Abstract
Our review covers the recent epigenetic data that are focused on matrix metalloproteinases (MMPs), their inhibitors (tissue inhibitors of MMPs; TIMPs) and collagen substrates. Twenty-four MMPs, four TIMPs and at least 28 collagen types are known in humans. The MMP activity regulates the functionality of multiple extracellular matrix proteins, cytokines, growth factors and cell signaling and adhesion receptors. Aberrantly enhanced MMP proteolysis affects multiple cell functions, including proliferation, migration and invasion. This aberrant MMP proteolysis is frequently recorded in cancer. Recent evidence, however, indicates that several MMPs function as tumor suppressors in cancer. Their inhibition could have pro-tumorigenic effects (making them anti-targets), counterbalancing the benefits of target inhibition and leading to adverse effects in cancer patients. The current epigenetic data suggest that there are distinct multi-layered epigenetic mechanisms that regulate MMPs, TIMPs and collagens. We show that in certain cancer types, epigenetic signatures of selected MMPs exhibit stem cell-like characteristics. Epigenetic mechanisms appear to play an especially important role in glioblastoma multiforme. Glioblastomas/gliomas synthesize de novo and then deposit collagens into the brain parenchyma. The collagen deposition, combined with an enhanced MMP activity in glioblastomas/gliomas, facilitates rapid invasion of tumor cells through the brain. It is tempting to hypothesize that the epigenetic mechanisms which control MMPs, TIMPs and collagens and, consequently, tumor cell invasion, represent promising drug targets and that in the near future these targets will be challenged pharmacologically.
Keywords: cancer, cell migration, collagen, DNA methylation, epigenetics, extracellular matrix, glioblastoma, glioma, histone modification, MMP
Introduction
Gene products involved in cell locomotion, angiogenesis, tumor progression and survival are all potential targets of epigenetic regulation via DNA methylation and histone modification mechanisms (1). In malignancies, DNA methylation is frequently dysregulated. Methylation of CpG islands (CpGI) inhibits transcription and represses tumor suppressor genes. Acetylation of the core histones H3, H4, H2A and H2B are normally associated with the activation of gene transcription (2). Methylation of histone lysines occurs in a form of mono-, di- and trimethylation and is reversed by enzymatic demethylation (3, 4). Methylation of the H3K4, H3K36 and H3K79 residues attracts the RNA polymerase II complex and, as a result, up-regulates gene expression (5, 6). Methylation of the H3K9, H3K27 and H4K20 lysine residues, however, leads to gene silencing (7-10). It was unclear, until recently, if and how pro-migratory genes including the extracellular matrix (ECM) proteins, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are controlled epigenetically (11).
ECM and collagens
A three-dimensional architecture of the ECM, a complex structural entity surrounding and supporting cells within mammalian tissues, includes structural proteins (collagen and elastin) and specialized proteins (fibronectin, laminin and tenascin) and proteoglycans (hyaluronan, chondroitin, keratin and dermatan sulfates). Complex multi-factorial relations among these proteins result in the diverse mechanical characteristics of the ECM. The ECM is modified in hypoxia (12, 13), inflammation (14) and in pathologies, including malignancy, atherosclerosis, arthritis, osteoporosis and fibrosis. Collagens are the most abundant proteins in both the ECM and the human body. There are at least 28 collagen types encoded by 49 COL genes (15) including fibril-forming (types I, II, III, V, VI and XI), fibril-associated (types IX, XII and XIV), anchoring (type VII) and network-forming collagens (types IV, VIII and X). There are multiple genetic diseases in humans which are caused by mutations in collagens including osteogenesis imperfecta (type I), Ehler-Danlos syndrome (types I and IV), arterial aneurysms (type III), Alport syndrome (type IV), Ullrich molecular dystrophy (type VI), certain dysplasias (types II, IX and XI) and additional pathologies.
The MMP family
MMPs play a well-documented role in the collagenolysis and in the general ECM proteolysis in disease. Recent scientific discoveries directly implicate a number of MMPs in multiple diseases of the cardiovascular, pulmonary, renal, endocrine, gastrointestinal, musculoskeletal, visual and hematopoietic systems in humans. Elevated MMP activity and the resulting aberrant ECM proteolysis are also characteristics of malignant lesions (16).
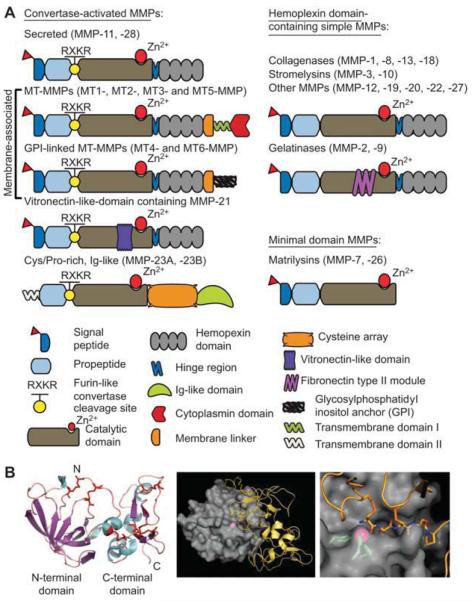
MMPs belong to a zinc endopeptidase, metzincin superfamily (Figure 1A) (17). This superfamily is distinguished by the presence of a conserved HEXXHXXGXX(H/D) sequence motif in the active site. The human MMP family is comprised of 24 zinc-containing enzymes which share several functional domains (18, 19). Six membrane type MMPs (MT-MMPs) are distinguished from soluble MMPs by an additional transmembrane domain and a short cytoplasmic tail (MT1-, MT2-, MT3- and MT5-MMP) or by a glycosylphosphatidyl inositol anchor (MT4 and MT6-MMPs). MMPs are multifunctional enzymes which degrade the ECM components (collagens, laminin, fibronectin, vitronectin, aggrecan, enactin, versican, perlecan, tenascin, elastin and many others), growth factors, cytokines and cell surface-associated adhesion and signaling receptors. Because of their potentially disastrous effect on the cell microenvironment, MMPs are normally expressed in small amounts. Their cellular localization and activity are tightly controlled at both the transcriptional and post-transcriptional levels by cytokines, including interleukins (IL-1, IL-4 and IL-6), growth factors (epidermal growth factor, hepatocyte growth factor and transforming growth factor-β), and tumor necrosis factor-α (20, 21). In a feedback loop, some of these regulatory factors are proteolytically regulated by MMPs (22).
Figure 1.
MMPs and TIMPs.
