Abstract
Background
Sensitization and activation of trigeminal neurons are implicated in the underlying pathology of migraine, acute sinusitis, and allergic rhinitis. Cell bodies of trigeminal neurons that provide sensory innervation of the dura and nasal mucosa reside in the trigeminal ganglion in association with satellite glial cells where they communicate via gap junctions. Gap junctions, channels formed by connexins, modulate the excitability state of both neurons and glia under pathological conditions. Tonabersat, a compound being tested as an antimigraine drug, is thought to block gap junction activity.
Objective
To investigate the cellular events within trigeminal ganglia that may account for the significant comorbidity of migraine and rhinosinusitis and determine the effect of tonabersat on neuron-satellite glia communication.
Methods
Sprague Dawley rats injected with True Blue were used to localize neuronal cell bodies in the ganglion and study neuronglia signaling via gap junctions in the trigeminal ganglion. Dye coupling studies were conducted under basal conditions and in response to tumor necrosis factor-alpha injection into the whisker pad and/or capsaicin injection into the eyebrow. Changes in connexin 26 and active p38 levels were determined by immunohistochemistry. In addition, the effect of tonabersat prior to chemical stimulation on gap junction activity and expression of connexins and active p38 was investigated.
Results
Injection of tumor necrosis factor-alpha, a cytokine implicated in the pathology of acute sinusitis and allergic rhinitis, into the V2 region was shown to lower the amount of capsaicin required to stimulate neurons located in the V1 region of the ganglion. While injection of tumor necrosis factor-alpha into the whisker pad or capsaicin injection into the eyebrow alone did not cause increased dye movement, the combination of both stimuli greatly increased neuron-satellite glia communication via gap junctions in both V1 and V2 regions. The change in gap junction activity was accompanied by increased expression of connexin 26 and active p38 levels in both neurons and satellite glia in V1 and V2 regions. Pretreatment with tonabersat inhibited gap junction communication between neurons and satellite glia and blocked the increase in connexin 26 and active p38 levels in response to injection of both tumor necrosis factor-alpha (V2) and capsaicin (V1).
Conclusions
We propose that increased levels of tumor necrosis factor-alpha, as reported during acute sinusitis and allergic rhinitis, reduces the amount of capsaicin necessary to stimulate V1 neurons that leads to cellular changes in both V1 and V2 regions. The cellular events observed in this study may help to explain, in part, the significant comorbidity reported with migraine and rhinosinusitis. In addition, we have provided evidence to suggest that tonabersat can prevent increased neuron-satellite glia signaling and, thus, may be useful in the treatment of migraine, acute sinusitis, and allergic rhinitis.
Keywords: connexins, gap junctions, p38, satellite glia, tonabersat, trigeminal
Migraine patients often report sinus pressure or pain and nasal congestion during severe migraine attacks and it has been suggested that sinus pathology can act as a trigger of migraine.1,2 Furthermore, patients suffering from acute allergic rhinitis experience headache and migraine at a much higher frequency than nonallergic subjects.3 While migraine and rhinosinusitis exhibit considerable comorbidity, the underlying cellular mechanisms are not well understood. However, it is well established that activation of trigeminal nerves and the peripheral and central release of neuropeptides are involved in mediating the inflammatory and nociceptive events characteristic of both migraine and rhinosinusitis.4–6 The trigeminal nerve consists of 3 major branches referred to as the ophthalmic (V1), maxillary (V2), and mandibular (V3) branches. Each branch provides somatosensory innervation of distinct regions of the head, face, nasal, and sinus cavities.7
Primary afferent neurons, whose cell bodies reside in the trigeminal ganglion, convey sensory information from peripheral tissues implicated in migraine and rhinosinusitis to the central nervous system (CNS). The pathophysiological events involved in migraine and rhinosinusitis involve both peripheral and central sensitization.4,8–10 Peripheral sensitization, which is the result of increased activity of trigeminal nociceptors, is thought to play a key role in the initiation of migraine and rhinosinusitis, while central sensitization, which involves enhanced excitability of second-order neurons, leads to pain.11 Peripheral sensitization is characterized by increased neuronal excitability and a lowering of the threshold for activation. In this context, activation is defined as causing changes in the cell that allow it to perform functions beyond those present in a basal state.12 It is now thought that glia cells that are closely associated with peripheral and central neurons can directly modulate the functional and excitability state of these neurons.12,13 Furthermore, neuronglia interactions are reported to be involved in all stages of inflammation and pain associated with several CNS diseases.14,15
Within the trigeminal ganglion, the cell bodies of neurons are completely surrounded by specialized glial cells known as satellite glia that together form distinct, functional units.13 Morphological studies have provided evidence that neurons and satellite glial cells extend processes that are thought to facilitate exchange of chemicals between neurons and glia.16,17 In addition, it was recently shown that trigeminal ganglion neurons and satellite glial cells can communicate directly via gap junctions.18
Gap junctions serve as intercellular conduits that allow for direct transfer of small molecular weight molecules, such as ions, that regulate cellular excitability, metabolic precursors, and second messengers.19,20 Gap junctions are found in most neurons and glial cells and function to facilitate neuron-neuron, glia-glia, and neuron-glia communication. Within the CNS, gap junctions are abundant and allow for extensive intercellular coupling between cells that form a communication network.19,21 Each cell contributes a hemichannel composed of 6 transmembrane proteins known as connexins. The connexin family includes more than 20 members.22 However, only 10 connexin proteins are known to be expressed by neuronal or glial cells.21 Connexins are dynamic membrane proteins that exhibit short half-lives.23 Changes in the expression of connexins and hence, communication through gap junctions, are associated with numerous CNS diseases including Alzheimer’s disease, as well as cortical spreading depression.19 Similarly, we have recently provided evidence of enhanced neuron to satellite glia communication occurring through gap junctions within trigeminal ganglion in response to inflammatory stimuli.18 The expression of connexin proteins involved in forming gap junctions between neuronal and satellite glial cells within the trigeminal ganglion under normal and disease states is not known. In addition, we have observed cross activation within the ganglion by which stimulation of neurons in one branch caused a rapid and sustained activation in the other branches, an example of intraganglionic communication.18 Based on our previous findings, we propose that neuronal-satellite glial cell signaling is involved in initiating and maintaining peripheral sensitization within the ganglion and, thus, contributes to the significant comorbidity reported for migraine, acute sinusitis, and allergic rhinitis.
In this study, we used an in vivo animal model to test whether treatment of V2 neurons by tumor necrosis factor-alpha (TNF-α), a cytokine whose levels are elevated in nasal secretions during allergic rhinitis, can reduce the amount of stimulus required for cellular changes in neurons located in the V1 region, and thus act as a potential trigger. Increased neuron-satellite glia communication via gap junctions, as well as increased levels of connexin 26 and active p38, was observed in neurons and glia located in both V1 and V2 regions in response to cotreatment with TNF-α and capsaicin. Another significant finding from our study was that pretreatment with the anti-migraine drug tonabersat decreased gap junction communication and the level of connexin 26, and blocked p38 activation in both neurons and satellite glia.
