Abstract
Background:
Gestational hypertension is a common complication of pregnancy. Recent evidence suggests that women with gestational hypertension have a high rate of sleep disordered breathing (SDB). Using laboratory-based polysomnography, we evaluated for the frequency of SDB in women with gestational hypertension compared to healthy women with uncomplicated pregnancies.
Methods:
In this single-center cross-sectional study, women with the diagnosis of gestational hypertension were screened in the Fetal Assessment Unit and Antepartum ward. Healthy subjects were recruited by local advertising. Subjects completed a series of questionnaires addressing sleep quality and daytime sleepiness, followed by full night polysomnography. The primary outcome was frequency of SDB (defined as a respiratory disturbance index ≥ 5) in the gestational hypertension and healthy groups.
Results:
A total of 34 women with gestational hypertension and singleton pregnancies and 26 healthy women with uncomplicated singleton pregnancies consented to participate in the study. The mean ages and gestational ages, but not the body mass indices, of the 2 groups were similar. The frequencies of SDB in the more obese gestational hypertension group and the healthy group were 53% and 12%, respectively (P < 0.001).
Interpretation:
Women with gestational hypertension may have a significantly higher frequency of SDB than do healthy women with uncomplicated pregnancies of similar gestational age. The relative causal contributions, if any, of SDB and obesity remain to be determined.
Citation:
Reid J; Skomro R; Cotton D; Ward H; Olatunbosun F; Gjevre J; Guilleminault C. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. SLEEP 2011;34(8):1033-1038.
Keywords: Sleep disordered breathing, pregnancy, gestational hypertension
INTRODUCTION
Hypertensive disorders in pregnancy are the most common medical complications of pregnancy and are an important cause of maternal and perinatal morbidity and mortality worldwide. Hypertensive disorders in pregnancy are divided into three categories: chronic hypertension, gestational hypertension (GH), and preeclampsia. Preeclampsia has classically been defined by as triad of hypertension, namely a resting BP > 140/90, proteinuria (24-h urinary protein level > 0.3 g), and edema occurring after 20 weeks' gestation in previously normotensive women. Preeclampsia is also referred to as “gestational hypertension with proteinuria” (GHP) and is a multisystem disease that can result in HELLP syndrome characterized by microvascular hemolysis, elevated liver enzymes, thrombocytopenia, and renal dysfunction with proteinuria, as well as peripheral and cerebral edema.1 GHP is associated with adverse maternal and fetal outcomes2–4 and commonly results in intrauterine growth retardation, early induction of labor, and caesarian section. While the effect of GH without proteinuria on maternal fetal outcomes is less clear, up to half of the women initially diagnosed with GH can progress to GHP.4–6 Furthermore, even without proteinuria, GH is linked to an increased risk of hypertension and adverse cardiovascular outcomes for the mother later in life.7
Sleep disordered breathing (SDB) is a recognized cause of hypertension in non-pregnant adults,8 and effective treatment of SDB can result in improved blood pressure control.9,10 In pregnancy, nasal congestion and snoring are common complaints, but occur even more frequently in women with GH or GHP11,12; these symptoms may be markers of SDB. The consequence of SDB can include intermittent hypoxemia13,14 and surges in sympathetic tone15; these events in the mother could potentially trigger or exacerbate maternal hypertension, placental hypoperfusion, and fetal hypoxia.16–18
By these mechanisms, there is physiologic plausibility for SDB to be a cause in initiating or exacerbating GH/GHP and its complications.18 In the last 10 years, there has been accumulating evidence to suggest that women with GH/GHP have a high prevalence of snoring and SDB.11,12,19–22 However, most investigations in this area have employed questionnaire-based assessments11,12,20,22 or small case series that have either not had appropriate comparison groups or relied on limited sleep monitoring technologies.21,23–25
Additionally, not all of these studies have found a strong association between GH/GHP and SDB.25 In a rigorous evaluation of women with uncomplicated pregnancies, Guilleminault et al.26 reported an association between abnormal breathing during sleep and a trend to higher (but still within normal limits) blood pressure. Most recently, in the largest case-control series to date, Champagne et al.27 found a strong association between GH and SDB. If those findings can be reproduced on a broader scale, this may expand the clinical armamentarium in the management of women with GH. By utilizing the gold-standard test for SDB—full-night polysomnography (PSG) in the sleep laboratory—we investigated the frequency of SDB in women with GH (with or without proteinuria) and in women with uncomplicated pregnancies of similar gestational age.
