Abstract
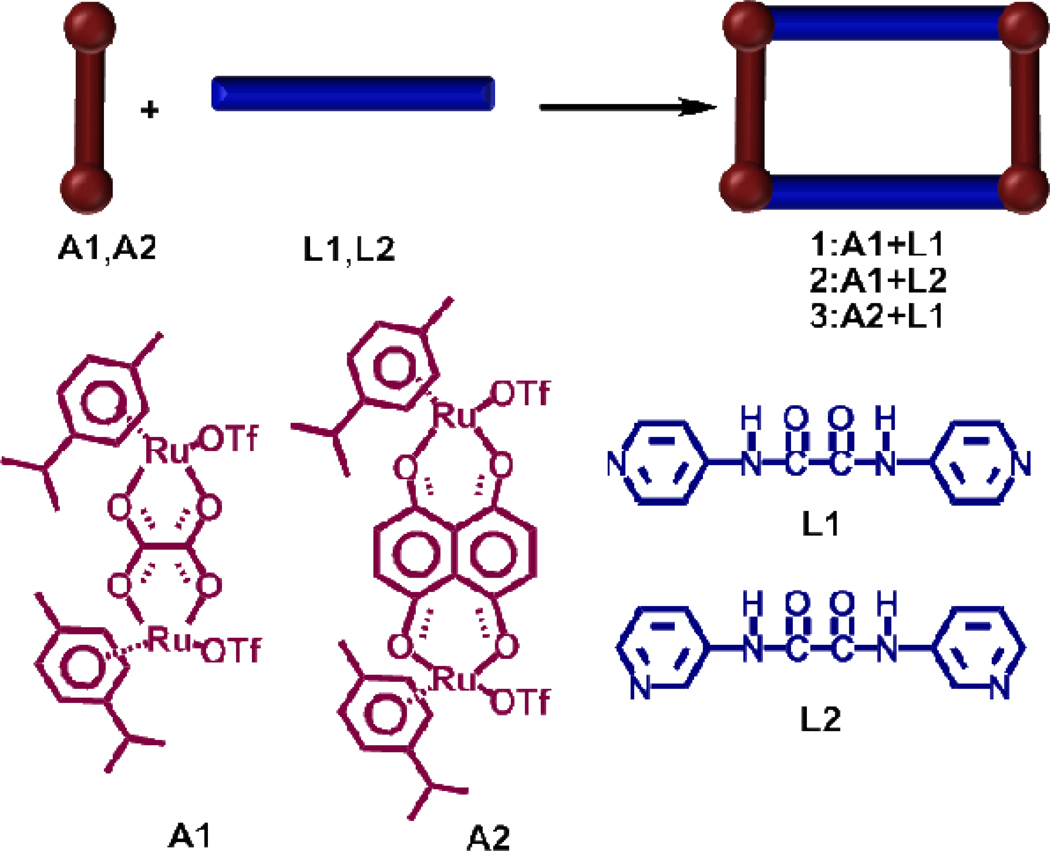
The synthesis of new 2+2 metalla-rectangles via coordination driven self-assembly of octahedral Ru(II) based acceptors and amide donors is described. To evaluate their in vitro cytotoxic properties, preliminary biological assays were carried out for various human cancer cell lines, and our results show that the cytotoxicity level of 3 is comparable or even greater in the cases of SK-hep-1 and HCT-15 than that of the reference drug cisplatin.
Literature from the last two decades chronicles the burgeoning understanding of the biological activities of proteins and enzymes chelated with metal centers, where the organic backbone acts as a scaffold to hold the metal ion in place for the requisite transformation.1 Medicinal inorganic chemistry is a fertile area of research, initially fueled by the discovery of cisplatin about four decades ago.2 Following the discovery of cisplatin, there has been a vast expansion of interest in the use of inorganic compounds for the treatment of tumors. Despite success as a therapeutic agent for a wide range of cancers, cisplatin has a number of drawbacks such as toxicity and resistance.3 This has led to the development of other platinum drugs, such as carboplatin, oxaliplatin, 4 and satraplatin,5 which have lower toxicity and are currently used for the treatment of various cancers.