METHODS
Animals
The animal care and procedures were conducted in accordance with institutional and National Institutes of Health guidelines. Sprague Dawley rats (Charles River Laboratories Inc., Wilmington, MA, USA) were housed in clean plastic cages on a 12-hour light/dark cycle with unrestricted access to food and water.
Dye Coupling Studies
Young male rats (200–225 g) were brought to the lab and kept in a quiet environment for about 30 minutes. Rats were anesthetized by an intraperitoneal (i.p.) injection of 0.3 mL of ketamine and xylazine solution (800 mg and 60 mg in 10 mL, respectively; Sigma, St. Louis, MO, USA). Rats were observed for about 15 minutes and were assessed for effects of anesthesia using writhing reflex and tonicity of the tail (tail flick reflex). Initially, rats were injected with a fluorescent dye, True Blue (25 μL, 2 mg/mL in dimethyl sulfoxide, DMSO; Invitrogen, Eugene, OR, USA) in the whisker pad or eyebrow regions to retrogradely label neuronal cell bodies within the V1 and V2 regions of the trigeminal ganglion. Bilateral injections were performed using a 50 μL Hamilton syringe (Hamilton Company, Reno, NV, USA) and a 261/2 G needle (Becton Dickinson, Franklin Lakes, NJ, USA). Initially, dye was injected subcutaneously into both right and left whisker pads (25 μL total volume; 4 injections per side) and also injected in the brow of each eye (25 μL total; 3 injections per side). After 5 days, the animal was sacrificed and both trigeminal ganglia removed and immediately placed in Neg 50 mounting medium (Richard-Allan Scientific, Kalamazoo, MI, USA) at −25°C for further analysis. For the labeling studies, the entire ganglion was sectioned from the dorsal to ventral surface in 20 μm tissue sections using a cryostat (Microm HM525, Richard Allan Scientific) set at −25°C. Up to 6 sections were placed on each Superfrost Plus microscope slide (Fischer Scientific, Pittsburgh, PA, USA). After fixing and permeabilizing, tissues were covered in Vectashield mounting medium (H-1000, Vector Laboratories, Burlingame, CA, USA), and images (40×, 100×, or 400×) were collected using an Olympus DP70 camera mounted on an Olympus BX41 fluorescent microscope and image analysis performed using Olympus Micro-Suite Five image processing software (Olympus, Center Valley, PA, USA). Multiple image alignment was utilized to view the entire ganglion in a single image at 40× magnification as described previously.18 For the gap junction studies, True Blue was bilaterally injected into the right and left whisker pads and eyebrows 5 days prior to injection of chemical agents to allow for retrograde transport of the dye to the neuronal cell bodies in the V1 and V2 regions of the ganglion. A single injection site was used to deliver TNF into the whisker pads. Specifically, the needle was inserted just posterior to the middle positioned vibrissa and ~6 μL was injected in 4 different regions in a radiating pattern that covered approximately 1 cm2. Both ganglia were then collected from untreated animals (n = 5) or animals injected with TNF-α (1 ng/mL in phosphate buffered saline [PBS], pH 7.4) in whisker pads for 120 minutes (n = 3) or injection of capsaicin in the eyebrows (1 nM in 100% DMSO) for 15 minutes (n = 3) and processed for microscopic analysis as described above. For the TNF-α/capsaicin study, animals (n = 4) were initially injected with TNF-α. After 2 hours, animals were then injected with capsaicin and ganglia collected 15 or 60 minutes following capsaicin treatment. For the tonabersat studies, animals (n = 3) were injected (i.p.) with 10 mg/kg tonabersat (cis-[-]-6-acetyl-4S-[3-chloro-4-fluorobenzoylamino]-3,4-dihydro-2,2-dimethyl-2H-1-benzopyran-3S-ol; kindly provided by Minster Pharmaceuticals) dissolved in 100% DMSO or an equal volume of DMSO 5 minutes prior to TNF-α injection.
Immunohistochemistry
For immunohistochemical studies, ganglia were collected from untreated animals or animals injected with TNF-α, capsaicin, or TNF-α and capsaicin as described above. Twenty μm sections from the medial region of the ganglion that contained all 3 major regions (section numbers 15–25) were fixed using 4% paraformaldehyde for 60 minutes, permeabilized with 0.3% Triton – ×100 for 60 minutes, and then incubated in 5% donkey serum for another 60 minutes. Sections were then incubated overnight at 4°C with primary antibodies against connexin 26 (1: 500 in PBS; Chemicon, Temecula, CA, USA) or active p38 MAP kinase (1: 200; Cell Signaling Technology, Danvers, MA, USA). Immunoreactive proteins were visualized following incubation with Rhodamine Red X-conjugated donkey anti-rabbit antibodies (diluted 1: 100 in PBS, Jackson Immuno-Research Laboratories, West Grove, PA, USA) for 1 hour at room temperature and then mounted in Vectashield medium (H-1200) containing 4′,6 diamidino-2-phenylindole to allow for identification of neuronal and glial cell nuclei. As a control, some sections were incubated for 1 hour at room temperature with only the Rhodamine Red X-conjugated antirabbit antibodies. Images were collected as described above.
Quantification of Staining Intensity
For intensity measurements, each image was converted to gray-scale prior to analysis using Olympus MicroSuite Five image processing software. The average gray value was measured in 6 regions of interest in the V1/V2 region that contained neurons and satellite glia and normalized to the average gray value in 3 regions of interest that contained only glial Schwann cells, which was used as a background level of staining. The data are reported as a ratio of average grayscale intensity of neurons and satellite glial cells vs Schwann cells ± SEM. The reported numbers are the average of the counts obtained by 2 laboratory staff who were blinded to the experimental design. Each experimental condition was repeated in at least 3 independent experiments. Statistical analysis was performed using the parametric two-sample t-test. Differences were considered statistically significant at P < .05. All statistical tests were performed using Minitab Statistical Software, Release 14.
