trans Med Imaging. doi: 10.1118/1.4773890. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Fatemi M, Kinnick R, Greenleaf JF. Comparison of stress field forming methods for vibro-acoustography. IEEE Trans Ultrason Ferroelectr Freq Control. 2004 Mar;51:313–21. [PubMed] [Google Scholar]
- 24.Herman BA, Harris GR. Models and regulatory considerations for transient temperature rise during diagnostic ultrasound pulses. Ultrasound Med Biol. 2002;28:1217–24. doi: 10.1016/s0301-5629(02)00558-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Aquino W, Alizad A, Urban MW, Kinnick RR, Greenleaf JF, Fatemi M. Thermal safety of vibro-acoustography using a confocal transducer. Ultrasound Med Biol. 2010;36 doi: 10.1016/j.ultrasmedbio.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva GT, Frery AC, Fatemi M. Image formation in vibro-acoustography with depth-of-field effects. Comput Med Imaging Graph. 2006 Jul;30:321–7. doi: 10.1016/j.compmedimag.2006.08.001. [DOI] [PubMed] [Google Scholar]