METHODS
This was a single-center cross-sectional study, which compared women with singleton pregnancies and the diagnosis of GH or GHP to healthy women with uncomplicated singleton pregnancies of similar gestational age.
Participants
Women ≥ 18 years of age with singleton pregnancies and the diagnosis of GH (with or without proteinuria) were recruited from the Fetal Assessment Unit and Antepartum ward of Royal University Hospital, Saskatoon, Saskatchewan, Canada, between February 2006 and February 2008. GH was defined according to standard criteria.28 Exclusion criteria included multiparity gestation, imminent delivery, severe underlying maternal or fetal conditions expected to worsen maternal or fetal outcomes, and maternal condition preventing safe transfer off of the obstetrical floor. Women with chronic hypertension were considered eligible, providing they met the criteria of chronic hypertension with superimposed gestational hypertension.28 If there was any question of the diagnosis, the treating physician was consulted for confirmation. Women with poorly controlled chronic hypertension were excluded. All other women with GH or the more complicated GHP were considered eligible for study inclusion and labeled collectively as “GH” for study analysis. Screening occurred on weekdays and occasional weekends. All potentially eligible women identified were screened for eligibility by chart review and if possible, by interview. Healthy subjects were recruited by local advertising (posters and handout information provided in the Fetal Assessment Unit and obstetricians offices). “Healthy” was defined as having an uncomplicated pregnancy and the absence of any underlying maternal condition expected to adversely affect the pregnancy. Eligible GH subjects were studied by PSG as soon as possible, usually within a few days of study enrollment. To fulfill the definition of GH, these women had to present after 20 weeks gestation; most were ≥ 32 weeks gestation. In attempt to generally match gestational age between the GH and healthy groups, interested healthy women who presented at earlier gestational ages were deferred until after 32 weeks. Written informed consent was obtained for all patients included in the study protocol, which was approved by the University of Saskatchewan Biomedical Ethics Review Board and registered with the National Institute of Health Clinical Trials Registry (Identifier NCT00259688).
Procedures
Baseline data were collected for age, height, weight, weight gain in pregnancy, body mass index (BMI), estimated pre-pregnancy weight, snoring history, as well as previous and present obstetrical history and general medical history. Subjects completed a series of short questionnaires which addressed both sleepiness and common signs or symptoms of SDB. These included the Epworth Sleepiness Score (ESS),29 the Pittsburgh Sleep Quality Index (PSQI),30 the Berlin Questionnaire,31 and a visual analogue scale of sleep quality which asked subjects to rate their current sleep on a horizontal scale between “worst” and “best.” This was converted to a percentage score, with worst being 0% and best being 100%. This was followed by a full-night diagnostic PSG (Sandman 8.0, Tyco Inc., Ottawa, Canada) performed in the Royal University Hospital Sleep Laboratory. PSG channels included electroencephalogram; electrooculogram; submental electromyogram (EMG); pulse oximetry; nasal airflow pressure sensor and oronasal thermistor airflow, chest movement by piezoelectric belt; snore vibration sensor; intercostal surface EMG; and anterior tibialis EMG; electrocardiogram. Studies were scored according the American Academy of Sleep Medicine criteria32 by a single registered sleep technician who was blinded to clinical data. As per the AASM criteria, respiratory events were categorized as apneas (a decrease in airflow ≥ 90% from baseline for ≥ 10 sec); hypopneas (decrease in airflow ' 30% for ≥ 10 sec and followed by a desaturation ≥ 4% from the pre-event baseline); and respiratory event-related arousals (RERAs), defined as a sequence of breaths lasting ≥ 10 sec, associated with flattening of the nasal pressure waveform leading to an arousal from sleep, (not meeting criteria for apnea or hypopnea). The total number of apneas, hypopneas, and RERAs divided by the hours of sleep was expressed as the respiratory disturbance index (RDI).