RESULTS
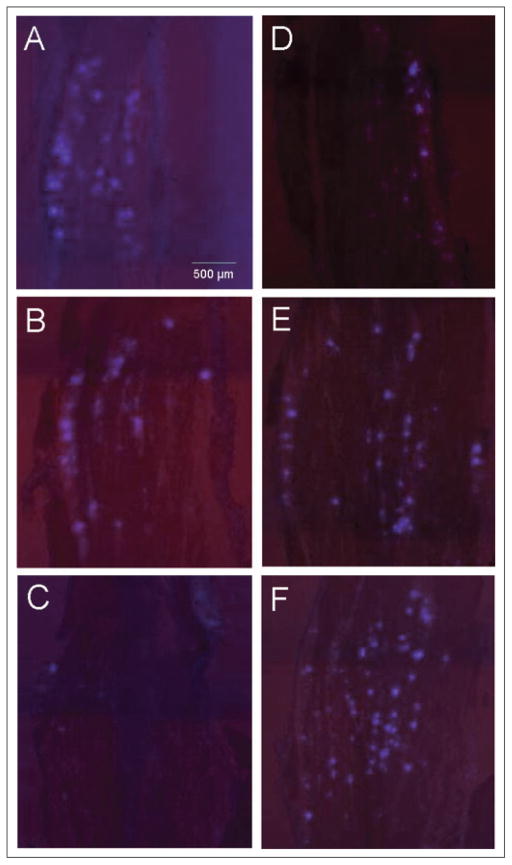
Initially, the retrograde tracer True Blue and multiple image alignment were used to localize neuronal cell bodies throughout the entire trigeminal ganglion that provide sensory innervation of the whisker pad and eyebrow regions under basal conditions. Trigeminal ganglia were obtained from untreated young male rats 5 days after bilateral injection of True Blue in the eyebrows or whisker pads. The entire ganglion was cryosectioned from the dorsal to the ventral side in 20 μm sections. The dorsal region included section numbers 6–9 while the medial region included section numbers 15–25 and the ventral region section numbers 40–50. Multiple image alignment was used to view the distribution of True Blue dye in cells throughout the V1 and V2 regions of the ganglion. The images shown correspond to representative anterior sections of the ganglion obtained from the dorsal (Fig. 1A,D), medial (Fig. 1B,E), and ventral (Fig. 1C,F) regions. Numerous dye-labeled neuronal cell bodies were observed in bands in the V1 (anteriomedial) and V2 (anteriolateral) regions of the ganglion in the dorsal (Fig. 1A) and medial (Fig. 1B) regions of the ganglion, but not the ventral (Fig. 1C) region, following injection of True Blue into the eyebrow. A different distribution pattern was observed after injection of True Blue into the whisker pad. While labeled neuronal cell bodies were observed exclusively in bands in the V2 region in the dorsal portion of the ganglion (Fig. 1D), the dye was observed in neuronal cell bodies in both the V2 and V1 regions in the medial (Fig. 1E) and ventral (Fig. 1F) sections of the ganglion. The number of labeled cells in the V1 region from the medial sections was greater than that observed in the V1 region from the ventral sections. Thus, somewhat surprisingly, there appears to be some overlap in the localization of neuronal cell bodies within bands in both V1 and V2 regions that provide innervation to facial structures thought to be primarily innervated by V1 (eyebrow) or V2 (whisker pad) nerves.
Fig 1.
Localization of neuronal cell bodies in trigeminal ganglia 5 days after injection of True Blue in the eyebrows or whisker pads of young male rats. The anterior portion of trigeminal ganglia obtained from animals injected in the eyebrow (A–C) or whisker pad (D–F) are shown at 40× magnification. Panels A and D are representative sections from the dorsal region of the ganglion, while B and E are from the medial region, and C and F are from the ventral region. Each ganglion section is oriented such that the top of the figure is the anterior most region and the right side is the lateral region. Dye-labeled neuronal cell bodies were seen in the V1 and V2 regions of the ganglion in the dorsal (A) and medial (B) regions of the ganglion, but not the ventral (C) region, following injection of True Blue into the eyebrow. After injection of True Blue into the whisker pad, labeled neuronal cell bodies not only were observed in the dorsal V2 (D) region, but were also located in both the V2 and V1 regions in the medial (E) and ventral (F) regions of the ganglion.
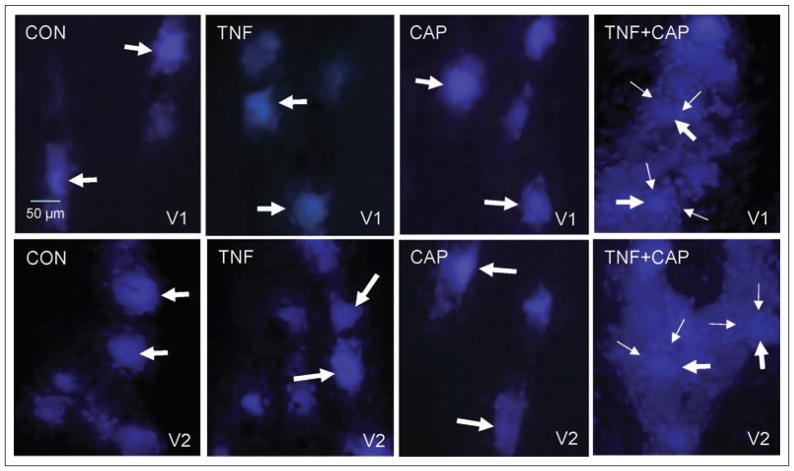
Having shown that neuronal cell bodies that provide innervation to the whisker pad are found in close association with neuronal cell bodies that provide innervation of the eyebrow, we wanted to test whether chemical stimulation of whisker pad afferents could lower the threshold for cellular changes in V1 neurons. To accomplish this goal, dye coupling studies were used to investigate whether neuronalglial cell signaling through gap junctions would be increased in response to an initial injection of TNF-α in the whisker pad followed by a later injection of capsaicin in the eyebrow when compared with normal basal conditions. For these studies, True Blue was injected into both the whisker pads and eyebrows and after 5 days the trigeminal ganglia were obtained from animals that were either unstimulated (control) or injected with 1 ng/mL TNF-α for 2 hours, 1 nM capsaicin for 15 minutes, or first TNF-α and then 2 hours later with capsaicin for an additional 15 minutes. This amount of TNF-α was chosen because this concentration of TNF-α (1 ng/mL) has been reported in nasal secretions obtained from patients with allergic rhinosinusitis.24 The concentration of capsaicin was chosen because in our in vivo model of inflammation this concentration does not stimulate the release of calcitonin gene-related peptide from trigeminal neurons (data not shown). In ganglia from control animals, the fluorescent dye was observed primarily in neuronal cell bodies within the V1 and V2 regions of the ganglion (Fig. 2). Similarly, the dye was localized predominantly in neuronal cell bodies in both regions of the ganglia from TNF-α- or capsaicin-injected animals. In contrast, a much more diffuse dye distribution pattern was observed in both the V1 and V2 regions of ganglia obtained from animals injected initially with TNF-α (whisker pad) and then capsaicin (eyebrow). Following treatment with TNF-α and capsaicin, the dye was not only observed in the cytosol of neuronal cell bodies but now clearly visible in surrounding glial cells as early as 15 minutes after capsaicin treatment. The amount of dye observed in the satellite glial cells was similar in both the V1 and V2 regions. Furthermore, the dye was readily visible in glia cells not directly adjacent to neuronal cells. Results from these dye coupling studies provide evidence that TNF-α injection in the whisker pad lowered the threshold for cellular changes in V1 neurons that resulted in increased dye coupling between neurons and satellite glial cells in both the V1 and V2 regions of the ganglion.
Fig 2.
