Abstract
Study Objectives:
Pulse transit time (PPT) has been introduced as a useful screening tool to diagnose sleep disordered breathing (SDB). Since the prevalence of SDB increases with age, the question is whether PTT could be used to diagnose SDB in the elderly. We assess the effectiveness of PTT for SDB screening in a large healthy elderly population.
Setting:
Community-based sample in home and research clinical settings.
Intervention:
N/A.
Participants:
Seven hundred eighty volunteers, free of cardiac and neurologic disease, aged 68.6 ± 1.0 years, underwent ambulatory polygraphy to measure the apnea-hypopnea index (AHI). The presence of SDB was defined as an AHI of 15 or greater. The PTT was continuously monitored during the nocturnal study, and the overall autonomic arousal index (AAI) was calculated.
Results:
SDB was diagnosed in 447 (57.3%) subjects. In these subjects, the Bland-Altman plot for AAI revealed an underestimation with a bias of −8.04 ± 16.55 events per hour (mean ± 95% confidence interval). Receiver operating characteristic curves constructed for an AHI of 15 or greater defined an area under the curve of 0.67 and a cutoff point to AAI 32.3 events per hour, giving a sensitivity of 70.5% and a specificity of 54.7%. For prediction of an AHI of at least 30, the area under the curve was equal to 0.74 for a cutoff point of 56.3 events per hour, giving a better specificity (94.7%) but a lower sensitivity (32.2%).
Conclusions:
In a healthy older population, the AAI showed moderate sensitivity for predicting SDB. This data does not allow us to use PTT as a screening tool for the diagnosis of SDB in the elderly.
Clinical Trial Registration:
NCT 00759304 and NCT 00766584.
Citation:
Chouchou F; Sforza E; Celle S; Pichot V; Maudoux D; Garcin A; Barthélémy JC; Roche F. Pulse transit time in screening sleep disordered breathing in an elderly population: the PROOF-SYNAPSE study. SLEEP 2011;34(8):1051-1059.
Keywords: Sleep-disordered breathing, screening, autonomic function, arousal, aging
INTRODUCTION
It is well known that the occurrence of apneas and hypopneas is associated with cyclic fluctuations of the autonomic nervous system,1,2 with parasympathetic activity increasing during apneas and sympathetic activation occurring at the end of apnea, in relation to arousal occurrence.2 Many studies have been conducted to verify the ability of the autonomic correlates of apnea, ie, heart-rate variability,3,4 changes in blood pressure,5 peripheral arterial tone,6 and pulse-wave amplitude7 to detect sleep disordered breathing (SDB) in a clinical setting. Among autonomic markers, pulse transit time (PTT)8–11 has been used for SDB screening and follow-up. The PTT is the time taken for the pulse wave to travel from the aortic valve to the pulse waveform detected by a plethysmographic finger probe. Thus, sympathetic activation increases blood pressure and vascular tone and stiffens the arterial wall, causing the PTT to shorten.12 The advantages of this method are that it is less invasive, more practical, and cheaper than currently used tests, ie, polysomnography, and is easier to use in an ambulatory setting. Studies conducted on PTT in a middle-aged population8–11 and children13–15 have demonstrated its good specificity (91%) and sensitivity (95%).
The prevalence of SDB is estimated to be 9% to 14% in middle-aged men and from 2% to 7% in middle-aged women16–20; the prevalence increases with age (> 65 y), with an estimated percentage between 32% and 49% in women and 42% and 57% in men.18,21,22 Therefore, the use of screening tools in an ambulatory setting may be valuable in the elderly to prompt further definitive evaluation of SDB and to prevent the associated cardiovascular risk.
To our knowledge, no clinical or epidemiologic studies have evaluated the use of PTT for SDB screening in the elderly. The principal aim of this study was therefore to determine the effectiveness of PTT for SDB screening in a large elderly population. In addition, we attempted to define which factors related to clinical characteristics or sleep quality may affect the reliability of PTT as a diagnostic tool.
METHODS AND MATERIALS
Population
The population under study was recruited from the PROOF study (PROOF: PROgnostic indicator OF cardiovascular and cerebrovascular events), which is an observational prospective cohort study aimed at analyzing the predictive value of autonomic nervous system activity for severe cardiovascular and cerebrovascular events. Volunteers were recruited in 2001 from the electoral list of the city of Saint-Etienne, France, according to their age (65 years), and several exclusion criteria concerning cardiovascular and general morbidity were applied.23 Criteria for inclusion in the study were (1) absence of diseases not allowing analysis of heart-rate variability (ie, permanent or paroxysmal atrial fibrillation, Shy-Drager syndrome, polyneuropathy, permanent ventricular or atrial pacing), (2) no use of antiarrhythmic drugs or digitalis, and (3) willingness to have polygraphy performed and 24-h blood pressure monitored. An ancillary study addressing the association between SDB, assessed by at-home polygraphic study, and cardiovascular and cerebrovascular morbidity during a 7-year follow-up was proposed to participants (SYNAPSE study: SYsteme Nerveux Autonome - Physiologie - Sommeil - Epidemiologie). The data used in this report were those gathered on the second examination of the cohort (from January 2003 to December 2004). All volunteers enrolled in the PROOF study were eligible for participation in the SYNAPSE study, except those with diagnosed SDB (n = 5).