Statistical Analysis
The primary endpoint was determined a priori to be the frequency of SDB, using a cutoff of RDI ≥ 5 events per hour. Secondary endpoints included diagnosis of SDB using more stringent cutoffs of RDI ≥ 10, ≥ 15, and ≥ 20, mean RDI, and scores on self-administered questionnaires. Our main outcome of interest was the frequency of SDB in GH women versus women with uncomplicated pregnancies. Means and standard deviations were calculated for continuous variables and means were compared using the Student t-test. Frequencies of categorical variables were identified and assessed by χ2 and Fisher exact test. The correlation between BMI and RDI among study subjects with GH was assessed using correlation coefficient. Logistic regression was performed to assess the association between SDB and the presence of GH. Microsoft Excel 2003 and SAS Version 9.1 were used for data collection and statistical analysis.
RESULTS
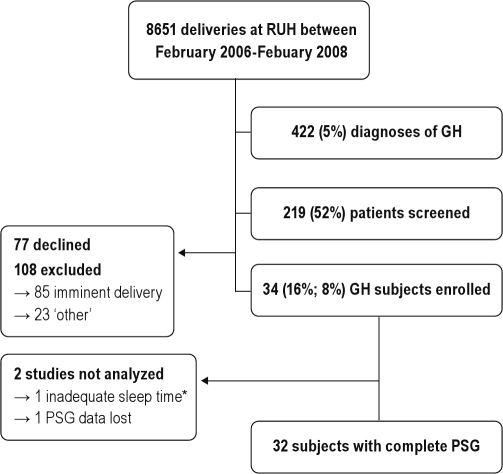
Between February 2006 and February 2008, a total of 8651 deliveries occurred at Royal University Hospital; of these there were 422 diagnoses of GH. We identified 219 (52%) of these women. The remaining 48% were missed mostly because they presented after hours or on weekends, and were either discharged or progressed to delivery before we could identify them. Of the 219 women screened, 34 (16% of those screened and 8% of all diagnoses of GH) consented to participate in the study protocol (Figure 1). Table 1 compares the group of GH women who consented to our study (68% of whom had proteinuria and therefore met criteria for GHP) versus the GH women who were screened but were either excluded or declined participation. Twenty-eight healthy subjects expressed interest during the same study period. One was excluded because of coexistent disease (diabetes). The other subject developed GH one week prior to her sleep study and was therefore studied as part of the GH group. This left 26 healthy pregnant participants. Table 2 compares the GH subjects we studied with the uncomplicated pregnancy cohort. The 2 groups were similar for maternal and gestational ages, but the GH women had a significantly higher mean BMI both at the time of evaluation (37.4 ± 7.3 vs 28.7 ± 4.4; P = 0.0001) and prior to pregnancy (31.9 ± 8.0 vs 23.2 ± 4.6; P < 0.0001). We found a much higher frequency of self-reported current, regular snoring (82% vs 38%, P = 0.0005) and nasal congestion (71% vs 36%, P = 0.008) in the GH women than the healthy cohort. The GH subjects described lower quality sleep as measured by the PSQI (9.7 ± 3.8 vs 6.4 ± 2.7, P = 0.0003) and visual analogue scales (42% ± 18% vs 59% ± 18%, P = 0.001), but there was no difference in daytime sleepiness between groups (ESS of 8.4 ± 3.4 vs 8.3 ± 3.6, P = 0.89). The majority of the GH women (79%) were taking at least one antihypertensive medication. The medications used were, in order of frequency, labetalol, α-methyldopa, and nifedipine.
Figure 1.
Recruitment of GH subjects. *This subject had a total of 4.5 minutes of discontinuous sleep before being transferred back to the labor and delivery ward.
Table 1.
Excluded/declined patients with GH compared to the GH study group
| GH Women: Excluded/Declined | GH women: studied | P-value | |
|---|---|---|---|
| N = 185 | N = 34 | ||
| Age | 28.9 ± 6.3 | 28.7 ± 6.1 | 0.97 |
| Gestational age | 36.3 ± 3.2 | 34.7 ± 3.2 | 0.004 |
| BMI | 35.9 ± 7.0 | 37.4 ± 7.3 | 0.3 |
| Pregnancy weight gain (kg) | 13.6 ± 6.3 | 11.8 ± 6.4 | 0.15 |
| Gestational diabetes | 28/150* (19%) | 3 (9%) | 0.20 |
| Snoring | 106/149* (71%) | 28 (82%) | 0.18 |
| Nasal congestion | 106/134* (79%) | 24 (71%) | 0.29 |
The number for which data was available is listed
Table 2.