Tumor necrosis factor-alpha (TNF-α) treatment of V2 neurons causes sensitization of capsaicin-responsive neurons in the V1 region of the trigeminal ganglion that results in increased neuron-satellite glia cell signaling through gap junctions in V1 and V2 regions. The cellular localization pattern of True Blue in the anterior portion of the trigeminal ganglion in the V1 region is shown in the top panels and the V2 region is shown in the bottom panels. Under basal conditions (control, CON), the dye was primarily located in the cytoplasm of neuronal cell bodies (large arrows) in the V1 and V2 regions of the ganglion. A similar pattern was observed in the V1 and V2 regions in response to TNF-α (TNF) or capsaicin (CAP) treatment. However, treatment with both TNF-α and capsaicin resulted in increased dye coupling between neuronal cell bodies (large arrows) and satellite glial cells (small arrows). Images obtained at 400× magnification.
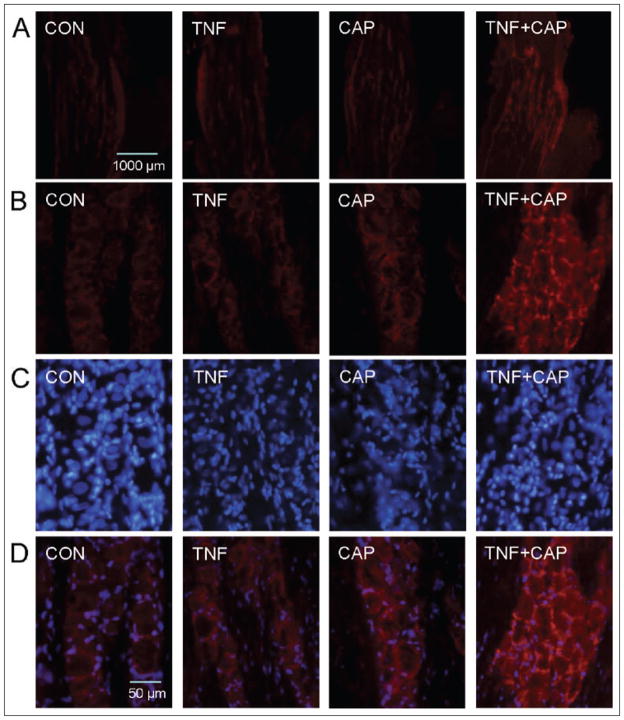
Having shown increased gap junction communication between trigeminal neurons and satellite glial cells in response to TNF-α and capsaicin treatment, the next goal was to identify which protein was involved in forming these channels. Gap junctions are channels that are formed by members of the connexin family of proteins that allow the direct passage of ions and other small molecules (<1 kDa) from one cell to another.21,23 An initial immunohistochemical screen was performed to identify which connexin proteins were expressed in trigeminal ganglion cells under basal conditions. Based on our results, we chose to focus our studies on the expression of connexin 26 as it was expressed in both neurons and satellite glial cells and observed more frequently in clusters or plaques located between these cell types than other connexins (data not shown). As seen in Figure 3A, low-level connexin 26 staining was observed in both the V1 and V2 regions of ganglia obtained from unstimulated control animals. A small increase in the level of connexin 26 was seen in ganglia from animals injected with TNF-α or capsaicin. However, connexin 26 expression was markedly increased in V1 and V2 regions following treatment with both chemical stimuli. While a small number of gap junction plaques were observed between neurons and satellite glial cells in ganglia obtained from untreated, TNF-α-, or capsaicin-treated animals, the number and intensity of connexin 26 plaques were greatly increased in response to treatment of TNF-α then capsaicin (Fig. 3B,D). Thus, expression of connexin 26 in trigeminal ganglion neurons and satellite glia was found to be increased in both V1 and V2 regions in response to TNF-α and capsaicin treatment.
Fig 3.
Expression of connexin 26 in trigeminal ganglion neurons and satellite glia is increased in both V1 and V2 regions of the trigeminal ganglion in response to tumor necrosis factor-alpha (TNF-α) and capsaicin treatment. A section of the anterior portion of the ganglia obtained from untreated animals (control, CON) or animals injected with TNF-α (TNF) for 2 hours in the whisker pad or capsaicin (CAP) for 15 minutes in the eyebrow or injection of both agents is shown in the top panel at 40× magnification (A). In control ganglion, low-level connexin 26 expression is visible within the V1 and V2 regions. Similarly, low-level expression of connexin 26 is observed in TNF-α- or capsaicin-treated animals. Increased connexin 26 expression was observed in V1 and V2 regions following treatment with both chemical stimuli. Panels B, C, and D correspond to staining at 400× magnification for connexin 26, nuclear dye 4′,6 diamidino-2-phenylindole (DAPI), or merged images of connexin 26 and DAPI, respectively. A small number of plaques were observed between neurons and satellite glial cells in ganglia obtained from untreated, TNF-, or capsaicin-treated animals. The number of connexin 26 plaques was greatly increased in response to treatment of TNF-α then capsaicin.
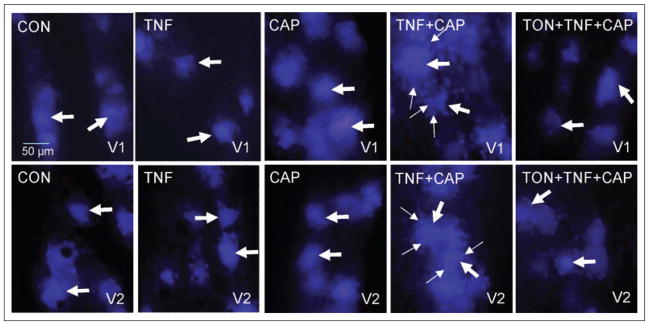
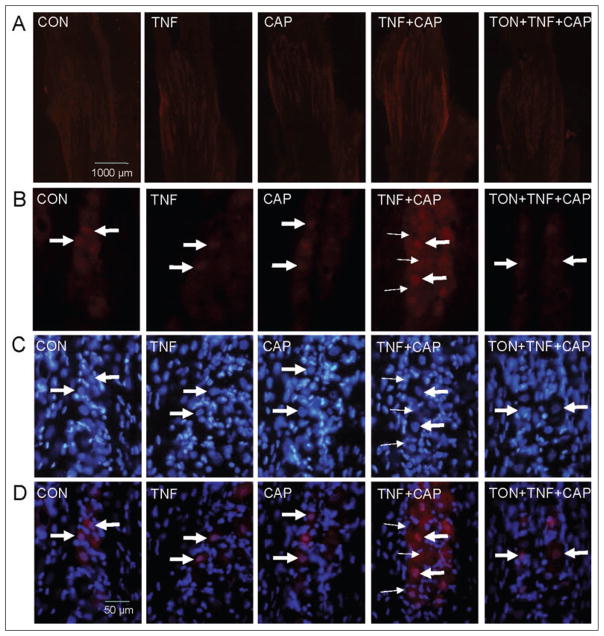
To determine whether tonabersat, which was proposed to function as a gap junction inhibitor,25 could inhibit the increased gap junction activity seen in response to TNF-α and capsaicin, dye coupling studies and fluorescent microscopy were used. For these studies, True Blue was injected into both the whisker pads and eyebrows and after 5 days the trigeminal ganglia were obtained from animals that were either unstimulated (control) or injected with 1 ng/ml TNF-α for 2 hours, 1 nM capsaicin for 60 minutes, first with TNF-α and then 2 hours later with capsaicin, or with 10 mg/kg tonabersat (i.p.) prior to the TNF-α and capsaicin injections. This amount of tonabersat was selected because this dose has been shown in animal models to block trigeminal nerve activation.26 In ganglia from control animals, the fluorescent dye was observed primarily in neuronal cell bodies that were localized within the V1 and V2 regions (Fig. 4). Similarly, the dye was localized predominantly in neuronal cell bodies in both regions of the ganglia from TNF-α- or capsaicin-injected animals. The number of labeled satellite glial cells in the V1 region was greater after TNF-α and capsaicin treatment when compared with control, TNF-α-, or capsaicin-treated ganglia. Pretreatment of animals with tonabersat greatly reduced stimulated dye movement between neurons and satellite glial cells in both the V1 and V2 regions of ganglia obtained from animals injected initially with TNF-α and then capsaicin. These data provide the first evidence to our knowledge that tonabersat can function to block gap junction signaling.