Ruthenium was noted as a promising metal for use in anticancer agents due to its similar kinetics to platinum. Its lower toxicity was assumed to be due to its ability to mimic iron, allowing it to bind to biomolecules such as serum albumin or transferrin.6 The anticancer activity for ruthenium complexes was investigated by Clarke;7 however, poor solubility restricted their clinical use. Since then, two of the most promising ruthenium complexes reported, NAMI-A and KP-1019, has entered clinical trials.8
Hartinger and Nazarov9 applied the same concept of multi-nuclearity that has been used to increase the cytotoxicity of Pt-based drugs, to ruthenium complexes and explored the cytotoxicity and structure-activity relationships (SAR) of multinuclear arene-complexes with the conclusion that nuclearity seems to correlate with cytotoxicity. Maeda et al.10 addressed the potential value of macromolecular drugs and noted that increased permeability leads to higher uptake of large molecules and proteins, and that the damaged lymphatic drainage of cancer cells leads to the retention of large molecules inside the cells. This suggests that large molecules could be selectively taken up and retained inside tumor cells, whereas if taken up by normal cells, they would be excreted through the healthy lymphatic system.11 Conceptually, this is an excellent consideration for the development of new anti-cancer agents. Large compounds are required in order to effectively exploit the size selective uptake of drugs into tumor cells, 12 and a coordination driven self-assembly approach may serve the purpose of designing large molecular tools for biological applications. Compared to classical methods, the simple synthesis of purposefully designed macromolecules without deleterious side product formation is a major advantage associated with the self-assembly process.13 The versatility of the complex architectures have been used in a variety of applications such as gas storage, catalysis and drug delivery.14
Recently, Therrien and coworkers exploited the self-assembly approach for the design of finite nano-cages assembled by the combination of organometallic half-sandwich ruthenium clips and a tripodal donor and explored their practical prospective use for guest encapsulation and biological significance.15 Nano-cages based on half-sandwich arene-ruthenium complexes are promising candidates for the treatment of cancer as they possess interesting features such as low toxicity and a balance of hydrophilicity and hydrohpobicity, a key issue for their transport in biological media. 15b While a number of reports have revealed the importance of metalla-rectangles in host-guest chemistry,16 only a few of these studies have documented their antitumor properties.17 With the established anti-cancer activity of arene ruthenium complexes and the current interest in self-assembled supramolecules for biological applications, we decided to explore the synthesis of multinuclear ruthenium complexes (Scheme 1) based on an amide scaffold and to investigate the cytotoxicity of these complexes against various cancer cell lines.
Scheme 1.
Synthetic route to the self-assembled metalla-rectangles 1–3.
The equimolar reaction of the arene-Ru acceptors A1 and A2 with the dipyridyl amide ligands L1 and L2 in nitromethane/methanol (1:1) produced the molecular rectangles 1 (86%), 2 (82%), and 3 (85%), respectively, in high yields (Scheme 1). These molecular rectangles were characterized by 1H NMR spectroscopy and HR-ESI-MS, and definitive structures for 1 and 2 were established by X-ray diffraction studies. The 1H NMR spectra of the molecular-rectangles 1 and 3 show two doublets (δ 7.86 and 7.75 for 1, δ 8.46 and 7.93 for 3) and four peaks for 2 at δ 8.50 (d), 8.25 (s), 7.60 (m) and 7.29 (m), for the pyridyl protons after self-assembly along with a sharp singlet at δ 11.46 (1), 11.23 (2), and δ 10.71 (3) for the ligand amide protons. The p-cymene protons were observed at δ 6.02 and 5.83 for 1, δ 6.00, 5.87 and 5.80 for 2, and δ 5.96 and 5.76 for 3. In case of complex 3 the aromatic protons of p-cymene are diastereotopic. The naphthoquinone protons of 3 were noted as a singlet at δ 7.28 ppm (Supporting Information).
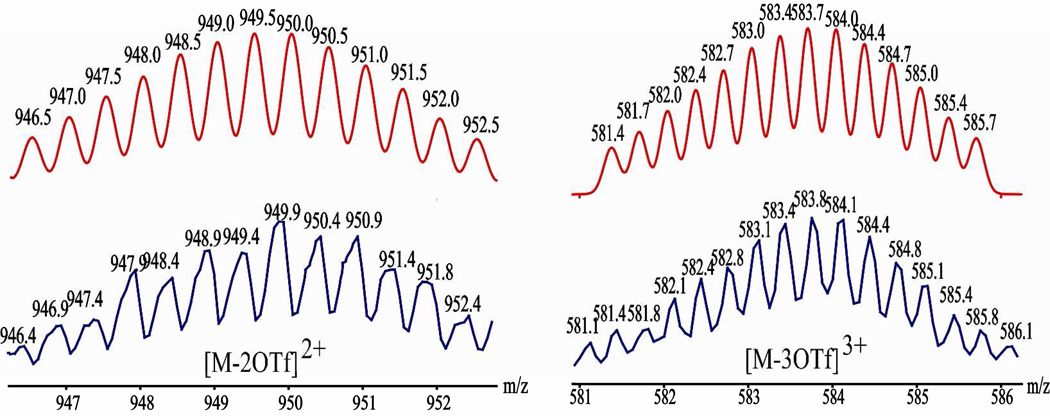
The formations of the molecular-rectangles 1 (see figure 2), 2 and 3 were further supported by HR-ESI-MS spectra. The charged states for 1 at m/z = 949.4 ([M – 2OTf]2+) and 583.8 ([M – 3OTf]3+), 2 at m/z = 583.8 ([M – 3OTf]3+), and 3 at m/z = 1050.1 ([M – 2OTf]2+) and 650.5 ([M – 3OTf]3+), were clearly observed and isotopically resolved. These peaks matched well with the theoretical distributions (Supporting Information).