From the original PROOF sample (1011 subjects), 854 volunteers free of previously diagnosed SDB were enrolled. Demographic characteristics—including sex, age, smoking status (past or current), body mass index, neck circumference, diabetes status, alcohol intake, and blood pressure measurements—were obtained for all subjects. All participants filled in the Epworth Sleepiness Scale (ESS)24 to assess daytime sleepiness. The Saint Mary's hospital questionnaire25–26 was used to subjectively evaluate the subjects' sleep quality and duration during the nocturnal study.
Both the PROOF and SYNAPSE studies were approved by the ethics committee (CCPPRB, Rhône-Alpes Loire) and sponsored by Saint-Etienne University Hospital (DRCI, CHU), France. The study was conducted in accordance with the Declaration of Helsinki, and all participants gave their written informed consent before the study began.
Polygraphic Study
Eight hundred and fifty-four subjects enrolled in the PROOF study underwent full-night recording using an ambulatory polygraphic device (HypnoPTT, Tyco Healthcare, Puritan Bennett, Pleasonton, CA). As part of the in-hospital clinical examination, an experienced technician instructed each participant how to place the sensors, and the recordings were then made that night. The polygraphy system recorded electrocardiographic tracings (1 lead), pulse oximetry, rib cage movements (transthoracic impedance), body position, nasal pressure, PTT, and R-R timing, following the American Academy of Sleep Medicine criteria.27 A second night of monitoring was performed when reported sleep latency exceeded 2 hours on the first night (n = 10) and when 1 respiratory parameter was missing (n = 45). A recording duration of at least 5 hours was required to validate the sleep study. Finally, 847 subjects had acceptable polygraphic recording.
All of the recordings were manually scored for apnea-hypopnea events by blinded investigators, according to standard criteria. Hypopnea was defined as a 50% or greater reduction in airflow from the baseline value, lasting at least 10 second and associated with at least a 3% oxygen desaturation. Apnea was defined as the absence of airflow on the nasal cannula lasting more than 10 seconds. The absence of rib cage movements associated with an apnea defined the event as central, whereas a progressive increase in PTT and thoracic movements defined the event as obstructive. The apnea-hypopnea index (AHI) was established as the ratio of the number of apneas and hypopneas per hour of recording. The recording time was defined by the start of time in bed until the end of time in bed, with subjects switching on the polygraph when they went to bed and switching it off when they woke up. The indexes of nocturnal hypoxemia used were the following: mean Sao2, the percentage of recording time in which the subjects Sao2 was below 90%, the minimum Sao2, and the oxygen desaturation index (ODI: the number of episodes of oxygen desaturation per hour of recording time during which blood oxygen fell by 3% or more). According to previously reported data obtained from elderly subjects, an AHI of at least 15 was considered as diagnostic of SDB.28–31 The cases were defined as mild (AHI ≥ 15) or moderate to severe (AHI ≥ 30).
PTT Measurement
The PTT was calculated as the time interval between the electrocardiographic R wave and a point on the pulse waveform (detected by a plethysmographic finger probe) that was 50% of the height of ascent of the pulse wave. The electrocardiogram and pulse were sampled at 500 Hz. PTT is typically about 250 milliseconds and is measured to an accuracy of 2 milliseconds.32 PTT values available with every heart beat were oversampled at 5 Hz. The PTT was continuously monitored and an autonomic activation index (AAI) was obtained from the PTT signal and was broken down into total, respiratory, and nonrespiratory autonomic activations. The scoring of autonomic activations was obtained using the manufacturer's analysis software.
Statistical Analysis
Data were analyzed using Statview (SAS Institute, Inc®, Cary, NC) and Sigmaplot (Systat Software, Inc®, Chicago, IL) software. Data are presented as mean ± SD. Significance was taken to be a P value of greater than 0.05.
The clinical and polygraphic characteristics of subjects without SDB (AHI < 5 and 5 ≤ AHI < 15), as well as those with mild (15 ≤ AHI < 30) and moderate to severe (AHI ≥ 30) SDB were compared using the Kruskall-Wallis test. The associations between AHI and AAI were explored by multivariate regression analysis for continuous variables. Measurements of sensitivity, specificity, positive predictive value, negative predictive value, Bland-Altman analysis plot,33 and the receiver operating characteristic curves34 were constructed for the AAI to determine the cutoff threshold values having the best sensitivity and specificity35 to minimize false positive and negative.
RESULTS
Analyses were performed on recordings from 780 of the 847 subjects who all had acceptable recordings. PPT data were not available for 67 of the 847 subjects because of too many artifacts or ectopic beats on electrocardiogram. These 780 subjects were aged 68.6 ± 1.0 years, 448 being women (57.4%) and 332 men (42.6%). Comparison of demographic characteristics of included and excluded subjects at the time of the first examination (Table 1) showed that clinical data of the 780 included volunteers did not differ from the overall PROOF sample, allowing exclusion of a selection bias and, therefore, generalization to the overall PROOF sample.
Table 1.