Fig 4.
Tonabersat blocks increased neuron-satellite glia signaling through gap junctions in response to tumor necrosis factor-alpha (TNF-α) and capsaicin treatment. The cellular localization pattern of True Blue in the anterior portion of the trigeminal ganglion in the V1 region is shown in the top panels and the V2 region is shown in the bottom panels. Under basal conditions (control, CON), the dye was primarily detected in neuronal cell bodies (large arrows) in the V1 and V2 regions of the ganglion. A similar pattern was observed in the V1 and V2 regions in response to 2 hour treatment with TNF-α (TNF) or 60 minute treatment with capsaicin (CAP). The increased amount of dye coupling between neurons and satellite glia was greatly reduced in both V1 and V2 regions by pretreatment with tonabersat (TON). Neuronal cell bodies are indicated by large arrows while satellite glial cells are indicated by small arrows. Images were obtained at 400× magnification.
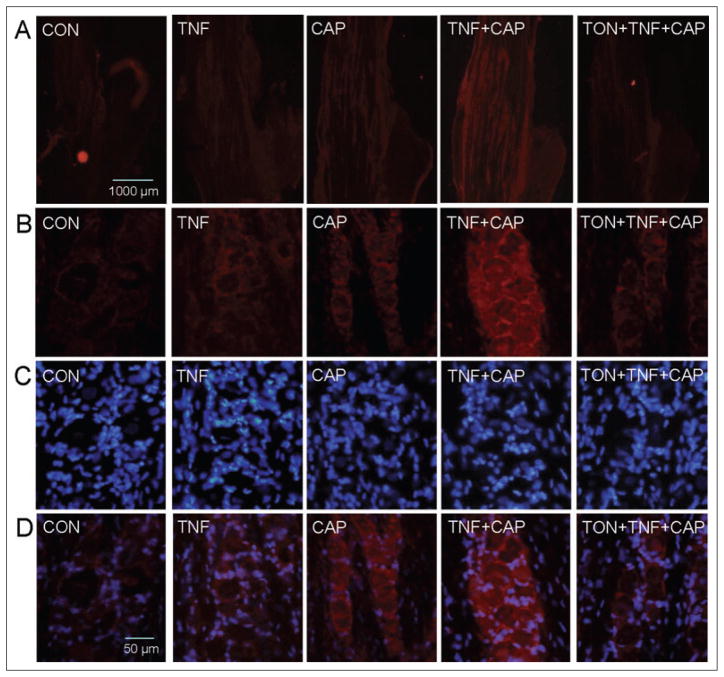
To determine whether the inhibitory effect of tonabersat on gap junction communication might involve changes in connexin 26 expression, ganglia were obtained from animals that were either unstimulated or injected with 1 ng/mL TNF-α for 2 hours, 1 nM capsaicin for 60 minutes, first with TNF-α and then 2 hours later with capsaicin for an additional 60 minutes, or with 10 mg/kg tonabersat (i.p.) prior to the TNF-α and capsaicin injections. While the pattern of connexin 26 staining in V1 and V2 regions was similar in control ganglia and ganglia obtained from TNF-α- or capsaicin-treated animals, the intensity of staining was slightly increased following individual treatments (Fig. 5). Interestingly, pretreatment of animals with tonabersat greatly decreased the level of connexin 26 staining in V1 and V2 regions compared with animals treated with TNF-α and capsaicin. As seen at higher magnification (Fig. 5B,D), not only was the intensity of connexin 26 greatly reduced by tonabersat pretreatment but the number of plaques observed between neurons and satellite glial cells in ganglia obtained from TNF-α- and capsaicin-treated animals was much lower. These data provide evidence that tonabersat inhibits stimulated dye coupling and hence gap junction communication between neurons and satellite glial cells by downregulation of connexin 26 levels and decreasing the number of gap junction plaques.
Fig 5.
Tonabersat inhibits the number of connexin 26 plaques observed between trigeminal ganglion neurons and satellite glia in response to tumor necrosis factor-alpha (TNF-α) and capsaicin. A section of the anterior portion of the ganglia obtained from untreated animals (control, CON) or animals injected with TNF-α (TNF) for 2 hours in the whisker pad or capsaicin (CAP) for 60 minutes in the eyebrow or injection of both agents or pretreatment with tonabersat (TON) prior to injections is shown in the top panel at 40× magnification (A). Control ganglion or ganglion obtained from TNF-α- or capsaicin-treated animals exhibited low-level connexin 26 expression in the V1 and V2 regions. Similarly, low-level expression of connexin 26 was observed in TNF-α-or capsaicin-treated animals. Pretreatment of animals with tonabersat greatly decreased the level of connexin 26 staining in V1 and V2 regions compared with animals treated with both TNF-α and capsaicin. Panels B, C, and D correspond to staining at 400× magnification for connexin 26, nuclear dye 4′,6 diamidino-2-phenylindole (DAPI), or merged images of connexin 26 and DAPI, respectively. A small number of plaques were observed between neurons and satellite glial cells in ganglia obtained from untreated, TNF-α-, or capsaicin-treated animals. The increased number of connexin 26 plaques seen in response to treatment of TNF-α then capsaicin was reduced to untreated control levels by pretreatment with tonabersat.
We had previously shown that stimulation of trigeminal neurons leads to increased levels of active p38 mitogen-activated protein (MAP) kinase,18,27,28 which is a key regulatory protein involved in signal transduction in response to inflammatory stimuli that result in changes in gene expression.29,30 To determine whether TNF-α sensitization of capsaicin-responsive neurons could lead to increased expression of p38, animals were injected with TNF-α, capsaicin, or both agents as described for Figure 5 and ganglion sections stained for the active phosphorylated form of p38. A low level of active p38 was observed in the V1 and V2 regions of ganglia obtained from unstimulated control animals or animals treated with TNF-α or capsaicin (Fig. 6A). In contrast, the level of p38 was greatly increased in all the neuronal bands located in the V1 and V2 regions of the ganglion in response to TNF-α and capsaicin. Importantly, a greater amount of active p38 staining was localized in the nucleus of neuronal and satellite glial cells in ganglia of animals treated with both TNF-α and capsaicin when compared with control or TNF-α- or capsaicin-only-treated animals (Fig. 6B,D). Pretreatment of animals with tonabersat greatly decreased active p38 levels and the amount of nuclear staining in both neurons and satellite glial cells in V1 and V2 regions when compared with animals treated with both TNF-α and capsaicin. Tonabersat treatment lowered p38 levels to those observed in ganglia obtained from untreated control animals or animals injected with only TNF-α or capsaicin. These results demonstrate that tonabersat greatly inhibits the level of active p38 in trigeminal ganglion neurons and satellite glia in response to TNF-α and capsaicin.