Figure 2.
Calculated (red) and experimental (blue) HR-ESI-MS spectra of the metalla-rectangle 1.
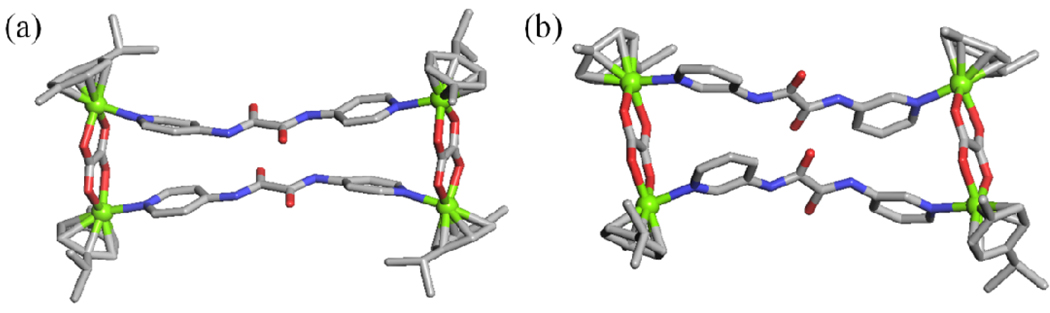
The molecular structures of 1 and 2 were further confirmed by X-ray crystallography (Figure 3). Single crystals of 1 and 2 suitable for X-ray analysis using synchrotron radiation were obtained by slow vapor diffusion of diethyl ether into the nitromethane/methanol solution of 1 and 2. X-ray structural analysis provided unambiguous proof of the molecular rectangles 1 and 2.
Figure 3.
X-ray crystal structure of the molecular rectangles 1 (a) and 2 (b). Solvent molecules, counter anions and hydrogen atoms are omitted for clarity (Color codes: green = Ru, red = O, blue = N and grey = C).
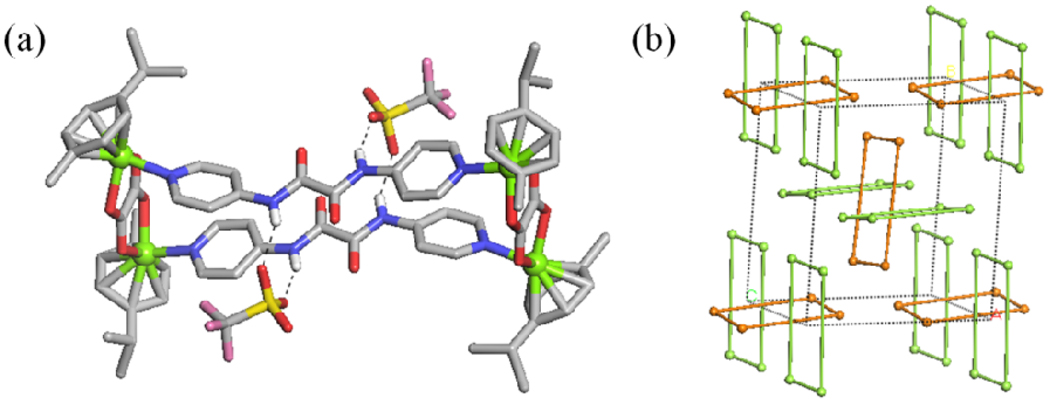
In the case of 1, there are two crystallographically independent M2L2 metalla-rectangles. Each amide group of molecular rectangles has hydrogen bond interactions with oxygen of triflate anions (Figure 4a). If Ru atoms are considered as connecting nodes, the Ru centers are connected to two neighboring Ru centers to form the molecular rectangles in 1 (Figure 4b). Molecular rectangles adopt the A–B cross packing conformation with an angle of 86.4° along the a axis.
Figure 4.
(a) Hydrogen bond interactions between amide group of M2L2 metalla-rectangles and oxygen of triflate anions, (b) the crystal packing structure of 1.
Previous reports revealed the importance of arene-ruthenium complexes in cancer treatment, therefore we also screened the growth-inhibitory activity of these metalla-rectangles 1–3 with respect to SK-hep-1 (liver cancer), HeLa (ovary cancer), HCT-15 (colon cancer), A-549 (lung cancer), and MDA-MB-231 (breast cancer) human cancer cell lines in vitro. The IC50 data are shown in Table 1.
Table 1.