(A) Domain structure of MMPs. The structure of MMPs is made up of the following homologous domains: (1) a signal peptide; directs MMPs to the secretory or plasma membrane insertion pathway, (2) a prodomain; confers latency to MMPs, (3) a catalytic domain with the active site zinc atom, (4) a hemopexin-like domain; in coordination with the catalytic domain controls the interactions with substrates, (5) a flexible hinge region; links the catalytic and the hemopexin domain and provides each domain mobility relative to the other. The membrane-type MMPs contain an additional transmembrane domain and a short cytoplasmic tail domain (MMP-14, MMP-15, MMP-16 and MMP-24) or a glycosylphosphatidyl inositol linkage, which attaches MMP-17 and MMP-25 to the cell surface. MMP-2 and MMP-9 contain fibronectin-like type II repeats which assist in collagen substrate binding. A hemopexin domain is absent in MMP-7 and MMP-26. Several MMPs are activated in the course of their secretion pathway by the proprotein convertases, including furin, which recognize the RXKR cleavage motif. MMP-1/Collagenase-1, MMP-2/Gelatinase-1, MMP-3/Stromelysin-1, MMP-7/Matrilysin-1, MMP-8/Collagenase-2, MMP-9/Gelatinase-2, MMP-10/Stromelysin-2, MMP-11/Stromelysin-3, MMP-12/Metalloelastase, MMP-13/Collagenase-3, MMP-14/MT1-MMP, MMP-15/MT2-MMP, MMP-16/MT3-MMP, MMP-17/MT4-MMP, MMP-18/Collagenase-4, MMP-19/RASI-1, MMP-20/Enamelysin, MMP-21/X-MMP, MMP-23/no alternative name, MMP-24/MT5-MMP, MMP-25/MT6-MMP, MMP-26/Matrilysin-2, MMP-27/no alternative name, MMP-28/Epilysin. (B) Structure of TIMPs and MMPs. Left panel, Three-dimensional structure of TIMP-2 (PDB entry 1BR9) (116). The N-terminal, inhibitory domain (residues 1–110) is folded into a beta-barrel. The C-terminal domain (residues 111–194) contains a parallel stranded beta-hairpin and a beta-loop-beta motif. Six disulfide bridges (shown as orange sticks) stabilize the structure. Middle panel, three-dimensional structure of the complex formed by the catalytic domain of MMP-14 with the N-terminal inhibitory domain of TIMP-2 (PDB entry 1BUV) (117). MMP-14 and TIMP-2 are shown as surface and cartoon models, respectively. Active site zinc is shown as a magenta ball. Disulfide bridges are orange. Right panel, close-up of the MMP-14/TIMP-2 complex. MMP-14, a surface model with three active site histidine residues and active site zinc shown as green sticks and magenta ball, respectively. The N-terminal residues of TIMP-2 are shown as sticks. Disulfide bridges are orange. Recently, MMP-18 has been renamed MMP-19.
Activation and inhibition of MMPs
MMPs are synthesized as latent proenzymes. In the proenzymes, the active site zinc is coordinated by the three active site histidines and also by the cysteine residue of the N-terminal inhibitory prodomain (23). To become active proteinases, the proenzymes require proteolytic processing that removes the N-terminal inhibitory prodomain and exposes the catalytic site of the MMP enzyme. The activation of MMPs occurs both intracellularly and extracellularly (24, 25). Several MMPs (MMP-11, MMP-28 and MT-MMPs), exhibit the furin cleavage motif RXK/RR in their propeptides. These MMPs are processed by the furin-like proprotein convertases in the trans-Golgi network (Figure 1A). Additional proteolytic cleavages, however, are required to inactivate the inhibitory prodomain. If the prodomain is released by furin alone, the resulting MT1-MMP enzyme remains inhibited by its non-covalently associated intact prodomain (26). Activation of soluble MMPs is mediated by serine proteases, including plasmin, by MT-MMPs (e.g., activation of the MMP-2 proenzyme by MT1-MMP) or by other active MMPs (e.g., activation of the proenzymes of MMP-1 and -9 by MMP-3).
Because of the overlapping cleavage preferences, there is functional redundancy among MMPs. As a result, MMP knockouts in mice, with the exception of MT1-MMP, are non-lethal and do not exhibit a strong phenotype. MT1-MMP knockout mice, however, develop dwarfism, bone malformations and die before adulthood, thus supporting the role of MT1-MMP in both cell migration during gastrulation and collagen turnover (26-30). Mice lacking both MMP-2 and MT1-MMP die immediately after birth (31).
Once activated, MMPs are inhibited by TIMPs (Figure 1B). Four different TIMPs are known in humans (TIMP-1, -2, -3 and -4) (32, 33). MMP/TIMP balance is a significant factor in the regulation of the net proteolytic activity of MMPs. Structurally, TIMPs contain two domains. The inhibitory N-terminal domain binds non-covalently to the active site of the active MMPs, blocking access of substrates to the catalytic site. The C-terminal domain of TIMP-1 and -2 binds to the hemopexin domain of the proenzymes of MMP-9 and -2, respectively.
Because of their important functions, MMPs are considered promising drug targets in cancer. To date, all clinical trials of wide-specificity inhibitors of MMPs, however, have failed with the exception of doxycycline for periodontal disease (34). In these trials, the inhibition of the tumor-suppressing MMPs might counterbalance the benefits of suppressing the tumor-promoting MMPs. Only after the failure of clinical trials, MMP-8 and -26 and, albeit less conclusively, MMP-3, -9, -11, -12 and -19, have been identified as potential tumor suppressors (35-43).
DNA methylation
The major epigenetic cues include DNA methylation and histone modification. DNA methyltransferases (DNMT1, DNMT3a and DNMT3b) (44, 45) recognize CpG dinucleotides and methylate cytosines on either one (hemimethylated DNA) or both DNA strands. DNA methylation can be reestablished on an unmethylated strand after DNA replication is completed because of the ability of DNMT1 to methylate hemimethylated CpG sites. Because of the high mutation rate leading to the CG suppression in the course of eukaryotic evolution, CpGs are under-represented in the eukaryotic genomes compared with other dinucleotide combinations (46, 47). CpGs are frequently clustered in the specific regions called CpGIs. CpGIs are considered strong if the observed: expected CpG ratio exceeds 0.6 (48). The promoter sequence of 70% of the annotated human genes contains CpGI regions (48, 49). The binding of the Sp1, RARE and GATA methylation-sensitive transcription factors with the methylated DNA sequences is normally repressed. As a result, methylation of CpGIs causes transcriptional silencing of the down-stream genes. Genome-wide methylation profiling at base resolution allows establishing the genomic distribution of methylated sequences – the methylome. The methylome pattern is unique in the individual cell types and altered in malignancies relative to normal cells (50, 51).
Histone modifications
Covalent modifications of the core histones represent a chromatin-level epigenetic regulation. There are several histone modifications: lysine acetylation, lysine and arginine methylation, serine and threonine phosphorylation, lysine ubiquitination, proline isomerization and arginine deimination (5). Acetylation of H3, H4, H2A and H2B is normally associated with the activation of gene transcription (2). Acetyl groups are added by histone acetyltransferases (HATs). Histone acetylation can be removed by histone deacetylases (HDACs). Methylation of the lysine and arginine residues in the histone tails may lead to either transcriptional activation or repression depending on the position of the modified residue on the histone tail. Methylation of histone lysines occurs as mono-, di- and trimethylation and is reversed by enzymatic demethylation (3, 4). Methylation of the H3K4, H3K36 and H3K79 residues attracts the RNA polymerase II complex (POL2) and, as a result, up-regulates gene expression (5, 6). In contrast, methylation of the H3K9, H3K27 and H4K20 lysine residues promotes interactions of the modified histones with the heterochromatin protein 1 (HP1) or its homologues. These events normally lead to gene silencing (7, 8). The co-existing H3K27 and H3K4 methylation constitutes a bivalent mark, a specific characteristic of the temporarily silenced developmental genes. This bivalent mark creates a balance that can be readily misbalanced by a developmental stimulus resulting in a rapid activation of the respective loci (52-54). This bivalent epigenetic mark is a known feature of embryonic stem (EC) cells (52, 53).