Baseline data GH: study group compared to healthy cohort
| Healthy (n = 26) | GH (n = 34) | P-value | |
|---|---|---|---|
| Age | 29.6 ± 4.6 | 28.7 ± 6.1 | 0.6 |
| Gestational age | 34.7 ± 2.6 | 34.7 ± 3.2 | 0.9 |
| Pre pregnancy BMI | 23.2 ± 4.6 | 31.9 ± 8.0 | < 0.001 |
| Pregnancy BMI (kg/m2)¶ | 28.7 ± 4.4 | 37.4 ± 7.3 | < 0.001 |
| Pregnancy weight gain (kg) | 13.4 ± 3.9 | 11.8 ± 6.4 | 0.26 |
| Neck circumference (cm) | 34.2 ± 3.2 | 38.6 ± 3.4 | < 0.001 |
| Proteinuria (≥ 0.3 g/day) | NA* | 23 (68%) | NA |
| Chronic hypertension | 0* | 3 (9%) | NA |
| Gestational diabetes | 0* | 3 (9%) | NA |
| Snoring pre pregnancy | 5 (19%) | 19 (56%) | 0.03 |
| Snoring during pregnancy | 10 (38%) | 28 (82%) | < 0.001 |
| Nasal congestion | 9/25♦ (36%) | 24 (71%) | 0.008 |
| ESS Score | 8.3 ± 3.6 | 8.4 ± 3.4 | 0.89 |
| PSQI Score | 6.4 ± 2.7 | 9.7 ± 3.8 | < 0.001 |
| Berlin Questionnaire – high risk for SDB | 1 (4%) | 18 (53%) | < 0.001 |
| Sleep quality by VAS (%) | 59 ± 8 | 42 ± 18 | 0.001 |
| Systolic blood pressure | 106 ± 12 | 142 ± 16 | < 0.001 |
| Diastolic blood pressure | 68 ± 10 | 86 ± 12 | < 0.001 |
| ≥ 1 antihypertensive med | 0* | 27 (79%) | NA |
| ≥ 2 antihypertensive meds | 0* | 5 (5%) | NA |
BMI, Body mass index; ESS, Epworth Sleepiness Score; PSQI, Pittsburg Sleep Quality Index, a higher score represents more disturbed sleep; VAS, visual analogue scale, where 0% represents “worst” sleep and 100% represents “best” sleep by subjective assessment.
BMI at the time of assessment.
By requirement these subjects were healthy.
Data on nasal congestion was unavailable for one subject in the healthy cohort.
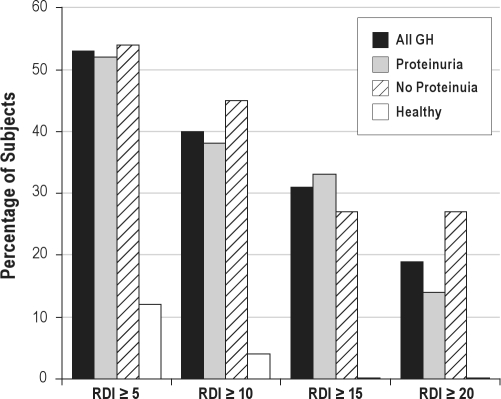
Polysomnographic data comparison is presented in Table 3. Two PSG recordings (one GH and one healthy) were inadvertently deleted, and one GH study was discarded because of minimal sleep time. Women with GH had less total sleep time (252 ± 81 min vs 311.8 ± 54.2 min, P = 0.003), lower sleep efficiency (62% ± 19.5% vs 71.9% ± 10.3%, P = 0.04), and a lower percentage of REM sleep (8.4% ± 5.1% vs 12.6% ± 5.1%, P = 0.003) than the healthy women. Furthermore, the GH women had a higher apnea-hypopnea index (AHI; 3.13 ± 6.2 vs 0.5 ± 1.3, P = 0.04) and higher RDI (11.5 ± 11.2 vs 2.3 ± 3.1, P = 0.0002) than the healthy women. The unadjusted odds ratio (OR) for SDB in subjects with GH was 8.3 (95% CI 2.1–33.4; P = 0.003). There was a highly significant difference in BMI between the GH and healthy groups, so much so that when adjusted for BMI, there was no association between SDB and GH. Looking at our primary endpoint, there was a significantly higher frequency of SDB (defined as RDI ≥ 5) in the GH group compared to the healthy group (53% vs 12%, P = 0.001). When using more stringent thresholds of RDI ≥ 10, RDI ≥ 15, and RDI ≥ 20, the GH women still had a much higher frequency of SDB (40% vs 4%, P = 0.001; 31% vs 0%, P = 0.005; and 19% vs 0%, P = 0.03, respectively), and this was not influenced by the presence or absence of proteinuria (Figure 2).