Cytotoxicity of the Ru-complexes 1–3 with human cancer cells in vitro.
| compound | IC50 µM[a] | ||||
|---|---|---|---|---|---|
| SK-hep-1 | HeLa | HCT-15 | A-549 | MDA-MB-231 | |
| 1 | >200 | >200 | >200 | >200 | >200 |
| 2 | >200 | >200 | >200 | >200 | >200 |
| 3 | 4.2±0.11 | 10.2±0.21 | 3.7±0.10 | 3.2±0.11 | 2.8±0.03 |
| Cisplatin | 6.3±0.06 | 10.5±0.18 | 5.6±0.09 | 2.4±0.08 | 2.7±0.05 |
IC50 drug concentration necessary for 50% inhibition of cell viability. Data are shown as means ± SD.
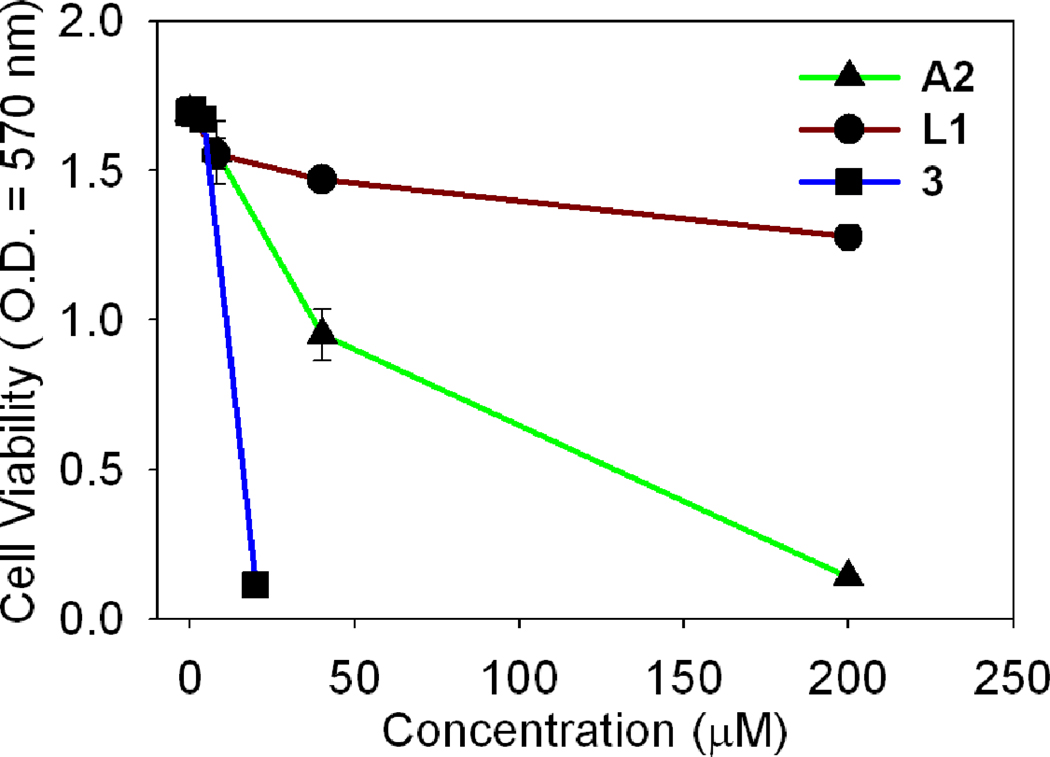
Cancer cells were exposed to increasing concentrations of the metalla-rectangles 1–3 for 24 h, and cell survival was established using the MTT assay. The metalla-rectangles 1 and 2 were inactive against these cancer cell lines, but the ruthenium metalla-rectangle 3 showed a low micro-molar range of inhibition against all of the cancer cell lines. The data indicate that the metalla-rectangle 3 (IC50 = 3.7 µM and 4.2 µM) was more potent compared with cisplatin (IC50 = 5.6 µM and 6.3 µM) especially with the HCT-15 and SK-hep-1 cancer cell lines, respectively. This result suggests that the metalla-rectangle 3 is most competent for inhibiting the proliferation of colon and liver cancer cell lines. To obtain the proper working concentrations, the cytotoxicity of the different compounds, arene-ruthenium acceptor A2, amide donor L1, and metalla-rectangle 3 were examined with the A-549 cancer cell line. As shown in Figure 5, the amide donor L1 alone does not give a measurable IC50 value even at the 200 micromolar concentration, whereas the acceptor A2 has moderate cytotoxicity at a high concentration level (200 µM), and the metalla-rectangle 3 displayed more than 10 fold enhancement compared with A2 on the A-549 cancer cell line.
Figure 5.
Cytotoxic effects of A2, L1 and the metalla-rectangle 3 on the A-549 cancer cell line.