Clinical and anthropometric data for excluded and included subjects in the analysis of the PROOF-SYNAPSE studies
| Excluded volunteers in our analysis (± SD)a | Included volunteers in our analysis (± SD)a | Pb | |
|---|---|---|---|
| n | 231 | 780 | |
| Age (years) | 65.6 ± 0.8 | 65.8 ± 1.1 | 0.17 |
| Body mass index (kg/m2) | 25.0 ± 3.7 | 25.4 ± 4.0 | 0.26 |
| Epworth (/24) | 5.5 ± 3.6 | 5.5 ± 3.4 | 0.86 |
| Systolic blood pressure (mmHg) | 142.5 ± 18.7 | 143.4 ± 18.4 | 0.53 |
| Diastolic blood pressure (mmHg) | 84.7 ± 7.4 | 86.0 ± 8.5 | 0.05 |
| Alcohol intake (drink per day) | 1.6 ± 2.2 | 1.9 ± 2.2 | 0.08 |
| Women (%) | 67.8 | 57.4 | NS |
| Hypertension (%) | 27.1 | 36.7 | NS |
| Smoker (past or current (%)) | 23.7 | 25.8 | NS |
The original PROOF study included 1011 subjects. Of these, 847 with no previous diagnosis of sleep disordered breathing and acceptable polygraphic recording were enrolled in the current study; PPT data were not available for 67 subjects because of too many artifacts or ectopic beats on electrocardiogram.
P values were derived using the Mann-Whitney test.
SDB, defined as an AHI of at least 15, was diagnosed in 447 subjects (57.3%), 223 women (49.9%) and 224 men (50.1%). Among the SDB group, 270 subjects (34.6%) had an AHI of at least 15 but less than 30, and 177 (22.7%) had an AHI of at least 30. As expected, compared with subjects without SDB, participants with SDB were more likely to be overweight and had larger neck circumferences. Their clinical and anthropometric data are reported in Table 2, and polysomnographic data are in Table 3.
Table 2.
Clinical and anthropometric data for subjects with and without sleep disordered breathing according to apnea-hypopnea index severity
| No. | AHI < 5 |
5 ≤ AHI < 15 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70 | 263 | |||||||||||||
| Mean | SD | Median | Min | Max | IRQ | % | Mean | SD | Median | Min | Max | IRQ | % | |
| Age, y | 68.6 | 1.3 | 68.0 | 62.5 | 71.0 | 1.8 | 68.4 | 1.1 | 68.0 | 65.0 | 72.0 | 1.0 | ||
| BMI, kg/m2 | 24.1 | 3.4 | 23.6 | 18.6 | 34.2 | 4.6 | 24.7 | 3.4 | 24.3 | 16.6 | 36.7 | 4.0 | ||
| Neck circumference, cm | 35.0 | 3.3 | 34.0 | 29.0 | 43.0 | 4.0 | 36.2 | 3.8 | 35.0 | 29.0 | 54.0 | 6.0 | ||
| ESS score | 4.4 | 3.4 | 3.0 | 0.0 | 16.0 | 4.0 | 5.2 | 3.3 | 5.0 | 0.0 | 18.0 | 4.0 | ||
| Blood pressure, mm Hg | ||||||||||||||
| Systolic | 138.3 | 16.2 | 138.0 | 104.0 | 198.0 | 24.3 | 138.2 | 17.3 | 135.0 | 98.0 | 206.0 | 21.0 | ||
| Diastolic | 84.7 | 7.7 | 84.0 | 65.0 | 108.0 | 10.0 | 85.4 | 8.8 | 84.5 | 65.0 | 110.0 | 10.0 | ||
| Alcohol intake, drink/d | 1.2 | 1.8 | 0.0 | 0.0 | 6.0 | 3.0 | 1.5 | 2.0 | 0.0 | 0.0 | 10.0 | 3.0 | ||
| Women | 78.6 | 66.2 | ||||||||||||
| Hypertension | 30.0 | 38.4 | ||||||||||||
| Past or current smoking | 12.9 | 27.4 | ||||||||||||
| Diabetes | 4.3 | 4.2 | ||||||||||||
| No. | 15 ≤ AHI < 30 |
AHI ≥ 30 |
P valuea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 270 | 177 | ||||||||||||||
| Mean | SD | Median | Min | Max | IRQ | % | Mean | SD | Median | Min | Max | IRQ | % | ||
| Age, y | 68.6 | 1.0 | 69.0 | 65.0 | 71.0 | 1.0 | 68.6 | 1.1 | 69.0 | 65.0 | 72.0 | 1.0 | NS | ||
| BMI, kg/m2 | 25.7 | 4.0 | 25.3 | 15.0 | 42.8 | 4.8 | 26.9 | 3.6 | 26.5 | 20.3 | 37.3 | 4.8 | < 0.0001 | ||
| Neck circumference, cm | 37.3 | 3.9 | 37.0 | 29.0 | 51.0 | 6.0 | 39.6 | 3.6 | 40.0 | 32.0 | 47.0 | 5.0 | < 0.0001 | ||
| ESS score | 6.0 | 3.7 | 5.0 | 0.0 | 19.0 | 6.0 | 6.6 | 3.8 | 6.0 | 0.0 | 20.0 | 6.0 | < 0.0001 | ||
| Blood pressure, mm Hg | |||||||||||||||
| Systolic | 140.5 | 17.7 | 140.0 | 103.0 | 191.0 | 20.0 | 139.0 | 14.6 | 140.0 | 106.0 | 175.0 | 24.0 | < 0.0001 | ||
| Diastolic | 86.9 | 8.4 | 87.0 | 64.0 | 126.0 | 10.25 | 86.4 | 8.3 | 86.5 | 65.0 | 110.0 | 10.0 | 0.0551 | ||
| Alcohol intake, drink/d | 2.0 | 2.3 | 0.0 | 0.0 | 10.0 | 4.0 | 2.3 | 2.4 | 3.0 | 0.0 | 10.0 | 4.0 | 0.0001 | ||
| Women | 57.0 | 40.0 | |||||||||||||
| Hypertension | 45.2 | 54.0 | |||||||||||||
| Past or current smoking | 27.0 | 26.9 | |||||||||||||
| Diabetes | 4.4 | 9.7 | |||||||||||||
AHI, apnea-hypopnea index; SD, standard deviation; Min, minimum; Max, maximum; IRQ, interquartile range; BMI, body mass index; ESS, Epworth Sleepiness Scale (maximum score of 24).