Fig 6.
Tonabersat inhibits the level of active p38 in trigeminal ganglion neurons and satellite glia in response to tumor necrosis factor-alpha (TNF-α) and capsaicin. A section of the anterior portion of the ganglia obtained from untreated animals (control, CON) or animals injected with TNF-α (TNF) for 2 hours in the whisker pad or capsaicin (CAP) for 60 minutes in the eyebrow or injection of both agents or pretreatment with tonabersat (TON) prior to injections is shown in the top panel at 40× magnification (A). Control ganglion and ganglion obtained from TNF-α- or capsaicin-treated animals exhibited low levels of active p38 in V1 and V2 regions. In contrast, levels of active p38 were increased in response to TNF-α and capsaicin. Pretreatment of animals with tonabersat greatly decreased the level of p38 staining in V1 and V2 regions compared with animals treated with both TNF-α and capsaicin. Panels B, C, and D correspond to staining at 400× magnification for active p38, nuclear dye 4′,6 diamidino-2-phenylindole (DAPI), or merged images of active p38 and DAPI, respectively. Increased nuclear p38 staining was observed in animals injected with TNF-α and capsaicin when compared with untreated control animals or animals injected with either TNF-α or capsaicin alone. The increased level of nuclear and cytoplasmic p38 staining seen in response to TNF-α then capsaicin treatment was reduced to control levels by pretreatment with tonabersat.
A summary of the changes in connexin 26 and active p38 levels in the V1 and V2 regions of the trigeminal ganglion is shown in the Table. The average intensity of the staining in regions of interest containing neurons and glia in V1 and V2 was measured in ganglia from control animals and animals injected with TNF-α, capsaicin, TNF-α and capsaicin, or both stimulatory agents and tonabersat. Analysis of the V1 and V2 regions showed that while connexin 26 and active p38 intensities were not significantly different from control levels when treated with TNF-α or capsaicin alone, the staining intensities for connexin 26 and p38 were significantly (P < .001) increased in response to TNF-α and capsaicin injections. However, pretreatment with tonabersat prevented the significant increase in connexin 26 and p38 levels.
Table.
Quantification of Neuronal and Glial Cx 26 and Active p38 Staining Intensities in the V1 and V2 Regions of the Trigeminal Ganglion
| Cx26 | p38 | |||
|---|---|---|---|---|
| Control | 1.933 | 0.127 | 1.892 | 0.104 |
| Capsaicin | 2.052 | 0.083 | 2.189 | 0.061 |
| TNF-α | 1.933 | 0.104 | 2.192 | 0.151 |
| Capsaicin/TNF-α | 3.742 | 0.224* | 3.239 | 0.072* |
| Capsaicin/TNF-α/tonabersat | 1.912 | 0.204 | 2.053 | 0.066 |
P < .001 when compared with control values.
The values, which are the ratio of the staining intensity of neuron and satellite glial cells to the intensity of Schwann cells in 6 independent fields, are reported as the average staining intensity SEM. Each experimental condition was repeated a minimum 3 times.
TNF-α = tumor necrosis factor-alpha.
DISCUSSION
Although there is significant comorbidity associated with diseases involving trigeminal nerves, the cellular basis for this prevalence has not been investigated. In this study, we wanted to test the hypothesis that the unique cellular morphology of the trigeminal ganglion allows cell to cell signaling within the ganglion, which mediates activation of other branches leading to comorbidity as reported for migraine and allergic rhinitis and acute sinusitis. It has been reported that patients with self-described or physician diagnosed “sinus headache” are often mis-diagnosed, especially if the migraine attack is accompanied by rhinosinus pathology.2 As suggested in a recent study, recurrent mucosal edema and inflammation typically observed in patients with seasonal allergic rhinitis may act as a migraine trigger in susceptible individuals.31 Specifically, in this study, we wanted to determine whether stimulation of V2 neurons with TNF-α, a cytokine whose levels are elevated in nasal secretions during allergic rhinitis and acute sinusitis,24,32–34 would result in a lowering of the threshold for cellular changes in V1 neurons. Sensitization and activation of trigeminal neurons originating in the V1 region of the ganglion that provide sensory innervation of the meningeal blood vessels are thought to be involved in the pathology of migraine.35 Data from our study showed that while TNF-α or capsaicin treatment alone was not sufficient to cause cellular changes in V1 neurons, the combination of both chemical stimuli greatly increased gap junction communication between trigeminal neurons and satellite glial cells and increased active p38 levels in both cell types. Somewhat surprisingly, the increase in gap junction signaling was observed not only in the V1 region but also in the V2 region of the ganglion. This result is in agreement with our previously published finding that stimulation of V3 neurons could cause increased levels of active signaling proteins in neuronal and satellite glial cells in other regions of the ganglion.18 Thus, based on results from our studies, it appears that this type of intraganglion signaling may be a normal response to peripheral stimulation of trigeminal neurons. An important question to address is how this is possible. Increased expression of signaling molecules across different regions of the trigeminal ganglion is likely to involve neuron to glia signaling via gap junctions and paracrine signaling.18 Another possible mechanism may involve the distribution and organization of neuronal cell bodies within the trigeminal ganglion. In this study, we found that there was some overlap of retrogradely labeled neurons that were thought to be primarily localized only in distinct regions of the ganglion. For example, injection of True Blue in the whisker pads, which are thought to be innervated solely by V2 neurons, not only labeled neuronal cell bodies organized in bands in the V2 region but also labeled neuron cell bodies in the V1 region. Similarly, we found that following injection of True Blue into the eyebrows, which are thought to be solely innervated by V1 neurons, the dye was localized in V1 as well as V2 regions of the ganglion. The observed overlapping distribution of neuronal cells in bands within the V1 and V2 regions would allow for a coordinated response to inflammatory stimuli that may account for why cellular changes in one branch leads to coordinated changes in the other region of the trigeminal ganglion as seen in this study. Taken together, we have provided evidence that may help, at least in part, to explain how rhinosinusitis or acute sinusitis may act as a trigger of migraine as well as to provide a possible explanation for the significant comorbidity seen between migraine and rhinosinus diseases.