The work of Yan et al. (55) was the first demonstrating that recruitment of HDAC2 at the promoter of MMP-9 reduced H3/H4 acetylation levels and MMP-9 expression. As a result of the more recent massive research efforts, volumes of the whole genome epigenetic information, including those on MMPs, have been deposited in the public databases, such as the Gene Expression Omnibus (GEO) repository (56). Specifically for this review, we have analyzed these available, albeit incomplete, epigenetic data for selected MMPs, TIMPs and collagens. We now conclude that epigenetic cues play a significant role in regulating the ECM functionality in malignancy.
Epigenetic control of MMPs in the 11q22.3 genomic region
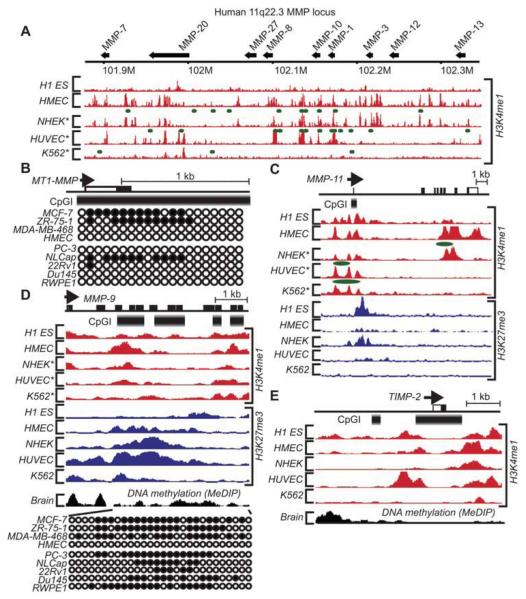
Knowledge of epigenetic regulation of MMPs is still limited. Genomic data indicate that the promoter region of 13 out of 24 MMPs exhibit pronounced CpGIs (an observed:expected CpG ratio >0.6) (Table 1). Nine MMPs (MMP-1, -3, -7, -8, -10, -12, -13, -20, and -27) are clustered in the 11q22.3 region of chromosome 11 (Figure 2A). Chromosomal abnormalities and multiple polymorphisms in the 11q22.3 region predispose to prostate and lung cancer (57, 58) and coronary artery aneurysm formation in patients with Kawasaki’s disease (59). The approximately 0.43 Mb 11q22.3 region is largely deficient of CpGIs. This parameter suggests a limited effect of DNA methylation on MMPs from this cluster. However, there is evidence that methylation of CpGIs is sufficient to impose the selective epigenetic control on MMPs. Thus, the DNA methyltransferase inhibitor, 5-aza-2′-deoxycytidine (5-Aza), reduced the level of methylation of a single CpG site in the MMP-1 promoter and increased the expression of MMP-1 in human amnion fibroblasts. Intriguingly, this CpG site overlaps with the single nucleotide polymorphism (SNP) that is associated with the risk of premature fetal membrane rupture (60). A dual knock-out of the DNA methyltransferases DNMT1 and DNMT3b in mice induced the expression of MMP-3 but not of MMP-1 and -2. The DNA methyltransferase inhibitors, 5-Aza and zebularine, also selectively induced MMP-3 in colon cancer cells (61). The similar treatment of lymphoma cells was without any effect (61).
Table 1.
Human MMP and TIMP genes.
| Gene | Alternative name | Chromosomal location |
CpGI positiona |
|---|---|---|---|
| MMP-1 | Interstitial collagenase/collagenase-1 | 11q22.3 | – |
| MMP-2 | Gelatinase A | 16q13-q21 | −0.4 to +1.1 (1.5) |
| MMP-3 | Stromelysin-1 | 11q22.3 | – |
| MMP-7 | Matrilysin-1 | 11q22.3 | – |
| MMP-8 | Neutrophil collagenase/collagenase-2 | 11q22.3 | – |
| MMP-9 | Gelatinase B | 20q11.2 | +0.95 to +3.7 (4.65)b |
| MMP-10 | Stromelysin-2 | 11q22.3 | – |
| MMP-11 | Stromelysin-3 | 22q11.23 | −0.3 to +0.55 (0.85) |
| MMP-12 | Macrophage metalloelastase | 11q22.3 | – |
| MMP-13 | Collagenase-3 | 11q22.3 | – |
| MMP-14 | MT1-MMP | 14q11-q12 | −0.1 to +1.3 (1.4) |
| MMP-15 | MT2-MMP | 16q13-q21 | −0.9 to +2.1 (3.0) |
| MMP-16 | MT3-MMP | 8q21 | −1.3 to +0.7 (2.0) |
| MMP-17 | MT4-MMP | 12q24.3 | −0.8 to +1.25 (2.05) |
| MMP-19 | Stromelysin-4 | 12q14 | – |
| MMP-20 | Enamelysin | 11q22.3 | – |
| MMP-21 | 10q26.2 | +0.1 to +2.2 (2.3)b | |
| MMP-23B | 1p36.3 | −0.2 to +2.1 (2.3)c | |
| MMP-24 | MT5-MMP | 20q11.2 | −0.3 to +0.8 (1.1) |
| MMP-25 | MT6-MMP | 16p13.3 | −1.7 to +0.5 (2.2) |
| MMP-26 | Matrilysin-2 | 11p15 | −0.8 to −0.45 (0.35) |
| MMP-27 | 11q24 | – | |
| MMP-28 | Epilysin | 17q11-q21 | −0.3 to +1.3 (1.6) |
| TIMP-1 | Xp11.3-p11.23 | +0.3 to +0.6 (0.3) | |
| TIMP-2 | 17q25 | −0.5 to +0.9 (1.4) | |
| TIMP-3 | 22q12.3 | +0.5 to +1.5 (1.0) | |
| TIMP-4 | 3p25 | +0.25 to +0.5 (0.25) |
CpGI position (in kb) is shown relative to the transcriptional start site. The CpGI size is in parenthesis.
Multiple CpGIs exist within the indicated region.
CpGI overlaps with the whole gene region.
Figure 2.
Epigenetic signature of the selected MMPs and TIMP-2 in normal and cancer cells.
The H3K4me1 and H3K27me3 methylation profiling data of human embryonic H1 stem cells (H1 ES), mammary epithelial cells (HMEC), normal epidermal keratinocytes (NHEK), umbilical vein endothelial cell (HUVEC) and leukemia cells (K562) are derived from the ENCODE database (53, 65-67). The DNA methylation data of breast carcinoma cells (MCF-7, ZR-75-1 and MDA-MB-468), HMEC, prostate carcinoma cells (PC-3, LnCap, 22Rv1 and Du145) and normal prostate cells (RWPE1) are derived from (68). DNA methylation data of the frontal cortex gray matter from the human brain are derived from (118). Color peaks correspond to the enrichment levels of H3K4me1 (red) and H3K27me3 (blue) across the genome (66). Green color marks the POL2-association sites (66). *POL2 data are not available. (A) The 11q22.3 locus includes MMP-1, -3, -7, -8, -10, -12, -13, -20, and -27. The arrows show the size and the transcription orientation of the MMP genes. The numbers below the line show the coordinates in chromosome 11. (B) DNA methylation profiling of the MT1-MMP gene. Each circle represents the methylation data of the individual probe. Open and closed circles represent unmethylated and methylated sites, respectively (68). Open and closed bars correspond to the non-coding and coding regions of the gene, respectively. The arrowhead indicates the transcriptional start site. The thick gradient bar represents CpGI. The panels (C), (D) and (E) show the epigenetic signature of the MMP-9, MMP-11 and TIMP-2 genes, respectively. MeDIP, methylated DNA immunoprecipitation.