On the observation of these results, it seems that the length of spacer is a significant factor for designing the antitumor compounds as selectively complex 3 (dhnq bridged) showing the high activity and complexes 1 and 2(oxalate bridged) are inactive against cancer cell lines.15d The biocompatible amide donor L1 coupled with the ruthenium acceptor A2 synergically increases the cytotoxicity of the metalla-rectangle 3 as the ligand introduces conjugation and flexibility in order to enhance the cytotoxicity as compared with the acceptor A2 . On the basis of our previous findings we can suggest the possible mode of action for cytotoxicity. 18 The cytotoxic behaviour of metalla-rectangle 3 could be due to its ability to interfere with cellular function, leading to apoptosis. The complex 3 could inhibit tumor cell proliferation by arresting the cell cycle process, preventing the mitotic entry of the cell.
In summary, the three compounds reported herein represent a new set of molecular rectangles constructed by the coordination driven self-assembly approach, and these complexes were evaluated for in vitro anti-cancer activity. The complex 3 proved to be highly cytotoxic, with an activity competitive with cisplatin. The detailed mechanistic study is under process.
Experimental Section
General Details
The arene-ruthenium acceptors A115d, A219a and the donor L1, L219b were prepared according to reported methods. Deuterated solvents were purchased from Cambridge Isotope Laboratory (Andover, MA). NMR spectra were recorded on a Bruker 300 MHz spectrometer. 1H NMR chemical shifts are reported relative to residual solvent signals. Mass spectra for the self-assemblies were recorded on a Micromass Quattro II triple-quadrupole mass spectrometer using electrospray ionization with a MassLynx operating system. From the single crystals of 1 and 2, the diffraction data were collected at 100 K on an ADSC Quantum 210 CCD diffractometer with synchrotron radiation (λ = 0.90000 Å) at the Macromolecular Crystallography Beamline 6B1, Pohang Accelerator Laboratory (PAL), Pohang, Korea. The raw data were processed and scaled using the program HKL2000. The structure was solved by direct methods, and the refinements carried out with full-matrix least-squares on F2 with the appropriate software implemented in the SHELXTL program package.
Cancer Cell Growth Inhibition Assay (MTT assay)
The cells were grown at 37 °C and 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum (FBS) 1% penicillin streptomycin. Cell suspensions of different cells were seeded into 96-well plates at a concentration of 5×104 cells per well (90 µL per well and 10 µL sample). MTT was first prepared as a stock solution of 5mg/ml in phosphate buffer (PBS, pH 7.2) and was filtered. 10 µL of MTT solution was added to each well. After incubation for 4 h at 37 °C and 5% CO2, 100 µL of DMSO (dimethylsulfoxide) was added to each well. After the 96 well plates was read by an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm for absorbance density values to determine the cell viability and the percentage of surviving cells was calculated from the ratio of absorbance of treated to untreated cells. The IC50 values for the inhibition of cell growth were determined by fitting the plot of the logarithmic percentage of surviving cells against the logarithm of the drug concentration using a linear regression function.
Synthesis of the Metalla-Rectangle 1
A mixture of the donor L1 (2.42mg, 0.01 mmol) and the ruthenium triflate acceptor A1 (8.58 mg, 0.01 mmol) in CH3NO2-CH3OH solution (1:1, 1.0 mL) was stirred for 10 h at room temperature. Upon addition of diethyl ether, a yellow crystalline powder of 1 was afforded. Yield 86%. Anal. Calcd for C72H76F12N8O24Ru4S4·(CD3)2SO: C, 38.95; H, 3.89; N, 4.91. Found: C, 38.64; H, 3.87; N, 4.80. 1H NMR [300 MHz, (CD3)2SO]: δ (ppm) 11.46 (s, NH), 7.86 (d, J = 6.9 Hz, 8H, Hα), 7.75 (d, J = 6.9 Hz, 8H, Hβ), 6.02 (d, J = 6.3 Hz, 8H, Hcym), 5.83(d, J = 6.3 Hz, 8H, Hcym), 2.76(sept, 4H, CH(CH3)2), 2.12 (s, 12H, CH3), 1.26 (d, J = 6.9Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2SO]: δ (ppm) 170.2, 158.7, 153.3, 147.4, 116.1, 102.7, 98.9, 83.6, 81.0, 30.7, 21.9, 17.5 ; MS (ESI) for 1 (C72H76F12N8O24Ru4S4): 949.9 [M –2OTf]2+; 583.8 [M – 3OTf]3+.