Table 3.
Polysomnographic data: GH study group compared to healthy cohort
| Healthy (n = 25)* | GH (n = 32)♦ | P-value | |
|---|---|---|---|
| Total sleep time (min) | 311.8 ± 54.2 | 252 ± 81 | 0.003 |
| Sleep efficiency (%) | 72.3 ± 10.4 | 62 ± 19.5 | 0.04 |
| Percentage REM sleep | 12.6 ± 5.1 | 8.4 ± 5.1 | 0.003 |
| Percentage stage 3/4 sleep | 21.0 ± 6.9 | 22.4 ± 9.9 | 0.56 |
| Arousal Index | 11.1 ± 7.0 | 16.5 ± 12.2 | 0.07 |
| Mean SpO2 (%) | 95.9 ± 1.5 | 95.5 ± 1.2 | 0.37 |
| ODI – NREM | 0.7 ± 1.7 | 1.4 ± 2.2 | 0.27 |
| ODI – REM | 1.1 ± 4.5 | 9.4 ± 15.7 | 0.02 |
| AHI (events/h) | 0.5 ± 1.3 | 3.1 ± 6.2 | 0.04 |
| RERA index (events/h) | 1.9 ± 2.1 | 8.3 ± 8.4 | < 0.001 |
| RDI | 2.3 ± 3.1 | 11.5 ± 11.2 | < 0.001 |
| REM RDI | 5.9 ± 9.8 | 28.1 ± 39.4 | 0.006 |
| RDI ≥ 20 events/h | 0 | 6 (19%) | 0.03 |
| RDI ≥ 15 events/h | 0 | 10 (31%) | 0.005 |
| RDI ≥ 10 events/h | 1 (4%) | 13 (40%) | 0.001 |
| RDI ≥ 5 events/h | 3 (12%) | 17 (53%) | 0.001 |
ODI, oxygen desaturation index; AHI, apnea hypopnea index; RERA, respiratory event related arousal; RDI, respiratory disturbance index (the total number of apneas, hypopneas and RERAs per hour of sleep).
25 of 26 studies were available for analysis.
32 of 34 studies were available for analysis.
Figure 2.
Frequency of sleep disordered breathing using 4 separate RDI criteria. RDI, respiratory disturbance index; Proteinuria is defined as ' 3 grams/d on 24-h collection. All GH n = 32; Proteinuria n = 21; No Proteinuria n = 11; Healthy n = 25.
DISCUSSION
We found that the frequency of SDB is high in unselected women with GH, and is much higher than in women with uncomplicated pregnancies of the same gestational age. Consistent with previous reports,11,12 we also found that women with GH complained of snoring and nasal congestion at a much higher rate. Although some of our GH subjects had moderate to severe obstructive sleep apnea, many had relatively mild SDB with recurrent arousals but minimal or no episodes of arterial oxygen desaturation. We feel this is an important point, because simple monitoring techniques such as overnight oximetry and other bedside testing devices rely heavily on the presence of either frank apneas or oxygen desaturation events to detect SDB. Such testing strategies are not sensitive enough to detect subtle decrements in airflow, and many of our subjects with SDB would not have been diagnosed using these limited technologies. This may explain the discrepancies in the literature and why many previous studies of women with GH have reported the presence of subtle airflow abnormalities23,24 but not clear SDB.21,23–25
We used the most sensitive diagnostic cutoff (RDI ≥ 5) for SDB applied in common practice. While this is lower than the diagnostic threshold used by some authors, we think it is justified because even mild SDB has been shown to have hemodynamic consequences in the non-pregnant population; this may be particularly true in preeclamptic women, in whom the hemodynamic responses to obstructive respiratory events of SDB are exaggerated.15,21 Subtle obstructive respiratory events typical of SDB have been shown to precipitate transient blood pressure spikes, and women with GH demonstrate an exaggerated hypertensive response to these events.15,18,21 In fact, recent data suggest that short-term relief of even very mild SDB, including isolated flow limitation or snoring, with nasal continuous positive pressure (CPAP) is associated with improvements in hemodynamic status, including blood pressure in preeclamptic patients.23,33,34 Furthermore, during normal pregnancy there is a progressive rightward shift of the oxyhemoglobin dissociation curve,35 which facilitates off-loading of oxygen to peripheral tissues, most importantly the placenta. Not only does this rightward shift not occur in preeclampsia, but there is actually a shift to the left,35 which impairs oxygen off-loading and thereby renders the fetus particularly vulnerable to even subtle obstructive respiratory events of the preeclamptic mother. In this clinical context, even our low RDI cutoff of ≥ 5 may have been too high.