DNA methylation of the regulatory genes may indirectly affect the expression of MMPs in malignancy. Thus, aberrant methylation of fibulin-5 selectively increases the level of MMP-7 and stimulates the migration of lung cancer cells (62). Similarly, protein kinase PKD1 is highly expressed in the normal ductal epithelial mammary cells in which it performs as a suppressor of MMP-2, -7, -9, -10, -11, -13, -14 and -15. In turn, the PDK1 gene is highly methylated and repressed in invasive breast tumors, while the MMP expression and the invasive cell phenotype are stimulated (63).
Epigenetic profiling of the 11q22.3 MMP cluster in highly migratory glioma U251 cells recorded its selective epigenetic stimulation, including histone H3 hyperacetylation and a gain in the H3K4me2 mark, of the actively transcribed MMP-7, -10 and -13 genes, but not of the MMP-1, -3, -8, -13, -20, and -27 genes. In the non-migratory breast carcinoma MCF-7 cells, in turn, hypoacetylation, a loss of H3K4me2 and a gain in the repressive H3K27me3 mark were observed resulting in epigenetic silencing of MMPs of the 11q22.3 locus (64). In contrast with other MMPs, MMP-8 was epigenetically silenced in both breast carcinoma and glioma cells, providing evidence for the stringent epigenetic control of this proteinase. The global epigenetic profiling data of human H1 embryonic stem cell line (H1 ES), mammary epithelial cells (HMEC), normal epidermal keratinocytes (NHEK), umbilical vein endothelial cell (HUVEC) and leukemia cells (K562) are now available from the Broad Institute and the ENCODE group (53, 65-67). According to these data, there is a differential deposition of the H3K4 methylation marks in the H1 ES and K562 cells relative to HMEC, NHEK and HUVEC (Figure 2A). In NHEK and HUVEC, H3K4me1, a marker of the transcriptionally active genes, strongly associates with the POL2 sites. The H3K4me1 profiles are similar in H1 ES and K562 cells. This similarity is not entirely surprising because K562 cells have multiple features of the multipotent hematopoietic stem cells. The density of the repressive histone marks, including H3K27 methylation, is limited in the 11q22.3 locus (data not shown). Overall, epigenetic signatures of MMPs of the 11q22.3 genomic region exhibit striking similarity with the developmentally regulated genes in ES cells.
Epigenetic regulation of the pro-invasive MT1-MMP/MMP2 axis
Experimental evidence suggests that there is an inverse correlation between the promoter methylation and both the expression levels of the pro-invasive MT1-MMP and MMP-2 and cell migration efficiency. The MT1-MMP and MMP-2 promoter regions are hyper-methylated in non-migratory MCF-7 cells. As a result, these cells do not synthesize MT1-MMP and MMP-2. In contrast, hypo-methylation was recorded in the MT1-MMP and MMP-2 promoter regions in the highly migratory glioma cells (11). According to the methylated DNA immunoprecipitation (MeDIP) profiling, the promoter methylation also inversely correlates with the MT1-MMP expression in prostate and breast cancer cells (Figure 2B) (68). These data correlate well with the inverse correlation between promoter methylation and expression levels of MMP-2 in prostate cancer cells reported by Shukeir et al. (69).
In agreement, 5-Aza increases both the levels of MT1-MMP and MMP-2 and cell invasion in pancreatic cancer cells (70). Furthermore, H3K27 methylation is a clear mark of the inactive MMP-2 gene in non-migratory MCF-7 cells, but not in migratory U251 cells. Interestingly, the role of histone H3 acetylation (H3ac) and H3K4 methylation appears to be limited in regulating MMP-2 and MT1-MMP (11). In agreement, trichostatin A, an inhibitor of HDACs, had no effect on MT1-MMP (71). Hyperacetylation of migration-associated MMP genes explains why HDAC inhibitors were inefficient in repressing migration and invasion of cancer cells.
Regulation of anti-tumorigenic MMPs
The specific epigenetic regulation of the anti-tumorigenic MMPs remains insufficiently understood. There are no clear repressive epigenetic marks in MMP-3, -8 and -12 (Figure 2A) (64). Stimulatory H3K4me1 mark was observed in several primary cell types and associated with activation of the MMP-3 and -8 transcriptional activity. It is, however, unknown if the H3K4me1 mark alone is sufficient or if other factors play a primary role in transcriptional silencing of these three MMPs in malignancy.
The precise epigenetic regulation of MMP-11 that may perform as both an activator and suppressor in malignancies (72, 73) also remains unclear. The ENCODE data show that there are two H3K4me1 and H3K27me3 deposition sites in the MMP-11 gene (Figure 2C). The first site overlaps with the first exon and the CpGI. Only in H1 ES cells the H3K4me1 and H3K27me3 deposition is co-localized in this region. The second H3K4me1 site is close to exon 6.
The regulation pattern of MMP-9 is likely unique among MMPs. The extensive CpGIs are present in the MMP-9 gene coding region (Figure 2D) suggesting an important role of DNA methylation in regulating MMP-9. In agreement, DNA methylation of the promoter and MMP-9 silencing were reported in lymphomas (74) and carcinomas (75). These early observations correlate well with the more recent MeDIP data (68). According to our data, MMP-9 is epigenetically repressed in glioma U251 cells compared with breast carcinoma MCF-7 cells. In MCF-7 cells the elevated expression of MMP-9 correlated with the presence of H3ac and H3K4 methylation. These data agree with the anti-tumorigenic role of MMP-9 expressed by glioma cells (76).
Epigenetic regulation of TIMPs
The available data strongly suggest that epigenetic regulation of TIMPs is different from that of MMPs. There is also a significant difference in the regulation of the different TIMPs. There are pronounced CpGIs in the TIMPs’ promoters. DNA methylation plays a significant role in regulating all TIMP species including the suppression of TIMP-3 in malignancies (77, 78). Because of its localization in the X chromosome, TIMP-1 (but not other TIMPs) is a subject for epigenetic regulation and dosage control primarily among females (79).
In contrast with other TIMPs, there are two CpGIs in the TIMP-2 promoter (Figure 2E). The major CpGI overlaps with the first exon. This CpGI is generally unmethylated, but undergoes aberrant hypermethylation in some cancers (80-82). The additional, highly methylated, CpGI is localized 1.5kb upstream of the promoter (11). The presence of these two CpGIs provides an opportunity to epigenetically balance the levels of TIMP-2 in cancer. Transcriptional activity of the TIMP-2 gene also correlates with the presence of the stimulatory H3ac, H3K4me1 and H3K4me2 marks and low levels of the repressive H3K27me3 (Figure 2E) (64).