Synthesis of the Metalla-Rectangle 2
A mixture of the donor L2 (2.42 mg, 0.01 mmol) and the acceptor A1 (8.58 mg, 0.01 mmol) in CH3OH-CH3NO2 solution (1:1, 1.0 mL) was stirred for 10 h at room temperature. Upon the addition of diethyl ether, a yellow crystalline solid of 2 was precipitated and collected. Yield 82 %. Anal. Calcd for C72H76F12N8O24Ru4S4· (CD3)2SO: C, 38.95; H, 3.89; N, 4.91. Found: C, 38.73; H, 3.81; N, 5.07. 1H NMR [300 MHz, (CD3)2SO]: δ (ppm) 11.23 (s, NH), 8.50 (m, 4H, H1), 8.25 (s, 4H, H4), 7.60 (m, 4H, H2), 7.29 (m, 4H, H3), 6.00 (d, J= 6.3 Hz, 8H, Hcym), 5.87(dd, J = 6.6 Hz, 3JH,H = 5.4 Hz, 8H, Hcym), 2.82 (sept, 4H, CH(CH3)2), 2.12 (s, 12H, CH3), 1.30 (d, J = 6.9Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2SO]: δ (ppm) 170.4, 158.2, 147.0, 135.7, 129.5, 126.1, 122.8, 100.6, 96.3, 82.4, 81.6, 30.6, 22.0, 17.6 ; MS (ESI) for 2 (C72H76F12N8O24Ru4S4): 583.8 [M – 3OTf]3+.
Synthesis of the Metalla-Rectangle 3
A mixture of the donor L1 (2.42 mg, 0.01 mmol) and the acceptor A2 (8.58 mg, 0.01 mmol) in CH3OH-CH3NO2 solution (1:1, 1.0 mL) was stirred for 10 h at room temperature. After completion of the reaction the mixture was concentrated and green powdered product 3 was precipitated upon addition of diethyl ether. Yield 85%. Anal. Calcd for C88H84F12N8O24Ru4S4: C, 44.07, H, 3.53, N, 4.67. Found: C, 43.86, H, 3.71, N, 4.87. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 10.71 (s, NH), 8.46 (d, J = 6.9 Hz, 8H, Hα), 7.93 (d, J = 6.9 Hz, 8H, Hβ), 7.28 (s, 8H, Hq), 5.96 (d, J = 6.3 Hz, 8H, Hcym), 5.76 (d, J = 6.3 Hz, 8H, Hcym), 2.93 (sept, 4H, CH(CH3)2), 2.17 (s, 12H, CH3), 1.36 (d, J = 6.9Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2SO]: δ (ppm) 170.3, 158.3, 152.2, 146.9, 137.3, 115.9,110.9, 102.1, 99.2, 84.3, 82.2, 30.1, 21.9, 16.6 ; MS (ESI) for 3 (C88H84F12N8O24Ru4S4): 1050.1 [M – 2OTf]2+, 650.5 [M – 3OTf]3+.
Supplementary Material
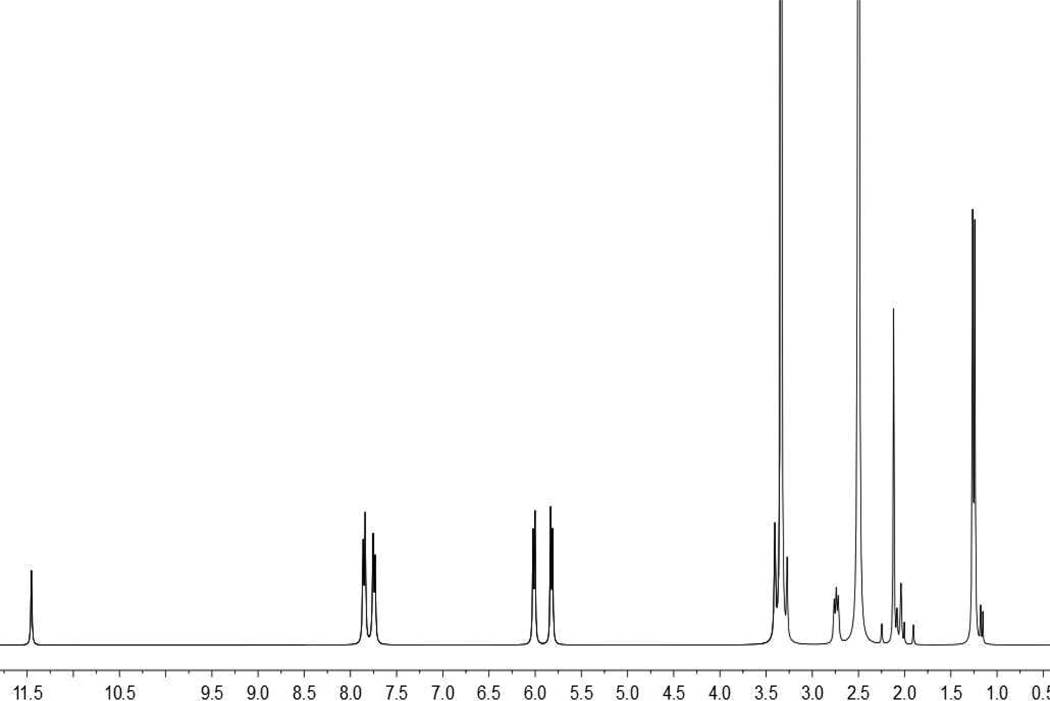
Figure 1.
1H NMR spectrum of the metalla-rectangle 1.