P values were derived using the Kruskall-Wallis test.
Table 3.
Polygraphic data for subjects with and without sleep disordered breathing, stratified according to apnea-hypopnea index severity
| No. | AHI < 5 |
5 ≤ AHI < 15 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70 | 263 | |||||||||||
| Mean | SD | Median | Min | Max | IRQ | Mean | SD | Median | Min | Max | IRQ | |
| AHI | ||||||||||||
| Total | 3.6 | 1.3 | 3.7 | 0.8 | 4.9 | 1.9 | 10.8 | 3.4 | 10.8 | 5.0 | 14.9 | 6.1 |
| Obstructive | 3.3 | 1.1 | 3.4 | 0.8 | 5.0 | 1.6 | 9.9 | 3.1 | 9.8 | 5.0 | 15.0 | 5.7 |
| Central | 0.3 | 0.4 | 0.1 | 0.0 | 1.6 | 0.4 | 0.9 | 1,0 | 0.5 | 0.0 | 6.2 | 1.1 |
| ODI | 2.2 | 3.3 | 1.4 | 0.0 | 22.9 | 1.5 | 4.3 | 3.3 | 3.6 | 0.0 | 22.3 | 4.2 |
| Min Sao2 | 92.1 | 2.7 | 93.0 | 81.0 | 96.0 | 2.0 | 91.1 | 2.8 | 91.0 | 74.0 | 96.0 | 3.0 |
| Sao2< 90% | 1.6 | 6.0 | 0.0 | 0.0 | 32.0 | 0.0 | 1,0 | 5.4 | 0.0 | 0.0 | 60.0 | 0.2 |
| PMD | 472.2 | 59.8 | 476.5 | 313.0 | 608.0 | 81.0 | 473.8 | 63.6 | 483.0 | 302.0 | 711.0 | 75.8 |
| AAI | ||||||||||||
| Total | 27.5 | 14.9 | 25.5 | 0.0 | 65.7 | 16.6 | 32.8 | 14.2 | 31.7 | 2.8 | 86.0 | 19.5 |
| Respiratory related | 5.6 | 4.3 | 4.6 | 0.0 | 27.2 | 3.7 | 9.2 | 4.7 | 8.3 | 0.0 | 64.3 | 6.7 |
| Nonrespiratory related | 21.9 | 13.3 | 20.9 | 0.0 | 55.8 | 21.3 | 23.5 | 12.2 | 22.5 | 1,0 | 74.0 | 16.6 |
| No. | 15 ≤ AHI < 30 |
AH I ≥ 30 |
P valuea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 270 | 177 | ||||||||||||
| Mean | SD | Median | Min | Max | IRQ | Mean | SD | Median | Min | Max | IRQ | ||
| AHI | |||||||||||||
| Total | 23.6 | 15.1 | 22.5 | 15.0 | 29.9 | 8.4 | 48.6 | 15.1 | 44.4 | 30.7 | 108.6 | 19.3 | |
| Obstructive | 21.8 | 4.4 | 21.4 | 15.1 | 30.0 | 8.0 | 43.1 | 11.7 | 39.9 | 30.1 | 83.3 | 15.1 | < 0.0001 |
| Central | 1.8 | 2.1 | 1.2 | 0.0 | 12.3 | 2.0 | 5.5 | 6.6 | 3.1 | 0.0 | 37.8 | 6.5 | < 0.0001 |
| ODI | 9.3 | 5.8 | 8.4 | 0.0 | 26.8 | 8.2 | 21.0 | 11.8 | 19.2 | 11.8 | 56.7 | 16.0 | < 0.0001 |
| Min Sao2 | 89.0 | 4.3 | 90.0 | 61.0 | 95.0 | 5.0 | 87.4 | 4.8 | 88.0 | 61.0 | 95.0 | 5.3 | < 0.0001 |
| Sao2< 90% | 2.3 | 7.7 | 0.1 | 0.0 | 100.0 | 1.0 | 3.5 | 6.7 | 1.0 | 0.0 | 45.0 | 3.6 | < 0.0001 |
| PMD | 459.8 | 75.7 | 464.5 | 301.0 | 643.0 | 89.0 | 451.6 | 78.4 | 462.0 | 300.0 | 633.0 | 85.5 | 0.0242 |
| AAI | |||||||||||||
| Total | 36.6 | 14.7 | 36.2 | 4.1 | 94.0 | 19.5 | 48.3 | 16.2 | 46.7 | 16.6 | 98.4 | 23.2 | < 0.0001 |
| Respiratory related | 15.4 | 6.4 | 15.1 | 1.3 | 39.5 | 8.5 | 28.6 | 11.2 | 26.4 | 4.3 | 69.2 | 13.7 | < 0.0001 |
| Nonrespiratory related | 21.2 | 11.1 | 19.9 | 1.3 | 66,0 | 15.8 | 19.6 | 9.8 | 17.6 | 4.5 | 58.7 | 14.1 | 0.0092 |
AHI, apnea-hypopnea index; ODI, oxygen desaturation index; Minimum SaO2, minimum recorded oxygen saturation; SaO2 < 90%, percentage of recording time spent with an oxygen saturation less than 90%; PMD, polygraphic monitoring duration; AAI, autonomic arousal index.