Epigenetics of collagens
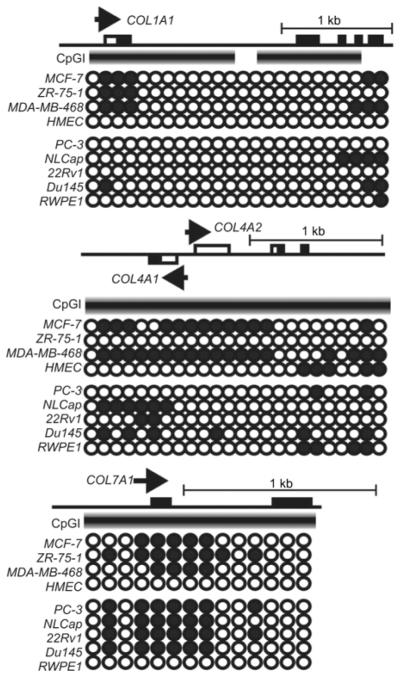
The presence of pronounced CpGIs near the transcriptional start sites is a feature of many collagen genes. This feature suggests that DNA methylation plays an important role in regulating collagens. Our analysis of the MeDIP data demonstrates that the DNA methylation patterns of COL1A1 and COL4A1/A2 vary in cancer and normal cells (68). On a similar note, a loss of type VII anchoring collagen is associated with an increased risk of epidermal cancer (43). The MeDIP data suggest that DNA hypermethylation is the cause of the COL7A1 silencing the mammary and prostate cancer cell lines (Figure 3).
Figure 3.
DNA methylation profiling of the COL1A1, COL4A1/A2 and COL7A1 collagen genes.
Each circle represents the methylation data of the individual probe. Open and closed circles represent unmethylated and methylated sites, respectively (68). The arrowhead indicates the transcriptional start site. The thick gradient bar represents CpGI. Open and closed bars correspond to the non-coding and coding regions of the gene, respectively. The cell line abbreviations are shown in the legend of Figure 2.
In contrast to many other tissues, the level of collagen is limited in brain parenchyma (83). Strikingly, collagen expression is significantly up-regulated in GBM (64, 83, 84). The data suggest that this up-regulation and subsequent deposition of collagens by tumor lesions facilitate the invasion and spread of GBM cells through the brain (64, 84). It is becoming increasingly clear that histone modifications (H3ac and H3K4me2) play a significant role in the up-regulation of collagens including type I collagen in GBM (64). Conversely, an abundant H3K27me3 deposition was recorded in the inactive collagen genes in MCF-7 cells.
Transcriptional activity of the collagen genes in glioma cells also correlates with the expression level of TGFBI. TGFBI encodes an RGD-containing adhesion protein that binds to type I, II and IV collagens and that plays an important role in cell-matrix interactions (85, 86). In agreement with the deposition of collagens by GBM, the collagen internalization receptor urokinase-type plasminogen activator receptor-associated protein (UPARAP)/Endo180/CD280 is also highly expressed in GBM (84). UPARAP also mediates the collagenolytic activity of MT1-MMP (87). UPARAP mediates the invasion of GBM cells through collagen-containing matrices. Taking together, these studies suggest that GBM-derived collagen is an important constituent of the tumor microenvironment in the brain and that epigenetic regulation of the ECM collagens plays a role in facilitating GBM cell migration through the brain tissues. It is tempting to hypothesize that the specific epigenetic mechanisms which control collagens and consequently, brain cancer spread, represent novel and promising drug targets and that these targets can be challenged pharmacologically.
MicroRNAs
MicroRNAs (miRNA) are small, approximately 22 nucleotides, endogenous noncoding RNAs. miRNAs modulate target gene expression by binding predominantly with the 3′-untranslated region (UTR) of target mRNA sequences. The mature miRNA species binds an RNA-induced silencing complex and then represses protein synthesis either by blocking translation or by causing transcript degradation (88-91). Because a perfect complimentarity is not required for the miRNA binding with a target mRNA, over 1000 currently known miRNAs can regulate a majority of genes in the human genome (1). The miRNA expression profiles are significantly different in cancer vs. normal cells. A growing number of miRNAs have been reported to contribute to the regulation of cell functions, including tumor cell invasion. Many miRNAs function as tumor oncogenes or anti-oncogenes.
Evidence is emerging that miRNAs are directly involved in the regulation of ECM components (92). Our data suggest that miRNAs play an important function in highly migratory glioma cells. The total concentration of miRNAs is three-fold higher in highly migratory glioma U251 cells compared to that in non-migratory breast carcinoma MCF-7 cells (64). It appears that glioma cells efficiently employ the miRNA mechanisms to impose an additional level of control in the process of cell migration.
Some recent data are summarized in Table 2. Thus, miR-21 targets MMPs and, as a result, promotes glioma cell invasion (93). The known targets of miRNAs in cancers include MMP-2, MMP-9 MMP-13, MMP-14, and MMP-16 and also their cleavage targets such as type I, II and IV collagens. Frequently, miRNAs (miR-181b, -21 and -221/222) repress the expression of the genes coding for TIMPs, such as the ECM-associated TIMP-3. The repression of TIMP-3 expression increases the activity of invasion-promoting MMPs. Currently, a more in-depth analysis of miRNA is being performed in many laboratories and volumes of data linking miRNAs with MMPs, TIMPs, collagens and ECM maintenance will be generated in the very near future.
Table 2.
miRNAs, MMPs and TIMPs.
| miRNA | Target gene | Cell system | Ref. |
|---|---|---|---|
| miR-10b | MMP-14 | GBM | (94) |
| miR-143 | MMP-13 | Osteosarcoma | (95) |
| miR-146b | MMP-16 | GBM | (96) |
| miR-181b | TIMP-3 | Hepatocellular carcinoma | (97) |
| miR-206 | MMP-2/MMP-9 | Breast carcinoma | (98) |
| miR-216a | Type II collagen | Renal mesangial cells | (99) |
| miR-218 | MMP-9 | GBM | (100) |
| miR-21 | TIMP-3 | Cholangiocarcinoma, GBM, breast carcinoma | (93, 101, 102) |
| miR-212/132 | MMP-9 | Mammary stromal cells | (103) |
| miR-221/222 | TIMP-3 | Hepatocellular and non-small cell lung carcinomas | (104) |
| miR-27b | MMP-13 | Osteoarthritis chondrocytes | (105) |
| miR-29 | Type I/III collagens | Systemic sclerosis | (106) |
| miR-29a | Type IV collagen | Human kidney HK2 cells | (107) |
| miR-29b | Multiple collagens and MMP-2 | Renal fibrosis | (108, 109) |
| miR-29b | Type I/IV collagens and MMP-2 | Prostate carcinomas | (110) |
| miR-29b | Type I collagen | Stellate cells | (111) |
| miR-29b | MMP-2/MMP-9 | Human aortic smooth muscle cell | (112) |
| miR-451 | MMP-2/MMP-9 | GBM | (113) |
| miR-488 | MMP-2 | Mesenchymal cells | (114) |
| miR-675 | Type II collagen | Chondrocytes | (115) |
Expert opinion
The available, albeit limited, information suggests that the multiple epigenetic mechanisms are engaged in the selective regulation of the transcriptional activity of MMPs, TIMPs and their ECM collagen substrates in cancer compared with normal tissue. The resulting misbalance among MMPs, TIMPs and collagens contributes to cancer cell migration and invasion. Additional comprehensive epigenetic, genome-wide transcriptional and proteomics profiling studies of the DNA methylation and histone modification cues are required to elucidate further a sophisticated regulatory network that governs ECM homeostasis in both normal tissue and disease.