Acknowledgments
This work was supported by the World Class University (WCU) program (R33-2008-000-10003) and Priority Research Centers program (2009-0093818) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. We also thank Pohang Accelerator Laboratory in Korea for their help in the X-ray analysis. P.J.S thanks the NIH (GM-57052) for financial support.
Footnotes
Supporting Information Available: 1H and 13C NMR spectra for 1–3, HR-ESI-MS data for 2 and 3 and crystallographic data for the metalla-rectangles 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Orvig C, Abrams MJ. Chem. Rev. 1999;99:2201. doi: 10.1021/cr980419w. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg B, Camp LV, Grimley EB, Thomson AJ. J. Biol. Chem. 1967;242:1347. [PubMed] [Google Scholar]
- 3.Melchart M, Sadler PJ. Bioorganometallics. Vol. 1. Weinheim: Wiley - VCH; 2006. [Google Scholar]
- 4.(a) Reedijk J. Proc. Natl Acad. Sci. 2003;100:3611. doi: 10.1073/pnas.0737293100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guo Z, Sadler PJ. Angew. Chem. Int. Ed. 1999;38:1512. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Reedijk J. Plat. Met. Rev. 2008;52:2. [Google Scholar]
- 6.Allardyce CS, Dyson PJ. Plat. Met. Rev. 2001;45:62. [Google Scholar]
- 7.Clarke MJ. Met. Ions Biol. Syst. 1980;11:231. [PubMed] [Google Scholar]
- 8.Dyson PJ, Sava G. Dalton Trans. 2006:1929. doi: 10.1039/b601840h. [DOI] [PubMed] [Google Scholar]
- 9.(a) Mendoza-Ferri M-G, Hartinger CG, Eichinger RE, Stolyarova N, Severin K, Jakupec MA, Nazarov AA, Keppler BK. Organometallics. 2008;27:2405. [Google Scholar]; (b) Mendoza-Ferri MG, Hartinger CG, Mendoza MA, Groessl M, Egger AE, Eichinger RE, Mangrum JB, Farrell NP, Maruszak M, Bednarski PJ, Klein F, Jakupec MA, Nazarov AA, Severin K, Keppler BK. J. Med. Chem. 2009;52:916. doi: 10.1021/jm8013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Maeda H. Cancer. Res. 1986;46:6387. [PubMed] [Google Scholar]
- 11.Maeda H. Advan. Enzyme Regul. 2001;41:189. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 12.(a) Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Nature Biotech. 2005;23:1517. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]; (b) Tomalia DA, Reyna LA, Svenson S. Biochem. Soc. Trans. 2007;35:61. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]; (c) Boas U, Christensen JB, Heegaard PMH. Dendrimers in Medicine and Biotechnology: New Molecular Tools. RSC Publishing; 2006. [Google Scholar]; (d) Aulenta F, Hayes W, Rannard S. Eur. Polym. J. 2003;39:1741. [Google Scholar]; (e) Wolinsky JB, Grinstaff MW. Adv. Drug Deliv. Rev. 2008;60:1037. doi: 10.1016/j.addr.2008.02.012. [DOI] [PubMed] [Google Scholar]; (f) Agarwal A, Saraf S, Asthana A, Gupta U, Gajbhiye V, Jain NK. Int. J. Pharm. 2008;350:3. doi: 10.1016/j.ijpharm.2007.09.024. [DOI] [PubMed] [Google Scholar]; (g) Gillies ER, Fréchet JMJ. Drug Discov. Today. 2005;10:35. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 13.(a) Ghosh K, Hu J, Yang H-B, Northrop BH, White HS, Stang PJ. J. Org. Chem. 2009;74:4828. doi: 10.1021/jo900580w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang M, Vajpayee V, Shanmugaraju S, Zheng Y-R, Zhao Z, Kim H, Mukherjee PS, Chi K-W, Stang PJ. Inorg. Chem. 2011;50:1506. doi: 10.1021/ic1020719. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chi K-W, Addicott C, Moon M-E, Lee HJ, Yoon SC, Stang PJ. J. Org. Chem. 2006;71:6662. doi: 10.1021/jo060971i. [DOI] [PubMed] [Google Scholar]; (d) Zheng Y-R, Zhao Z, Wang M, Ghosh K, Pollock JB, Cook TR, Stang PJ. J. Am. Chem. Soc. 2010;132:16873. doi: 10.1021/ja106251f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhao G-Z, Chen L-J, Wang C-H, Yang H-B, Ghosh K, Zheng Y-R, Lyndon MM, Muddiman DC, Stang PJ. Organometallics. 2010;29:6137. [Google Scholar]; (f) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; (g) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (h) Holliday BJ, Mirkin CA. Angew. Chem. Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]; (i) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (j) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:349. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (k) Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Raymond KN. Nature. 2009;460:585. [Google Scholar]; (m) Pluth MD, Bergman RG, Raymond KN. Acc. Chem. Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (n) Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Chem. Soc. Rev. 2009;38:1450. doi: 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]; (o) Mounir M, Lorenzo J, Ferrer M, Prieto MJ, Rossell O, Avile`s FX, Moreno V. J. Inorg. Biochem. 2007;101:660. doi: 10.1016/j.jinorgbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.(a) Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’Keeffe M, Yaghi OM. Science. 2003;300:1127. doi: 10.1126/science.1083440. [DOI] [PubMed] [Google Scholar]; (b) Trembleau L, Rebek J., Jr Science. 2003;301:1219. doi: 10.1126/science.1086644. [DOI] [PubMed] [Google Scholar]; (c) Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]; (d) Pluth MD, Bergman RG, Raymond KN. Science. 2007;316:85. doi: 10.1126/science.1138748. [DOI] [PubMed] [Google Scholar]
- 15.(a) Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew. Chem., Int. Ed. 2008;47:3773. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]; (b) Zava O, Mattsson J, Dyson PJ, Therrien B. Chem.–Eur. J. 2010;16:1428. doi: 10.1002/chem.200903216. [DOI] [PubMed] [Google Scholar]; (c) Mattsson J, Zava O, Renfrew AK, Sei Y, Yamaguchi K, Dyson PJ, Therrien B. Dalton Trans. 2010;39:8248. doi: 10.1039/c0dt00436g. [DOI] [PubMed] [Google Scholar]; (d) Barry NPE, Zava O, Furrer J, Dyson PJ, Therrien B. Dalton Trans. 2010;39:5272. doi: 10.1039/c001521k. [DOI] [PubMed] [Google Scholar]; (e) Barry NPE, Abd Karim NH, Vilar R, Therrien B. Dalton Trans. 2009:10717. doi: 10.1039/b913642h. [DOI] [PubMed] [Google Scholar]
- 16.(a) Barry NPE, Furrer J, Freudenreich J, Süss-Fink G, Therrien B. Eur. J. Inorg. Chem. 2010;725 [Google Scholar]; (b) Han Y-F, Lin Y-J, Jia W-G, Wang G-L, Jin G-X. Chem. Commun. 2008;1807 doi: 10.1039/b717554j. [DOI] [PubMed] [Google Scholar]; (c) Han Y-F, Jia W-G, Lin Y-J, Jin G-X. Angew. Chem. Int. Ed. 2009;48:6234. doi: 10.1002/anie.200805949. [DOI] [PubMed] [Google Scholar]; (d) Yan H, Süss-Fink G, Neels A, Stoeckli-Evans H. J. Chem. Soc., Dalton Trans. 1997:4345. [Google Scholar]; (e) Han Y-F, Lin Y-J, Jia L-H, Weng W-G, Jin G-X. Organometallics. 2007;26:5848. [Google Scholar]; (f) Zhang W-Z, Han Y-F, Lin Y-J, Jin G-X. Dalton Trans. 2009:8426. doi: 10.1039/b909357e. [DOI] [PubMed] [Google Scholar]; (g) Jia W-G, Han Y-F, Lin Y-J, Weng L-H, Jin G-X. Organometallics. 2009;28:3459. [Google Scholar]; (h) Linares F, Galindo MA, Galli S, Romero MA, Navarro JAR, Barea E. Inorg. Chem. 2009;48:7413. doi: 10.1021/ic900980y. [DOI] [PubMed] [Google Scholar]; (i) Zhang W-Z, Han Y-F, Lin Y-J, Jin G-X. Organometallics. 2010;29:2842. [Google Scholar]; (j) Yu W-B, Han Y-F, Lin Y-J, Jin G-X. Organometallics. 2010;29:2827. [Google Scholar]; (k) Yan H, Suss-Fink G, Neels A, Evans HS. J. Chem. Soc. Dalton Trans. 1997:4345. [Google Scholar]; (l) Barry NPE, Furrer J, Freudenreich J, Süss-Fink G, Therrien B. Eur. J. Inorg. Chem. 2010:725. [Google Scholar]
- 17.(a) Mattsson J, Govindaswamy P, Renfrew AK, Dyson PJ, Štěpnička P, Süss-Fink G, Therrien B. Organometallics. 2009;28:4350. [Google Scholar]; (b) Linares F, Procopio EQ, Galindo MA, Romero MA, Navarro JAR, Barea E. CrystEngComm. 2010;12:2343. [Google Scholar]
- 18.Vajpayee V, Yang YJ, Kang SC, Kim H, Kim IS, Wang M, Stang P, Chi K-W. Chem. Commun. 2011;47:5184. doi: 10.1039/c1cc10167f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Barry NPE, Therrien B. Eur. J. Inorg. Chem. 2009:4695. [Google Scholar]; (b) Tzeng B-I, Chen Y-F, Wu C-C, Hu C-C, Chang Y-T, Chen C-K. New J. Chem. 2007;31:202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.