Although we studied only 8% of the women who were diagnosed with GH during the enrollment period, and only 16% of the GH women who were screened; our data shows similar rates of snoring and nasal congestion as well as similar BMI between the GH group studied and those GH women who declined or were excluded (Table 1). We therefore think that the GH subjects we studied are representative of the general GH population. This is important because the high rate of obesity in our GH subjects is clearly a considerable confounder, and most of our subjects with SDB were obese (BMI ≥ 30). We still think that our findings are important because obesity is an established risk factor for GH,2,22,36,37 and therefore any study looking at unselected women with GH should be expected to enroll women with elevated BMIs. It may be that an increased frequency of SDB is one mechanism by which obesity and GH are linked; however, the correlation between BMI and RDI was only moderate (r = 0.54), suggesting there may be other factors at play. Certainly, in non-pregnant adults, BMI and neck circumference explain only 30% of the variability in the frequency of apnea-hypopnea indices,38,39 and only 60% of adults with SDB are obese.38–40 Further study is needed to better understand the interactions between BMI, GH, and SDB.
Another potential limitation of our study is that the total sleep and REM sleep times were short in both groups, particularly the GH women (Table 3). These low times increase the likelihood of error when calculating time-indexed values. However, since SDB normally worsens as the sleep period progresses and with subsequent REM periods, we believe that longer sleep times and more REM would only have amplified our results. With this in mind, it is noteworthy that during REM sleep both the RDI (28.1 ± 39.4 vs 5.9 ± 9.8, P = 0.006) and the oxygen desaturation index ≥ 3% (9.4 ± 15.7 vs 1.1 ± 4.5, P = 0.02) were much higher in GH women. In fact, as there was no clear external cause for worsened sleep quality in the GH group (no complaints of headache, cramping or other pain), we postulate that the short total and REM sleep times of the GH women may actually have been consequences of SDB-induced sleep disruption
Furthermore, this REM related deterioration is concerning because of its potential physiologic importance. Due to the decrease in muscle tone associated with this sleep stage,41 REM sleep is a uniquely vulnerable time for upper airway obstruction; and this REM-related deterioration appears to be especially pronounced in young women.42 Moreover, sympathetic nervous system activity is much higher in REM than in other sleep stages41; therefore, the cardiovascular consequences of obstructive respiratory events and oxygen desaturations during REM sleep may be particularly profound.
It could be argued that the high frequency of SDB we found represents a marker of GH disease, rather than a contributing factor. Gestational hypertension is a complex disorder that is incompletely understood, but current thinking is that vascular endothelial damage underpins the hypertension and end organ damage, and we are not proposing that SDB is the sole cause of GH or even that SDB always results in GH. Just as is seen with non-pregnant adults, pregnant women can have SDB and not develop hypertension15,27; we found this to be the case in 12% of our healthy subjects. However, SDB is a known cause of hypertension in non-pregnant adults,8,43–46 and it seems plausible that the same physiologic interaction can occur during pregnancy. We believe that the interaction between GH and SDB may be bidirectional: along with obesity, upper extremity and facial edema are common features of GH, and GH-mediated oropharyngeal edema may exacerbate a predisposition towards SDB; the development of SDB could then further aggravate the underlying microvascular inflammation of GH.15,18,21,47 However, our study was designed only to assess for frequency of SDB in GH and did not evaluate for a causal relationship.
The strength of our study is the rigorous design and screening process. This is largest study of SDB in GH versus healthy pregnant women to be done using the gold standard test—laboratory-based PSG. We believe that our study population is a fair representation of the larger group of women with GH. The incidence of GH at our hospital during the study period was 5% of all deliveries. This is at the low end of the reported range for GH and probably reflects strict adherence to the diagnostic criteria by obstetricians and family practitioners at our institution.