Outlook
Progress in the development of analytical technologies including DNA methylation, Chip-on-Chip, miRNA and gene expression microarrays and proteomics and bioinformatics will result in the volumes of the novel, systems biology level data. We believe that future genome-wide transcriptional, epigenetic and proteomics studies will lead to a much better understanding of how migration-associated genes are regulated in malignancy. As a result, opportunities will emerge to target these genes pharmacologically. This increased level of understanding will ultimately lead to the development of personalized medicine for cancer patients.
Highlights.
Epigenetic mechanisms play an important role in the regulation of MMPs, TIMPs and ECM.
MMPs and TIMPs are regulated differently.
The epigenetic control plays an important role in the regulation of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis.
The regulation pattern of MMP-9 is likely to be unique among MMPs.
In certain cancer types epigenetic signatures of selected MMPs exhibit stem cell-like characteristics.
DNA methylation and histone modification stimulate the expression of collagens in highly migratory cancer cells.
Glioblastomas/gliomas deposit collagen in the brain to stimulate migration of tumor cells through the brain parenchyma.
Epigenetic regulation of collagens is an important parameter of the brain tumors.
Specific epigenetic mechanisms that control collagens represent novel and promising drug targets in glioblastomas/gliomas.
Acknowledgments
We apologize to colleagues whose work was not included in our manuscript because of space limitations. This work was supported by National Institutes of Health Grants CA083017 and CA077470 (to A.Y.S.).
References
- 1.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–79. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 2.Fry CJ, Peterson CL. Chromatin remodeling enzymes: who’s on first? Curr Biol. 2001;11:R185–97. doi: 10.1016/s0960-9822(01)00090-2. [DOI] [PubMed] [Google Scholar]
- 3.Swigut T, Wysocka J. H3K27 demethylases, at long last. Cell. 2007;131:29–32. doi: 10.1016/j.cell.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Turner BM. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat Struct Mol Biol. 2005;12:110–2. doi: 10.1038/nsmb0205-110. [DOI] [PubMed] [Google Scholar]
- 5.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–60. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 7.Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–4. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 8.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–20. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–3. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 10.Peters AH, Mermoud JE, O’Carroll D, Pagani M, Schweizer D, Brockdorff N, Jenuwein T. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet. 2002;30:77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- 11.Chernov AV, Sounni NE, Remacle AG, Strongin AY. Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J Biol Chem. 2009;284:12727–34. doi: 10.1074/jbc.M900273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright JE, Keogh RJ, Tissot van Patot MC. Hypoxia and placental remodelling. Adv Exp Med Biol. 2007;618:113–26. doi: 10.1007/978-0-387-75434-5_9. [DOI] [PubMed] [Google Scholar]
- 13.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–23. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 15.Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339:247–57. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomis-Ruth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Mol Biotechnol. 2003;24:157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 18.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 19.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 20.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP) Curr Top Dev Biol. 2003;54:1–74. doi: 10.1016/s0070-2153(03)54004-2. [DOI] [PubMed] [Google Scholar]
- 22.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–6. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 23.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 25.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–7. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 26.Golubkov VS, Cieplak P, Chekanov AV, Ratnikov BI, Aleshin AE, Golubkova NV, Postnova TI, Radichev IA, Rozanov DV, Zhu W, Motamedchaboki K, Strongin AY. Internal cleavages of the autoinhibitory prodomain are required for membrane type 1 matrix metalloproteinase activation, although furin cleavage alone generates inactive proteinase. J Biol Chem. 2010;285:27726–36. doi: 10.1074/jbc.M110.135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 28.Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: a tethered collagenase. J Cell Physiol. 2004;200:11–9. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 29.Coyle RC, Latimer A, Jessen JR. Membrane-type 1 matrix metalloproteinase regulates cell migration during zebrafish gastrulation: evidence for an interaction with non-canonical Wnt signaling. Exp Cell Res. 2008;314:2150–62. doi: 10.1016/j.yexcr.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Golubkov VS, Chekanov AV, Cieplak P, Aleshin AE, Chernov AV, Zhu W, Radichev IA, Zhang D, Dong PD, Strongin AY. The Wnt/planar cell polarity protein-tyrosine kinase-7 (PTK7) is a highly efficient proteolytic target of membrane type-1 matrix metalloproteinase: implications in cancer and embryogenesis. J Biol Chem. 2010;285:35740–9. doi: 10.1074/jbc.M110.165159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh J, Takahashi R, Adachi E, Kondo S, Kuratomi S, Noma A, Alexander DB, Motoda H, Okada A, Seiki M, Itoh T, Itohara S, Takahashi C, Noda M. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene. 2004;23:5041–8. doi: 10.1038/sj.onc.1207688. [DOI] [PubMed] [Google Scholar]
- 32.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Otin C, Palavalli LH, Samuels Y. Protective roles of matrix metalloproteinases: from mouse models to human cancer. Cell Cycle. 2009;8:3657–62. doi: 10.4161/cc.8.22.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez-Fernandez A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN, Sweep FC, Puente XS, Lopez-Otin C. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–63. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- 36.Korpi JT, Kervinen V, Maklin H, Vaananen A, Lahtinen M, Laara E, Ristimaki A, Thomas G, Ylipalosaari M, Astrom P, Lopez-Otin C, Sorsa T, Kantola S, Pirila E, Salo T. Collagenase-2 (matrix metalloproteinase-8) plays a protective role in tongue cancer. Br J Cancer. 2008;98:766–75. doi: 10.1038/sj.bjc.6604239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decock J, Long JR, Laxton RC, Shu XO, Hodgkinson C, Hendrikx W, Pearce EG, Gao YT, Pereira AC, Paridaens R, Zheng W, Ye S. Association of matrix metalloproteinase-8 gene variation with breast cancer prognosis. Cancer Res. 2007;67:10214–21. doi: 10.1158/0008-5472.CAN-07-1683. [DOI] [PubMed] [Google Scholar]
- 38.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–55. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 39.Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, Pozzi A, Carbone DP, Schwartz DR, Moin K, Sloane BF, Matrisian LM. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–75. doi: 10.1158/0008-5472.CAN-05-4279. [DOI] [PubMed] [Google Scholar]