A major finding of this study was that neuronal-satellite glial cell signaling via gap junctions was greatly increased in both V1 and V2 regions of the trigeminal ganglion in response to TNF-α and then capsaicin treatment. Based on our studies, the gap junctions that allowed for direct dye coupling of neurons and satellite glial cells in trigeminal ganglion were likely composed of connexin 26 proteins. This is the first report of connexin 26 being expressed by neurons and satellite glia cells in the trigeminal ganglion. In previous studies, connexin 26 had been shown to be expressed by CNS neurons and astrocytes.21,23,36 The fact that we observed a marked increase in the number of plaques or clusters of gap junctions has important implications as it has been reported that plaque formation may be required for opening of gap junction channels.37 In addition, we observed some plaques and a small amount of dye coupling, and hence gap junction signaling, between neurons and satellite glial cells under basal conditions. However, most of the dye coupling and plaques observed under basal conditions involved neurons and adjacent satellite glial cells. In contrast, many more plaques and much more extensive dye spreading were observed in satellite glial cells associated with a given band of neuronal cells in both V1 and V2 regions following TNF-α and capsaicin injections. Our finding is in agreement with results from a study in dorsal root ganglion that demonstrated increased neuronglia dye coupling in response to peripheral stimulation with inflammatory mediators.38 Increased neuronalglial cell signaling via gap junctions is commonly reported in neuroinflammatory CNS diseases and is thought to play an important role in the underlying pathology of these diseases by increasing neuronal excitability.23 Furthermore, it was shown that gap junctions between glial astrocytes allow for a syncytium-like organization that is responsible for the observed coordinated response and long distance propagation of calcium waves in the CNS.39,40 It is well established that gap junctions facilitate the movement of small molecules (<1 kDa) and ions such as calcium from one cell to another that allows them to be functionally coupled. Based on our results, it is likely that a similar type of neuronglia organization facilitates activation-dependent coupling that functions to coordinate inflammatory response in the trigeminal ganglion. It is interesting to speculate that this organization within the ganglion that involves neuronal-satellite glia signaling may play a role in the significant comorbidity seen with disease involving trigeminal nerves.
Increased neuronal-glial cell interactions as seen in our study are likely to play an important role in peripheral sensitization, an event that is thought to cause the throbbing sensation of migraine and lead to central sensitization.11 Peripheral sensitization is characterized by a lower activation threshold in response to inflammatory stimuli and increased hyperexcitability of sensory neurons. It has been reported that levels of the active form of p38, a member of the MAP kinase family, are elevated in sensory neurons during inflammatory pain and likely mediate peripheral sensitization by regulating inflammatory gene expression.41,42 MAP kinases, such as p38, are activated by phosphorylation of threonine and tyrosine residues and then can translocate into the nucleus and regulate gene expression.43 In this study, we found increased levels of active p38 in the nucleus of both neurons and satellite glial cells in V1 and V2 regions following TNF-α and capsaicin injections. Based on studies in neurons and glia, increased expression of p38 has been shown to stimulate synthesis of multiple inflammatory mediators including cytokines, iNOS, and COX enzymes.42,43 Activation of the p38 pathway in neuronal and glial cells has been reported to contribute to persistent inflammatory and neuropathic pain and its activation in nociceptive neurons participates in generating pain hypersensitivity.42 In addition, inhibition of p38 has been shown to alleviate inflammatory pain and neuropathic pain in animal models.41 It is likely that increased p38 activity in neuronal and glial cells will contribute to peripheral sensitization of trigeminal primary sensory neurons and play an important role in the generation and maintenance of inflammatory pain in migraine, allergic rhinitis, and acute sinusitis. In our study, we found that tonabersat treatment blocked the stimulatory effect of TNF-α and capsaicin treatment on active p38 levels. It will be important to determine whether the inhibitory effect of tonabersat on p38 levels is mediated by a direct mechanism or an indirect mechanism by reducing gap junction signaling.
A novel finding from our study was that antimigraine drug tonabersat, a member of a family of novel benzoylaminobenz compounds, was shown to greatly diminish gap junction signaling between trigeminal neurons and satellite glial cells. To our knowledge, this is the first evidence to directly demonstrate that tonabersat can function to block gap junction communication between neurons and glial cells. Based on our results, we propose that the inhibitory effect of tonabersat on gap junction communication likely involves decreasing the expression of connexin 26 as tonabersat treatment reduced the level of connexin 26 staining and number of plaques to control levels. However, given the large number of connexin family members, it is probable that other connexin proteins may participate in the formation of gap junctions and, therefore, they may also be regulated by tonabersat. In addition, we showed that tonabersat treatment blocked the increased expression of active p38 in both trigeminal ganglion neurons and satellite glial cells. Thus, our results provide evidence to suggest that tonabersat should be able to prevent peripheral sensitization within the trigeminal ganglion. Interestingly, in other studies relevant to migraine pathology, tonabersat was shown to block plasma protein extravasation following trigeminal nerve stimulation and to inhibit cortical spreading depression and nitric oxide release.26,44–46 In addition, tonabersat was shown to block trigeminal parasympathetic reflexes, which are involved in migraine and rhinosinusitis.4,35 Whether tonabersat’s ability to block gap junction signaling in the trigeminal ganglion is involved in mediating any of these inhibitory effects remains to be determined.
In summary, we have provided evidence that TNF-α, whose levels are elevated during allergic rhinitis and acute sinusitis, causes changes in V1 neurons such that the amount of capsaicin required for stimulating these neurons is lowered. Stimulation of V1 neurons leads to increased gap junction signaling and increased p38 levels in neurons and satellite glial cells not only in the V1 but also in the V2 region of the ganglion. We propose that increased gap junction activity and active p38 levels in both neurons and satellite glia contribute to peripheral sensitization within the trigeminal ganglion and to the significant comorbidity seen with diseases involving the trigeminal nerves. Finally, we have provided evidence to suggest that tonabersat can inhibit key cellular events that contribute to peripheral sensitization in trigeminal ganglion, and therefore could be effective as a therapeutic for migraine, allergic rhinitis, and acute sinusitis.
Acknowledgments
We would like to thank Ms. Carrie Vause for her technical and administrative assistance. We also would like to thank the National Institutes of Health (DE017805), National Headache Foundation, and Minster Pharmaceuticals for their financial support.
