Abstract
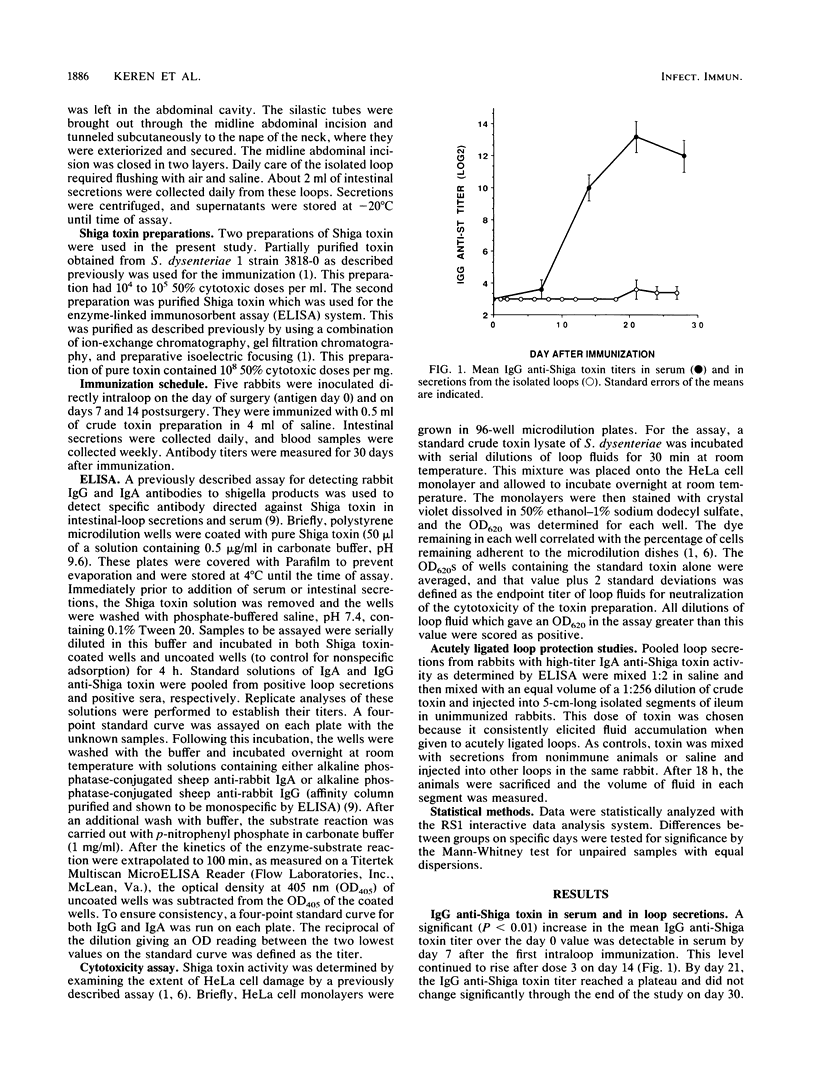
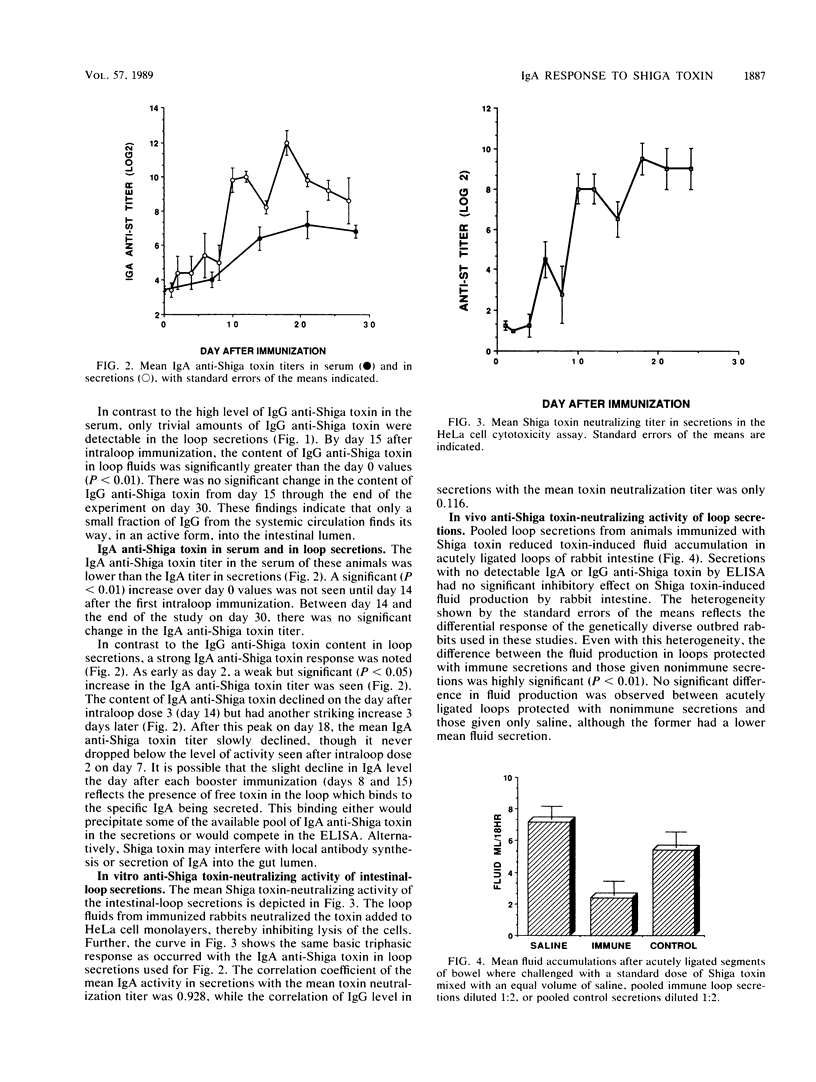
Although the role of Shiga toxin in dysentery is unknown, the toxin is cytotoxic to HeLa cells, causes fluid secretion in rabbit intestine, and is lethal to rabbits and mice when injected parenterally. In the present study, rabbits received three weekly doses of Shiga toxin directly into chronically isolated ileal loops. Within a week, secretions from these loops contained immunoglobulin A (IgA) anti-Shiga toxin. The titer of IgA anti-Shiga toxin increased after weekly doses 2 and 3. Little IgG anti-Shiga toxin was present in loop secretions, although high titers of IgG anti-Shiga toxin were found in the sera. These loop secretions were able to neutralize the cytotoxic effects of Shiga toxin in the HeLa cell assay. The capacity to neutralize the cytotoxicity of the toxin correlated strongly with the IgA anti-Shiga toxin titer in these same secretions. Pooled immune loop secretions were also able to significantly reduce fluid accumulation in acutely ligated loops in rabbits, while loop secretions from control rabbits could not. Shiga toxin elicited a strong secretory IgA response upon application to the intestine. Further, the mucosal antibodies produced functioned to prevent the toxic effects of Shiga toxin both in vitro and in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Griffin D. E., Rothman S. W., Doctor B. P. Purification and biological characterization of shiga toxin from Shigella dysenteriae 1. Infect Immun. 1982 Jun;36(3):996–1005. doi: 10.1128/iai.36.3.996-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Obrig T. G., Ussery M. A., Moran T. P. Shiga toxin from Shigella dysenteriae 1 inhibits protein synthesis in reticulocyte lysates by inactivation of aminoacyl-tRNA binding. Microb Pathog. 1986 Aug;1(4):325–334. doi: 10.1016/0882-4010(86)90065-3. [DOI] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Keusch G. T., Edson C., Thorley-Lawson D., Jacewicz M. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of Shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J Exp Med. 1984 Dec 1;160(6):1767–1781. doi: 10.1084/jem.160.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Fontaine A., Arondel J., Sansonetti P. J. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988 Dec;56(12):3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. R., Keren D. F., Yardley J. H., Brown G. No impairment of local intestinal immune response to keyhole limpet haemocyanin in the absence of Peyer's patches. Immunology. 1981 Mar;42(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G. T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986 Jun 1;163(6):1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Elliott H. L., Brown G. D., Yardley J. H. Atrophy of villi with hypertrophy and hyperplasia of Paneth cells in isolated (thiry-Vella) ileal loops in rabbits. Light-microscopic studies. Gastroenterology. 1975 Jan;68(1):83–93. [PubMed] [Google Scholar]
- Keren D. F. Enzyme-linked immunosorbent assay for immunoglobulin G and immunoglobulin A antibodies to Shigella flexneri antigens. Infect Immun. 1979 May;24(2):441–448. doi: 10.1128/iai.24.2.441-448.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Kern S. E., Bauer D. H., Scott P. J., Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. J Immunol. 1982 Jan;128(1):475–479. [PubMed] [Google Scholar]
- Keren D. F., McDonald R. A., Formal S. B. Secretory immunoglobulin A response following peroral priming and challenge with Shigella flexneri lacking the 140-megadalton virulence plasmid. Infect Immun. 1986 Dec;54(3):920–923. doi: 10.1128/iai.54.3.920-923.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Donohue-Rolfe A., Jacewicz M. Shigella toxin and the pathogenesis of shigellosis. Ciba Found Symp. 1985;112:193–214. doi: 10.1002/9780470720936.ch11. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Donohue-Rolfe A., Jacewicz M. Shigella toxin(s): description and role in diarrhea and dysentery. Pharmacol Ther. 1981;15(3):403–438. doi: 10.1016/0163-7258(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Mata L. J., McIver J. The pathogenesis of Shigella diarrhea. I. Enterotoxin production by Shigella dysenteriae I. J Clin Invest. 1972 May;51(5):1212–1218. doi: 10.1172/JCI106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Takeuchi A., Sprinz H. The pathogenesis of shigella diarrhea. II. Enterotoxin-induced acute enteritis in the rabbit ileum. J Infect Dis. 1972 Jul;126(1):92–95. doi: 10.1093/infdis/126.1.92. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M., Donohue-Rolfe A. Pathogenesis of shigella diarrhea: evidence for an N-linked glycoprotein shigella toxin receptor and receptor modulation by beta-galactosidase. J Infect Dis. 1986 Feb;153(2):238–248. doi: 10.1093/infdis/153.2.238. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. Pathogenesis of Shigella diarrhea. VII. Evidence for a cell membrane toxin receptor involving beta1 leads to 4-linked N-acetyl-D-glucosamine oligomers. J Exp Med. 1977 Aug 1;146(2):535–546. doi: 10.1084/jem.146.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. The pathogenesis of Shigella diarrhea. V. Relationship of shiga enterotoxin, neurotoxin, and cytotoxin. J Infect Dis. 1975 May;131 (Suppl):S33–S39. doi: 10.1093/infdis/131.supplement.s33. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Lindberg A. A., Brown J. E., Strömberg N., Westling-Ryd M., Schultz J. E., Karlsson K. A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987 Feb 5;262(4):1779–1785. [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Laveck G. D. Immunochemical and cytotoxic activities of Shigella dysenteriae 1 (shiga) and shiga-like toxins. Infect Immun. 1982 Mar;35(3):1151–1154. doi: 10.1128/iai.35.3.1151-1154.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Reisbig R., Eiklid K. Subunit structure of Shigella cytotoxin. J Biol Chem. 1981 Aug 25;256(16):8732–8738. [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E., GLADSTONE G. P. The neurotoxin of Shigella shigae. I. Production, purification and properties of the toxin. Br J Exp Pathol. 1953 Apr;34(2):202–216. [PMC free article] [PubMed] [Google Scholar]
- Wassef J. S., Keren D. F., Mailloux J. L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989 Mar;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F., Hamilton S. R., Brown G. D. Local (immunoglobulin A) immune response by the intestine to cholera toxin and its partial suppression with combined systemic and intra-intestinal immunization. Infect Immun. 1978 Feb;19(2):589–597. doi: 10.1128/iai.19.2.589-597.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