- 40.Savinov AY, Remacle AG, Golubkov VS, Krajewska M, Kennedy S, Duffy MJ, Rozanov DV, Krajewski S, Strongnin AY. Matrix metalloproteinase 26 proteolysis of the NH2-terminal domain of the estrogen receptor beta correlates with the survival of breast cancer patients. Cancer Res. 2006;66:2716–24. doi: 10.1158/0008-5472.CAN-05-3592. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 42.McCawley LJ, Wright J, LaFleur BJ, Crawford HC, Matrisian LM. Keratinocyte expression of MMP3 enhances differentiation and prevents tumor establishment. Am J Pathol. 2008;173:1528–39. doi: 10.2353/ajpath.2008.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martins VL, Vyas JJ, Chen M, Purdie K, Mein CA, South AP, Storey A, McGrath JA, O’Toole EA. Increased invasive behaviour in cutaneous squamous cell carcinoma with loss of basement-membrane type VII collagen. J Cell Sci. 2009;122:1788–99. doi: 10.1242/jcs.042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoder JA, Soman NS, Verdine GL, Bestor TH. DNA (cytosine-5)-methyltransferases in mouse cells and tissues. Studies with a mechanism-based probe. J Mol Biol. 1997;270:385–95. doi: 10.1006/jmbi.1997.1125. [DOI] [PubMed] [Google Scholar]
- 45.Xie S, Wang Z, Okano M, Nogami M, Li Y, He WW, Okumura K, Li E. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236:87–95. doi: 10.1016/s0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- 46.Coulondre C, Miller JH, Farabaugh PJ, Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978;274:775–80. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- 47.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987;196:261–82. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 49.Illingworth RS, Bird AP. CpG islands – ‘a rough guide’. FEBS Lett. 2009;583:1713–20. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Tost J. DNA methylation: an introduction to the biology and the disease-associated changes of a promising biomarker. Methods Mol Biol. 2009;507:3–20. doi: 10.1007/978-1-59745-522-0_1. [DOI] [PubMed] [Google Scholar]
- 51.Lopez J, Percharde M, Coley HM, Webb A, Crook T. The context and potential of epigenetics in oncology. Br J Cancer. 2009;100:571–7. doi: 10.1038/sj.bjc.6604930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, Fisher AG. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–8. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 54.Roh TY, Cuddapah S, Cui K, Zhao K. The genomic landscape of histone modifications in human T cells. Proc Natl Acad Sci USA. 2006;103:15782–7. doi: 10.1073/pnas.0607617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan C, Wang H, Toh Y, Boyd DD. Repression of 92-kDa type IV collagenase expression by MTA1 is mediated through direct interactions with the promoter via a mechanism, which is both dependent on and independent of histone deacetylation. J Biol Chem. 2003;278:2309–16. doi: 10.1074/jbc.M210369200. [DOI] [PubMed] [Google Scholar]
- 56.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Muertter RN, Edgar R. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res. 2009;37:D885–90. doi: 10.1093/nar/gkn764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun T, Gao Y, Tan W, Ma S, Zhang X, Wang Y, Zhang Q, Guo Y, Zhao D, Zeng C, Lin D. Haplotypes in matrix metalloproteinase gene cluster on chromosome 11q22 contribute to the risk of lung cancer development and progression. Clin Cancer Res. 2006;12:7009–17. doi: 10.1158/1078-0432.CCR-06-0464. [DOI] [PubMed] [Google Scholar]
- 58.Tsuchiya N, Narita S, Kumazawa T, Inoue T, Ma Z, Tsuruta H, Saito M, Horikawa Y, Yuasa T, Satoh S, Ogawa O, Habuchi T. Clinical significance of a single nucleotide polymorphism and allelic imbalance of matrix metalloproteinase-1 promoter region in prostate cancer. Oncol Rep. 2009;22:493–9. doi: 10.3892/or_00000462. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu C, Matsubara T, Onouchi Y, Jain S, Sun S, Nievergelt CM, Shike H, Brophy VH, Takegawa T, Furukawa S, Akagi T, Newburger JW, Baker AL, Burgner D, Hibberd ML, Davila S, Levin M, Mamtani M, He W, Ahuja SK, Burns JC. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55:779–84. doi: 10.1038/jhg.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Ogawa M, Wood JR, Bartolomei MS, Sammel MD, Kusanovic JP, Walsh SW, Romero R, Strauss JF., 3rd Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17:1087–96. doi: 10.1093/hmg/ddm381. [DOI] [PubMed] [Google Scholar]
- 61.Couillard J, Demers M, Lavoie G, St-Pierre Y. The role of DNA hypomethylation in the control of stromelysin gene expression. Biochem Biophys Res Commun. 2006;342:1233–9. doi: 10.1016/j.bbrc.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 62.Yue W, Sun Q, Landreneau R, Wu C, Siegfried JM, Yu J, Zhang L. Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer Res. 2009;69:6339–46. doi: 10.1158/0008-5472.CAN-09-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eiseler T, Doppler H, Yan IK, Goodison S, Storz P. Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 2009;11:R13. doi: 10.1186/bcr2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chernov AV, Baranovskaya S, Golubkov VS, Wakeman DR, Snyder EY, Williams R, Strongin AY. Microarray-based transcriptional and epigenetic profiling of matrix metalloproteinases, collagens, and related genes in cancer. J Biol Chem. 2010;285:19647–59. doi: 10.1074/jbc.M109.088153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ, 3rd, Gingeras TR, Schreiber SL, Lander ES. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–81. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaoz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Loytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Xu M, Haidar JN, Yu Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrimsdottir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, de Jong PJ. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeshima H, Yamashita S, Shimazu T, Niwa T, Ushijima T. The presence of RNA polymerase II, active or stalled, predicts epigenetic fate of promoter CpG islands. Genome Res. 2009;19:1974–82. doi: 10.1101/gr.093310.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shukeir N, Pakneshan P, Chen G, Szyf M, Rabbani SA. Alteration of the methylation status of tumor-promoting genes decreases prostate cancer cell invasiveness and tumorigenesis in vitro and in vivo. Cancer Res. 2006;66:9202–10. doi: 10.1158/0008-5472.CAN-06-1954. [DOI] [PubMed] [Google Scholar]
- 70.Sato N, Maehara N, Su GH, Goggins M. Effects of 5-aza-29-deoxycytidine on matrix metalloproteinase expression and pancreatic cancer cell invasiveness. J Natl Cancer Inst. 2003;95:327–30. doi: 10.1093/jnci/95.4.327. [DOI] [PubMed] [Google Scholar]
- 71.Ailenberg M, Silverman M. Trichostatin A-histone deacetylase inhibitor with clinical therapeutic potential-is also a selective and potent inhibitor of gelatinase A expression. Biochem Biophys Res Commun. 2002;298:110–5. doi: 10.1016/s0006-291x(02)02420-8. [DOI] [PubMed] [Google Scholar]
- 72.Andarawewa KL, Boulay A, Masson R, Mathelin C, Stoll I, Tomasetto C, Chenard MP, Gintz M, Bellocq JP, Rio MC. Dual stromelysin-3 function during natural mouse mammary tumor virus-ras tumor progression. Cancer Res. 2003;63:5844–9. [PubMed] [Google Scholar]
- 73.Brasse D, Mathelin C, Leroux K, Chenard MP, Blaise S, Stoll I, Tomasetto C, Rio MC. Matrix metalloproteinase 11/stromelysin-3 exerts both activator and repressor functions during the hematogenous metastatic process in mice. Int J Cancer. 2010;127:1347–55. doi: 10.1002/ijc.25309. [DOI] [PubMed] [Google Scholar]
- 74.Chicoine E, Esteve PO, Robledo O, Van Themsche C, Potworowski EF, St-Pierre Y. Evidence for the role of promoter methylation in the regulation of MMP-9 gene expression. Biochem Biophys Res Commun. 2002;297:765–72. doi: 10.1016/s0006-291x(02)02283-0. [DOI] [PubMed] [Google Scholar]
- 75.Patra SK, Bettuzzi S. Epigenetic DNA-methylation regulation of genes coding for lipid raft-associated components: a role for raft proteins in cell transformation and cancer progression. Oncol Rep. 2007;17:1279–90. review. [PubMed] [Google Scholar]