CONCLUSION
Snoring, nasal congestion, and poor quality sleep are very common in unselected women with GH. More importantly, more than 50% of women with GH had significant SDB, as opposed just 12% of women with uncomplicated pregnancies of the same gestational age. As SDB can cause physiologic stress on the mother and fetus,15,18,21,48 an evaluation for co-existent SDB should be considered in pregnant women diagnosed with GH.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Maryla Stiles, Lori Reid, Joe Mink, Hitesh Bhatt and Dr. Hyun Lim; as well as the staff in the Royal University Hospital Sleep Lab and Antepartum Ward for their assistance. The authors also acknowledge the Saskatoon Health Region for their support. All research was conducted at the Royal University Hospital Sleep Disorders Centre, University of Saskatchewan. This project was jointly funded by the Saskatchewan Health Research Foundation, The Lung Association of Saskatchewan and the University of Saskatchewan. The funding agencies had no role in the acquisition, storage or interpretation of the data. Dr. Reid had full access to all the data in the study and takes full responsibility for the integrity of the data and accuracy of the analysis.
REFERENCES
- 1.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–92. doi: 10.1016/s0029-7844(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM, Gordon T, Thom E, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995;172:642–8. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 3.Acien P, Lloret G, Lloret M. Perinatal morbidity and mortality in pregnancy hypertensive disorders: prognostic value of the clinical and laboratory findings. Int J Gynaecol Obstet. 1990;32:229–35. doi: 10.1016/0020-7292(90)90350-t. [DOI] [PubMed] [Google Scholar]
- 4.Barton JR, O'brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184:979–83. doi: 10.1067/mob.2001.112905. [DOI] [PubMed] [Google Scholar]
- 5.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002;287:3183–6. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- 6.Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105:1177–84. doi: 10.1111/j.1471-0528.1998.tb09971.x. [DOI] [PubMed] [Google Scholar]
- 7.Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–22. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 8.Canadian Hypertension Education Program. The 2008 Canadian Hypertension Education Program recommendations: the scientific summary -- an annual update. Can J Cardiol. 2008;24:447–52. doi: 10.1016/s0828-282x(08)70618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhimaraj A, Havaligi N, Ramachandran S. Rapid reduction of antihypertensive medications and insulin requirements after tracheostomy in a patient with severe obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:297–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsignore MR, Parati G, Insalaco G, et al. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:279–86. doi: 10.1164/rccm.2107117. [DOI] [PubMed] [Google Scholar]
- 11.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117:137–41. doi: 10.1378/chest.117.1.137. [DOI] [PubMed] [Google Scholar]
- 12.Calaora-Tournadre D, Ragot S, Meurice JC, et al. Obstructive Sleep Apnea Syndrom during pregnancy: prevalence of main symptoms and relationship with Pregnancy Induced-Hypertension and Intra-Uterine Growth Retardation. Rev Med Interne. 2006;27:291–5. doi: 10.1016/j.revmed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Bourne T, Ogilvy AJ, Vickers R, Williamson K. Nocturnal hypoxaemia in late pregnancy. Br J Anaesth. 1995;75:678–82. doi: 10.1093/bja/75.6.678. [DOI] [PubMed] [Google Scholar]
- 14.Charbonneau M, Falcone T, Cosio MG, Levy RD. Obstructive sleep apnea during pregnancy. Therapy and implications for fetal health. Am Rev Respir Dis. 1991;144:461–3. doi: 10.1164/ajrccm/144.2.461. [DOI] [PubMed] [Google Scholar]
- 15.Edwards N, Blyton DM, Kirjavainen TT, Sullivan CE. Hemodynamic responses to obstructive respiratory events during sleep are augmented in women with preeclampsia. Am J Hypertens. 2001;14:1090–5. doi: 10.1016/s0895-7061(01)02190-2. [DOI] [PubMed] [Google Scholar]
- 16.Sahin FK, Koken G, Cosar E, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet. 2008;100:141–6. doi: 10.1016/j.ijgo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Joel-Cohen SJ, Schoenfeld A. Fetal response to periodic sleep apnea: a new syndrome in obstetrics. Eur J Obstet Gynecol Reprod Biol. 1978;8:77–81. doi: 10.1016/0028-2243(78)90131-4. [DOI] [PubMed] [Google Scholar]