P values were derived using the Kruskall-Wallis test.
To evaluate sleep quality and duration according to SDB severity, the details of the 8 items of the subjective sleep estimation derived from the Saint Mary's Hospital Questionnaire were examined (Table 4). Overall, the questionnaire showed that subjects estimated their sleep quality and duration as relatively good and indicated that the subjects were not overly bothered by undergoing the polygram. No difference in sleep quality, sleep satisfaction, or sleep depth was found between groups. The estimated nocturnal sleep duration was shorter in subjects with mild SDB, who more often reported feeling tired when they finally awoke. Comparison between groups showed that, during the daytime, subjects with SDB tended to sleep longer, compared with subjects without SDB.
Table 4.
Subjective estimation of sleep quality and sleep duration using the St. Mary's Sleep Questionnaire, stratified according to severity of sleep disordered breathing
| Parameter | AHI < 5 | 5 ≤ AHI < 15 | 15 ≤ AHI < 30 | AHI ≥ 30 | P valuea |
|---|---|---|---|---|---|
| Sleep onset, h min | 22h06 to 5h12 | 21h18 to 6h06 | 21h18 to 5h56 | 21h18 to 6h00 | NS |
| Sleep depthb | 4.7 ± 1.7 | 4.7 ± 1.6 | 4.7 ± 1.7 | 4.9 ± 1.6 | NS |
| Estimated sleep duration, h | |||||
| Nocturnal | 6.9 ± 1.4 | 7.0 ± 1.8 | 6.6 ± 1.7 | 7.0 ± 1.6 | 0.04 |
| Diurnal | 0.2 ± 0.7 | 0.1 ± 0.2 | 0.2 ± 0.7 | 0.3 ± 0.7 | 0.0006 |
| Wake sensationc | 4.1 ± 1.0 | 4.1 ± 1.0 | 4.0 ± 1.0 | 4.2 ± 0.9 | 0.03 |
| Sleep qualityc | 4.1 ± 1.0 | 4.1 ± 1.0 | 4.0 ± 1.2 | 4.2 ± 1.1 | NS |
| Satisfied sleepd | 3.5 ± 1.3 | 3.5 ± 1.2 | 3.5 ± 1.3 | 3.6 ± 1.2 | NS |
| Sleep latency, h | 0.5 ± 0.5 | 0.6 ± 1.4 | 0.7 ± 2.2 | 0.5 ± 0.6 | NS |
Data are presented as mean ± SD.
P values were derived using the Kruskall-Wallis test.
Maximum score of 8.
Maximum score of 6.
Maximum score of 5.
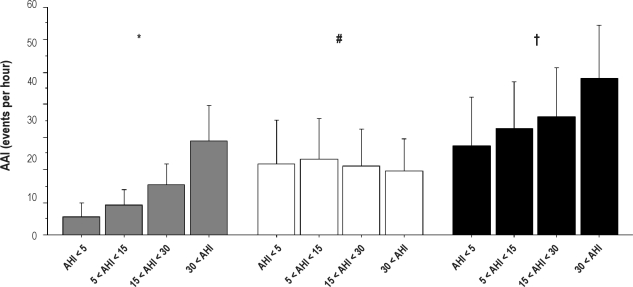
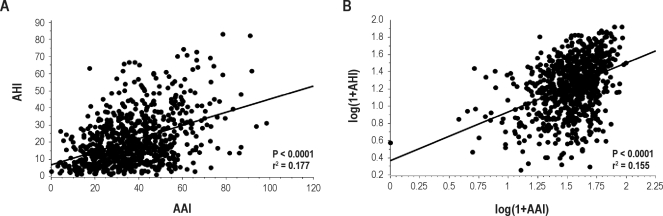
Concerning the AAI, there was a progressive rise in both overall (P < 0.0001) and respiratory (P < 0.0001) autonomic activations according to SDB severity, whereas spontaneous (P < 0.01) AAI decreased (Table 3 and Figure 4). The relationships among overall AAI and AHI and all clinical, anthropometric, and polygraphic data (Tables 1 and 2), as well as subjective sleep duration and quality (Table 4), were tested in a multivariate regression for continuous variables. In this analysis, AHI (t = 9.321, P < 0.0001) was significantly related to overall AAI. However, despite significant relationship between overall AAI and AHI (P < 0.0001), the correlation was low (r2 = 0.18) (Figure 1A). Moreover, logarithmic transformation did not improve the scatter of the relationship between AAI and AHI (Figure 1B) and the correlation coefficient remained similar with logarithmic values (correlation coefficient after transformation: r2 = 0.16).