- 76.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu MC, Choong DY, Hooi CS, Williams LH, Campbell IG. Genetic and epigenetic analysis of the TIMP-3 gene in ovarian cancer. Cancer Lett. 2007;247:91–7. doi: 10.1016/j.canlet.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 78.Masson D, Rioux-Leclercq N, Fergelot P, Jouan F, Mottier S, Theoleyre S, Bach-Ngohou K, Patard JJ, Denis MG. Loss of expression of TIMP3 in clear cell renal cell carcinoma. Eur J Cancer. 2010;46:1430–7. doi: 10.1016/j.ejca.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Anderson CL, Brown CJ. Epigenetic predisposition to expression of TIMP1 from the human inactive X chromosome. BMC Genet. 2005;6:48. doi: 10.1186/1471-2156-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pulukuri SM, Patibandla S, Patel J, Estes N, Rao JS. Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors. Oncogene. 2007;26:5229–37. doi: 10.1038/sj.onc.1210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galm O, Suzuki H, Akiyama Y, Esteller M, Brock MV, Osieka R, Baylin SB, Herman JG. Inactivation of the tissue inhibitor of metalloproteinases-2 gene by promoter hypermethylation in lymphoid malignancies. Oncogene. 2005;24:4799–805. doi: 10.1038/sj.onc.1208599. [DOI] [PubMed] [Google Scholar]
- 82.Ivanova T, Vinokurova S, Petrenko A, Eshilev E, Solovyova N, Kisseljov F, Kisseljova N. Frequent hypermethylation of 5′ flanking region of TIMP-2 gene in cervical cancer. Int J Cancer. 2004;108:882–6. doi: 10.1002/ijc.11652. [DOI] [PubMed] [Google Scholar]
- 83.Paulus W, Sage EH, Liszka U, Iruela-Arispe ML, Jellinger K. Increased levels of type VIII collagen in human brain tumours compared to normal brain tissue and non-neoplastic cerebral disorders. Br J Cancer. 1991;63:367–71. doi: 10.1038/bjc.1991.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, Isacke CM. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nacu N, Luzina IG, Highsmith K, Lockatell V, Pochetuhen K, Cooper ZA, Gillmeister MP, Todd NW, Atamas SP. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J Immunol. 2008;180:5036–44. doi: 10.4049/jimmunol.180.7.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shelton L, Rada JA. Inhibition of human scleral fibroblast cell attachment to collagen type I by TGFBIp. Invest Ophthalmol Vis Sci. 2009;50:3542–52. doi: 10.1167/iovs.09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Messaritou G, East L, Roghi C, Isacke CM, Yarwood H. Membrane type-1 matrix metalloproteinase activity is regulated by the endocytic collagen receptor Endo180. J Cell Sci. 2009;122:4042–8. doi: 10.1242/jcs.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–39. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 90.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 92.Wentz-Hunter KK, Potashkin JA. Role of miRNAs as key regulators in the neoplastic microenvironment. Mol Biol Int. 2011;2011:839872. doi: 10.4061/2011/839872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–80. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun L, Yan W, Wang Y, Sun G, Luo H, Zhang J, Wang X, You Y, Liu N, Yang Z. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9–18. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 95.Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T, Ito H, Oshimura M, Ochiya T. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther. 2011 doi: 10.1038/mt.2011.53. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S, Ge R, Jiang S, Li G, Chen Y, He ML, Kung HF, Lai L, Lin MC. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–65. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 97.Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. 2010;29:1787–97. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu H, Cao YD, Ye WX, Sun YY. Effect of microRNA-206 on cytoskeleton remodelling by downregulating Cdc42 in MDA-MB-231 cells. Tumori. 2010;96:751–5. doi: 10.1177/030089161009600518. [DOI] [PubMed] [Google Scholar]
- 99.Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells. J Biol Chem. 2010;285:34004–15. doi: 10.1074/jbc.M110.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song L, Huang Q, Chen K, Liu L, Lin C, Dai T, Yu C, Wu Z, Li J. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-beta. Biochem Biophys Res Commun. 2010;402:135–40. doi: 10.1016/j.bbrc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, Yang J, Paun B, Jin Z, Agarwal R, Hamilton JP, Abraham J, Georgiades C, Alvarez H, Vivekanandan P, Yu W, Maitra A, Torbenson M, Thuluvath PJ, Gores GJ, LaRusso NF, Hruban R, Meltzer SJ. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009;49:1595–601. doi: 10.1002/hep.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song B, Wang C, Liu J, Wang X, Lv L, Wei L, Xie L, Zheng Y, Song X. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res. 2010;29:29. doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PA, Thum T, Groner B, Chowdhury K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat Genet. 2010;42:1101–8. doi: 10.1038/ng.709. [DOI] [PubMed] [Google Scholar]
- 104.Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, Gasparini P, Gonelli A, Costinean S, Acunzo M, Condorelli G, Croce CM. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–71. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–43. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 107.Du B, Ma LM, Huang MB, Zhou H, Huang HL, Shao P, Chen YQ, Qu LH. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 2010;584:811–6. doi: 10.1016/j.febslet.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y, Liu W, Chao T, Zhang Y, Yan X, Gong Y, Qiang B, Yuan J, Sun M, Peng X. MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett. 2008;272:197–205. doi: 10.1016/j.canlet.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 109.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW, Jr, Ferreri NR, Yeo NC, Liang M. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–82. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steele R, Mott JL, Ray RB. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer. 2010;1:381–387. doi: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogawa T, Iizuka M, Sekiya Y, Yoshizato K, Ikeda K, Kawada N. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Biophys Res Commun. 2010;391:316–21. doi: 10.1016/j.bbrc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 112.Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J. 2011;25:1718–28. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- 113.Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Zhong Y, Kang C. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 114.Song J, Kim D, Jin EJ. MicroRNA-488 suppresses cell migration through modulation of the focal adhesion activity during chondrogenic differentiation of chick limb mesenchymal cells. Cell Biol Int. 2010;35:179–85. doi: 10.1042/CBI20100204. [DOI] [PubMed] [Google Scholar]
- 115.Dudek KA, Lafont JE, Martinez-Sanchez A, Murphy CL. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem. 2010;285:24381–7. doi: 10.1074/jbc.M110.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tuuttila A, Morgunova E, Bergmann U, Lindqvist Y, Maskos K, Fernandez-Catalan C, Bode W, Tryggvason K, Schneider G. Three-dimensional structure of human tissue inhibitor of metalloproteinases-2 at 2.1 A resolution. J Mol Biol. 1998;284:1133–40. doi: 10.1006/jmbi.1998.2223. [DOI] [PubMed] [Google Scholar]
- 117.Fernandez-Catalan C, Bode W, Huber R, Turk D, Calvete JJ, Lichte A, Tschesche H, Maskos K. Crystal structure of the complex formed by the membrane type 1-matrix metalloproteinase with the tissue inhibitor of metalloproteinases-2, the soluble progelatinase A receptor. EMBO J. 1998;17:5238–48. doi: 10.1093/emboj/17.17.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, Turecki G, Delaney A, Varhol R, Thiessen N, Shchors K, Heine VM, Rowitch DH, Xing X, Fiore C, Schillebeeckx M, Jones SJ, Haussler D, Marra MA, Hirst M, Wang T, Costello JF. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–7. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]