- 18.Jerath R, Barnes VA, Fadel HE. Mechanism of development of pre-eclampsia linking breathing disorders to endothelial dysfunction. Med Hypotheses. 2009;73:163–6. doi: 10.1016/j.mehy.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Edwards N, Middleton PG, Blyton DM, Sullivan CE. Sleep disordered breathing and pregnancy. Thorax. 2002;57:555–8. doi: 10.1136/thorax.57.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Chada D, Videla AJ, O'Flaherty ME, et al. Snoring, witnessed sleep apnoeas and pregnancy-induced hypertension. Acta Obstet Gynecol Scand. 2007;86:788–92. doi: 10.1080/00016340701281919. [DOI] [PubMed] [Google Scholar]
- 21.Yinon D, Lowenstein L, Suraya S, et al. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006;27:328–33. doi: 10.1183/09031936.06.00010905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ursavas A, Karadag M, Nalci N, Ercan I, Gozu RO. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration. 2008;76:33–9. doi: 10.1159/000107735. [DOI] [PubMed] [Google Scholar]
- 23.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–7. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 24.Connolly G, Razak AR, Hayanga A, Russell A, McKenna P, McNicholas WT. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J. 2001;18:672–6. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 25.Yin TT, Williams N, Burton C, et al. Hypertension, fetal growth restriction and obstructive sleep apnoea in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008 doi: 10.1016/j.ejogrb.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Guilleminault C, Querra-Salva M, Chowdhuri S, Poyares D. Normal pregnancy, daytime sleeping, snoring and blood pressure. Sleep Med. 2000;1:289–97. doi: 10.1016/s1389-9457(00)00046-0. [DOI] [PubMed] [Google Scholar]
- 27.Champagne K, Schwartzman K, Opatrny L, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33:559–65. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 28.Helewa ME, Burrows RF, Smith J, Williams K, Brain P, Rabkin SW. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ. 1997;157:715–25. [PMC free article] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 32.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: The American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 33.Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27:79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 35.Kambam JR, Handte RE, Brown WU, Smith BE. Effect of normal and preeclamptic pregnancies on the oxyhemoglobin dissociation curve. Anesthesiology. 1986;65:426–7. doi: 10.1097/00000542-198610000-00014. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–74. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Getahun D, Ananth CV, Oyelese Y, Chavez MR, Kirby RS, Smulian JC. Primary preeclampsia in the second pregnancy: effects of changes in prepregnancy body mass index between pregnancies. Obstet Gynecol. 2007;110:1319–25. doi: 10.1097/01.AOG.0000292090.40351.30. [DOI] [PubMed] [Google Scholar]
- 38.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840–51. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 39.Hoffstein V, Mateika S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J. 1992;5:377–81. [PubMed] [Google Scholar]
- 40.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 41.Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. [Google Scholar]
- 42.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–61. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endeshaw YW, Bloom HL, Bliwise DL. Sleep-disordered breathing and cardiovascular disease in the Bay Area Sleep Cohort. Sleep. 2008;31:563–8. doi: 10.1093/sleep/31.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grote L, Hedner J, Peter JH. Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J Hypertens. 2000;18:679–85. doi: 10.1097/00004872-200018060-00004. [DOI] [PubMed] [Google Scholar]
- 45.Khan NA, Hemmelgarn B, Herman RJ, et al. The 2008 Canadian Hypertension Education Program recommendations for the management of hypertension: part 2 - therapy. Can J Cardiol. 2008;24:465–75. doi: 10.1016/s0828-282x(08)70620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padwal RJ, Hemmelgarn BR, Khan NA, et al. The 2008 Canadian Hypertension Education Program recommendations for the management of hypertension: Part 1 - blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2008;24:455–63. doi: 10.1016/s0828-282x(08)70619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens. 2001;14:178S–85S. doi: 10.1016/s0895-7061(01)02086-6. [DOI] [PubMed] [Google Scholar]
- 48.Edwards N, Blyton DM, Hennessy A, Sullivan CE. Severity of sleep-disordered breathing improves following parturition. Sleep. 2005;28:737–41. doi: 10.1093/sleep/28.6.737. [DOI] [PubMed] [Google Scholar]