Figure 4.
Overall (black chart), respiratory (gray chart), and spontaneous (white chart) autonomic arousal index (AAI) according to the apnea-hypopnea index (AHI) group (mean ± SD). The Kruskall-Wallis test showed differences for overall, respiratory and spontaneous AAI according to AHI. *, respiratory AAI (P < 0.0001); #, spontaneous AAI (P = 0.0092); , overall AAI (P < 0.0001).
Figure 1.
Scatter plot of (A) the respiratory autonomic arousal index (AAI) and the apnea-hypopnea index (AHI) and (B) the logarithmic transformation of AAI and AHI.
To assess whether the presence of diabetes or hypertension would affect this relationship, the analysis was repeated in subjects having diabetes or hypertension. The relationship between AAI and AHI was preserved in both hypertensive (n = 239, r2 = 0.22, P < 0.0001) and diabetic (n = 43, r2 = 0.13, P < 0.012) subjects.
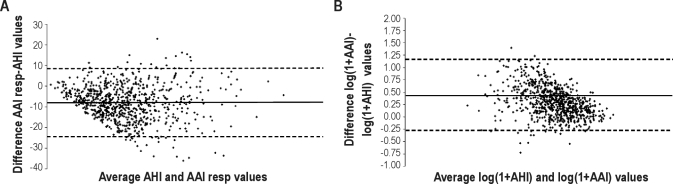
Using overall AAI and SDB screening, the Bland-Altman plot (Figure 2A) revealed systematic differences between AHI and overall AAI, with a mean of −8.04 ± 16.55 events per hour (mean ± 95% confidence interval [CI]), indicating a tendency of the AAI to underestimate the frequency of respiratory disorders (Figure 2B). An underestimation of AHI by AAI was present even when logarithmic values were used. In addition, the analysis of the Bland-Altman plot with logarithmic values revealed that the variance did not increase as a function of the mean. Moreover, a negative correlation appeared between the difference and the mean of the AAI and AHI after logarithmic transformation (raw values: r2 = 0.01, P = 0.007, logarithmic values: r2 = 0.17, P < 0.001).
Figure 2.
Bland-Altman plot of (A) the autonomic arousal index (AAI) and ambulatory polygraphy-derived apnea-hypopnea index (AHI) and (B) the logarithmic transformation of AAI and AHI. The solid line represents the mean bias between AAI and AHI, and the dotted line represents the 95% confidence interval.
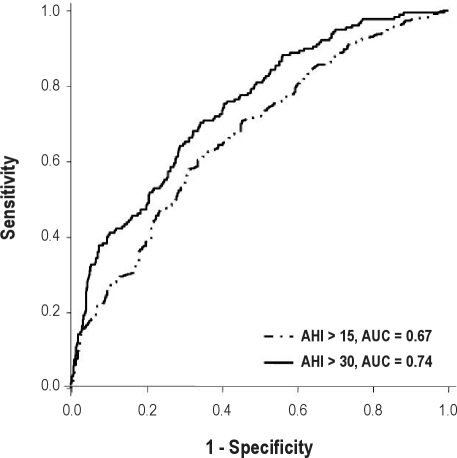
Similar results were obtained with receiver operating characteristic curves (Figure 3), confirming the low ability of AAI to discriminate SDB cases. For subjects with an AHI of at least 15, the area under the curve was 0.67 (standard error: 0.02, 95% CI: 0.62-0.71, P < 0.0001) and 0.74 for an AHI of at least 30 (standard error: 0.02, 95% CI: 0.70-0.78, P < 0.0001).
Figure 3.
Receiver operating characteristic curve for the autonomic arousal index (AAI) against the ambulatory polygraphy-derived apnea-hypopnea index (AHI). The area under the curve (AUC) indicates moderate accuracy of the pulse transit time in the diagnosis of mild and moderate to severe cases of sleep disordered breathing.
Analysis of the receiver operating characteristic curves allowed us to determinate the cutoff points for an AHI of at least 15 and for an AHI of at least 30 with the best sensitivity and specificity. For an AHI of at least 15, the AAI cutoff was 32.3 events per hour, resulting in a moderate specificity (54.7%) but higher sensitivity (70.5%). For an AHI of at least 30, the AAI cutoff was 56.3 events per hour, yielding a higher specificity (94.7%) but low sensitivity (32.2%). Finally, positive and negative posttest probabilities (PTP) were moderate for the threshold of both an AHI of at least 15 (positive PTP: 67.6% and negative PTP: 35.1%) and for an AHI of at least 30 (positive PTP: 76.2% and negative PTP: 27.5%).
DISCUSSION
The aim of the present study was to evaluate the use of PTT as a SDB screening tool in a large elderly population. The first interesting finding was that PTT had low level of effectiveness, especially for a threshold of 15 respiratory events per hour and, therefore, cannot be used alone for SDB screening in elderly populations. Second, the lack of a good agreement between AAI and AHI was not explained by clinical and anthropometric factors, nor by subjective sleep duration and sleep quality, but, rather, by a high number of spontaneous autonomic activations affecting sleep in subjects with and without SDB. In the absence of factors that explain the low accuracy level of the AAI, we can hypothesize that a sleep instability is present in this elderly population. This phenomenon and its clinical consequences remain to be determined in future studies.