Abbreviations
- CNS
central nervous system
- DMSO
dimethyl sulfoxide
- i.p
intraperitoneal
- MAP
mitogen-activated protein
- PBS
phosphate buffered saline
- TNF-α
tumor necrosis factor-alpha
Footnotes
Conflict of Interest: None
References
- 1.Cady R, Schreiber C. Sinus headache or migraine? Considerations in making a differential diagnosis. Neurology. 2002;58:S10–14. doi: 10.1212/wnl.58.9_suppl_6.s10. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber C, Hutchinson S, Webster C, Ames M, Richardson M, Powers C. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769–1772. doi: 10.1001/archinte.164.16.1769. [DOI] [PubMed] [Google Scholar]
- 3.Ku M, Silverman B, Prifti N, Ying W, Persaud Y, Schneider A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:226–230. doi: 10.1016/S1081-1206(10)60018-X. [DOI] [PubMed] [Google Scholar]
- 4.Baraniuk J. Neurogenic mechanisms in rhinosinusitis. Curr Allergy Asthma Rep. 2001;1:252–261. doi: 10.1007/s11882-001-0016-4. [DOI] [PubMed] [Google Scholar]
- 5.Goadsby P, Lipton R, Ferrari M. Migraine – current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 6.Hargreaves R, Shepheard S. Pathophysiology of migraine – new insights. Can J Neurol Sci. 1999;26:S12–19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 7.Shankland W. The trigeminal nerve. Part I:An overview. Cranio. 2000;18:238–248. doi: 10.1080/08869634.2000.11746137. [DOI] [PubMed] [Google Scholar]
- 8.Malick A, Burstein R. Peripheral and central sensitization during migraine. Funct Neurol. 2000;15:S28–35. [PubMed] [Google Scholar]
- 9.Sanchez del Rio M, Reuter U, Moskowitz M. Central and peripheral mechanisms of migraine. Funct Neurol. 2000;15:S157–162. [PubMed] [Google Scholar]
- 10.Yarnitsky D, Goor-Aryeh I, Bajwa Z, Ransil B, Cutrer F, Sottile A, et al. 2003 Wolff Award: Possible parasympathetic contributions to peripheral and central sensitization during migraine. Headache. 2003;43:704–714. doi: 10.1046/j.1526-4610.2003.03127.x. [DOI] [PubMed] [Google Scholar]
- 11.Dodick D, Silberstein S. Central sensitization theory of migraine: Clinical implications. Headache. 2006;46:S182–191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 12.Watkins L, Maier S. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hanani M. Satellite glial cells in sensory ganglia: From form to function. Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Watkins L, Milligan E, Maier S. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 15.Wieseler-Frank J, Maier S, Watkins L. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- 17.Pannese E. Perikaryal surface specializations of neurons in sensory ganglia. Int Rev Cytol. 2002;220:1–34. doi: 10.1016/s0074-7696(02)20002-9. [DOI] [PubMed] [Google Scholar]
- 18.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, et al. Neuronglia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 20.Nakase T, Naus C. Gap junctions and neurological disorders of the central nervous system. Biochim Biophys Acta. 2004;1662:149–158. doi: 10.1016/j.bbamem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Nagy J, Dudek F, Rash J. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Ebihara L. New roles for connexins. News Physiol Sci. 2003;18:100–103. doi: 10.1152/nips.01431.2002. [DOI] [PubMed] [Google Scholar]
- 23.Laird D. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bensch G, Nelson H, Borish L. Evaluation of cytokines in nasal secretions after nasal antigen challenge: Lack of influence of antihistamines. Ann Allergy Asthma Immunol. 2002;88:457–462. doi: 10.1016/S1081-1206(10)62382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silberstein S. Preventive treatment of migraine. Trends Pharmacol Sci. 2006;27:410–415. doi: 10.1016/j.tips.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Parsons A, Bingham S, Raval P, Read S, Thompson M, Upton N. Tonabersat (SB–220453) a novel benzopyran with anticonvulsant properties attenuates trigeminal nerve-induced neurovascular reflexes. Brit J Pharmacol. 2001;132:1549–1557. doi: 10.1038/sj.bjp.0703932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellamy J, Bowen E, Russo A, Durham P. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23:2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowen E, Schmidt T, Firm C, Russo A, Durham P. Tumor necrosis factor–a stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafers M, Svensson C, Sommer C, Sorkin L. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbin G, Roisin M, Zalc B. Tumor necrosis factor alpha activates the phosphorylation of ERK, SAPK/JNK, and P38 kinase in primary cultures of neurons. Neurochem Res. 2001;26:107–112. doi: 10.1023/a:1011086426652. [DOI] [PubMed] [Google Scholar]
- 31.Ku M, Silverman B, Prifti N, Ying W, Persaud Y, Schneider A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:226–230. doi: 10.1016/S1081-1206(10)60018-X. [DOI] [PubMed] [Google Scholar]
- 32.Repka-Ramirez S, Naranch K, Park Y, Clauw D, Baraniuk J. Cytokines in nasal lavage fluids from acute sinusitis, allergic rhinitis, and chronic fatigue syndrome subjects. Allergy Asthma Proc. 2002;23:185–190. [PubMed] [Google Scholar]
- 33.Bachert C, Wagenmann M, Hauser U. Proinflammatory cytokines: Measurement in nasal secretion and induction of adhesion receptor expression. Int Arch Allergy Immunol. 1995;107:106–108. doi: 10.1159/000236945. [DOI] [PubMed] [Google Scholar]
- 34.Bradding P, Mediwake R, Feather I, Madden J, Church M, Holgate S, et al. TNF alpha is localized to nasal mucosal mast cells and is released in acute allergic rhinitis. Clin Exp Allergy. 1995;25:406–415. doi: 10.1111/j.1365-2222.1995.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 35.Pietrobon D. Migraine: New molecular mechanisms. Neuroscientist. 2005;11:373–386. doi: 10.1177/1073858405275554. [DOI] [PubMed] [Google Scholar]
- 36.Saez J, Berthoud V, Branes M, Martinez A, Byer E. Plasma membrane channels formed by connexins: Their regulation and function. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 37.Bukauskas F, Jordan K, Bukauskiene A, Bennett M, Lampe P, Laird D, et al. Clustering of connexin 43-enhanced green flourescent protein gap junction channels and functional coupling in living cells. PNAS. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dublin P, Hanani M. Satellite glial cells in sensory ganglia: Their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Blomstrand F, Aberg N, Eriksson P, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92:255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- 40.Kielian T, Esen N. Effects of neuroinflammation on glia-glia gap junctional intercellular communication: A perspective. Neurochem Int. 2004;45:429–436. doi: 10.1016/j.neuint.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Ji R. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs. 2004;5:71–75. [PubMed] [Google Scholar]
- 42.Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- 43.Schramek H. MAP kinases: From intracellular signals to physiology and disease. News Physiol Sci. 2002;17:62–67. doi: 10.1152/nips.01365.2001. [DOI] [PubMed] [Google Scholar]
- 44.Bradley D, Smith M, Netsiri C, Smith J, Bockhorst K, Hall L, et al. Diffusion-weighted MRI used to detect in vivo modulation of cortical spreading depression: Comparison of sumatriptan and tonabersat. Exp Neurol. 2001;172:342–353. doi: 10.1006/exnr.2001.7809. [DOI] [PubMed] [Google Scholar]
- 45.Netsiri C, Bradley D, Takeda T, Smith M, Papadakis N, Hall L, et al. A delayed class of BOLD waveforms associated with spreading depression in the feline cerebral cortex can be detected and characterised using independent component analysis (ICA) Magn Reson Imaging. 2003;21:1097–1110. doi: 10.1016/s0730-725x(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 46.Read S, Hirst W, Upton N, Parsons A. Cortical spreading depression produces increased cGMP levels in cortex and brain stem that is inhibited by tonabersat (SB-220453) but not sumatriptan. Brain Res. 2001;891:69–77. doi: 10.1016/s0006-8993(00)03191-7. [DOI] [PubMed] [Google Scholar]