Previous studies conducted in middle-aged subjects8 and children14–15 have identified the possibility that the PTT can be used as a diagnostic tool for SDB screening, with a specificity of 91%, a sensitivity of 95%, and a negative predictive value of 95%. Despite the fact that the AAI was significantly different between subjects with and without SDB in our population, we found that the correlation between AAI and the overall AHI was low (r2 = 18, Figure 1). Moreover, the Bland-Altman analysis (Figure 2) indicated that the AAI had a low accuracy and understimated SBD severity. Finally, the reduced sensitivity (Table 5) identified with analysis of the receiver operating characteristic curve suggests that there are serious limitations to the use of PTT as a screening tool and that it should not be used alone to diagnose SDB in the elderly.
Table 5.
Sensitivity, specificity; positive and negative predictive value; true positive, false positive, true negative, false negative values; and negative and positive likelihood ratio of autonomic arousal index screening power based on the apnea-hypopnea index severity
| AHI ≥ 15 events/h |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sens (%) | Spe (%) | PPV (%) | NPV (%) | TP | FP | FN | TN | LR+ | LR- | |
| AAI threshold at 32.3 events/h | 70.47 | 54.65 | 67.60 | 57.96 | 315 | 151 | 183 | 182 | 1.55 | 1.29 |
| AHI ≥ 30 events/h |
||||||||||
| Sens (%) | Spe (%) | PPV (%) | NPV (%) | TP | FP | FN | TN | LR+ | LR- | |
| AAI threshold at 56.3 events/h | 32.20 | 94.69 | 64.04 | 82.63 | 57 | 32 | 120 | 571 | 6.06 | 0.72 |
Sens, sensitivity; spe, specificity; PPV positive predictive value; NPV, negative predictive value; TP, true positive; FP, false positive; TN, true negative; FN, false negative; LR− (negative) and LR+ (positive) likelihood ratio of autonomic arousal index (AAI) screening power. The cutoff values were determinated by receiver operating characteristic curves.
We attempted to define which factors may affect the reliability of PTT in an elderly population and found that overall AAI was not affected by clinical, polygraphic, or anthropometric factors, nor by subjective sleep duration or quality. The relationship with AHI was small, suggesting that the AAI is more reflective of the sleep instability related to age, in subjects both with and without SDB (Figure 4).
The low accuracy of the PTT as a screening tool in an elderly population may be explained by several factors related to the physiologic changes in sleep structure and cardiovascular activities that take place with aging. Firstly, physiologic changes in sleep quality and sleep continuity occur with aging,36 leading to an underestimation of wake time during sleep in absence of electroencephalographic control. Older people report having more fragmented sleep due to an increased number of spontaneous awakenings,37 a low arousal threshold, or the presence of other sleep disorders, such as periodic limb movement disorder.38 This was indirectly confirmed by our finding of a high spontaneous AAI in subjects with and without SDB and by the high level of false positives cases, indicating that autonomic activations occur during sleep independent of respiratory disorders. Secondly, as a consequence of the physiologic decrease in autonomic nervous system reactivity,39–40 the cardiovascular response to respiratory and arousing events is lower, compared with that of a middle-aged population, related to the physiologic downregulation of sympathetic activity. Finally, when we examined the frequency of respiratory events and the degree of nocturnal hypoxemia in our elderly subjects, we noted that, in general, they had lower levels of hypoxemia related to respiratory events, compared with a middle-aged population. Since hypoxemia contributes to oscillation in sympathetic and parasympathetic activity,41 less severe hypoxemia may lead to low vascular bed reactivity that affects the PTT values.
Some limitations of our study should be considered. Firstly, we assessed the effectiveness of PTT in subjects who did not undergo polysomnography; therefore, an underestimation of AHI could have occurred. Using polysomnography, we could have better estimated sleep and wake time and, therefore, increase the difference between PPT and AHI. However, we did not consider this as a major limitation considering that polygraphy is now used in epidemiologic studies.42 Secondly, the use of only manufacturer's criteria to define autonomic arousals may have induced a limitation. Algorithms based on an increase in PTT during obstructive events and a decrease during the end of the events could improve the effectiveness for the screening of obstructive sleep apnea. Thirdly, cutoff thresholds depend on the weight of false positive and negative cases, as well as on disease prevalence. Therefore, the results could vary in other populations and when using an unequal weight of the 2 types of errors. Finally, we validated PTT using healthy elderly volunteers who had few symptoms of SDB and a low oxygen desaturation index. Further studies will be necessary to replicate our results in a clinical setting.
In summary, the AAI as detected by PTT is unable, in an ambulatory setting, to establish the presence of SDB in a large, nonsymptomatic elderly population. The reduced sensitivity of PTT could be due to factors related to the physiologic changes in sleep quality and sleep continuity that occur in the elderly and affect sleep and the spontaneous AAI. Its clinical consequences remain to be determined in future studies.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by a grant from the French Ministry of Health (PHRC 1998 and PHRC 2002). The authors are indebted to the Cellule Projet Hospitalier de Recherche Clinique, Délégation de la Recherche Clinique et Innovation, CHU Saint-Etienne, and “L'Association de Recherche SYNAPSE” (Michel Segura, President).
ABBREVIATIONS
- AAI
autonomic arousal index
- AHI
Apnea-hypopnea index
- ESS
Epworth Sleepiness Scale
- SDB
Sleep disordered beathing
- PTT
pulse transit time
REFERENCES
- 1.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet. 1984;1:126–31. doi: 10.1016/s0140-6736(84)90062-x. [DOI] [PubMed] [Google Scholar]
- 2.Vanninen E, Tuunainen A, Kansanen M, Uusitupa M, Lansimies E. Cardiac sympathovagal balance during sleep apnoea episodes. Clin Physiol. 1996;16:209–16. doi: 10.1111/j.1475-097x.1996.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 3.Adachi H, Mikami A, Kumano-go T, et al. Clinical significance of pulse rate rise during sleep as a screening marker for the assessment of sleep fragmentation in sleep-disordered breathing. Sleep Med. 2003;4:537–42. doi: 10.1016/j.sleep.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Roche F, Pichot V, Sforza E, et al. Predicting sleep apnoea syndrome from heart period: a time-frequency wavelet analysis. Eur Respir J. 2003;22:937–42. doi: 10.1183/09031936.03.00104902. [DOI] [PubMed] [Google Scholar]
- 5.Davies RJ, Belt PJ, Roberts SJ, Ali NJ, Stradling JR. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74:1123–30. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- 6.Schnall RP, Shlitner A, Sheffy J, Kedar R, Lavie P. Periodic, profound peripheral vasoconstriction—a new marker of obstructive sleep apnoea. Sleep. 1999;22:939–46. [PubMed] [Google Scholar]
- 7.Pillar G, Bar A, Shlitner A, Schnall R, Shefy J, Lavie P. Autonomic arousal index: an automated detection based on peripheral arterial tonometry. Sleep. 2002;25:543–9. [PubMed] [Google Scholar]
- 8.Argod J, Pepin JL, Levy P. Differentiating obstructive and central sleep respiratory events through pulse transit time. Am J Respir Crit Care Med. 1998;158:1778–3. doi: 10.1164/ajrccm.158.6.9804157. [DOI] [PubMed] [Google Scholar]
- 9.Pitson DJ, Stradling JR. Autonomic markers of arousal during sleep in patients undergoing investigation for obstructive sleep apnoea, their relationship to EEG arousals, respiratory events and subjective sleepiness. J Sleep Res. 1998;7:53–9. doi: 10.1046/j.1365-2869.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith RP, Argod J, Pepin JL, Levy PA. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;54:452–7. doi: 10.1136/thx.54.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argod J, Pepin JL, Smith RP, Levy P. Comparison of esophageal pressure with pulse transit time as a measure of respiratory effort for scoring obstructive nonapneic respiratory events. Am J Respir Crit Care Med. 2000;162:87–93. doi: 10.1164/ajrccm.162.1.9907086. [DOI] [PubMed] [Google Scholar]
- 12.Pepin JL, Tamisier R, Borel JC, Baguet JP, Levy P. A critical review of peripheral arterial tone and pulse transit time as indirect diagnostic methods for detecting sleep disordered breathing and characterizing sleep structure. Curr Opin Pulm Med. 2009 Aug 29; doi: 10.1097/MCP.0b013e3283318585. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003;53:580–8. doi: 10.1203/01.PDR.0000057206.14698.47. [DOI] [PubMed] [Google Scholar]
- 14.Pepin JL, Delavie N, Pin I, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005;127:722–30. doi: 10.1378/chest.127.3.722. [DOI] [PubMed] [Google Scholar]
- 15.Brietzke SE, Katz ES, Roberson DW. Pulse transit time as a screening test for pediatric sleep-related breathing disorders. Arch Otolaryngol Head Neck Surg. 2007;133:980–4. doi: 10.1001/archotol.133.10.980. [DOI] [PubMed] [Google Scholar]
- 16.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 17.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnoea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 18.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnoea-hypopnoea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 19.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 20.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnoea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 21.Hoch CC, Reynolds CF, 3rd, Monk TH, et al. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep. 1990;13:502–11. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barthelemy JC, Pichot V, Dauphinot V, et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events: the ‘PROOF’ Study. Study design and population sample. Associations with sleep-related breathing disorders: the ‘SYNAPSE’ Study. Neuroepidemiology. 2007;29:18–28. doi: 10.1159/000108914. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary's Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4:93–7. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- 26.Leigh TJ, Bird HA, Hindmarch I, Constable PD, Wright V. Factor analysis of the St. Mary's Hospital Sleep Questionnaire. Sleep. 1988;11:448–53. doi: 10.1093/sleep/11.5.448. [DOI] [PubMed] [Google Scholar]
- 27.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnoea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips BA, Berry DT, Lipke-Molby TC. Sleep-disordered breathing in the healthy aged person. Fifth and final year follow-up. Chest. 1996;110:654–8. doi: 10.1378/chest.110.3.654. [DOI] [PubMed] [Google Scholar]
- 29.Celle S, Peyron R, Faillenot I, et al. Undiagnosed sleep-related breathing disorders are associated with focal brainstem atrophy in the elderly. Hum Brain Mapp. 2009;30:2090–7. doi: 10.1002/hbm.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche F, Gaspoz JM, Pichot V, et al. Association between CRP and unrecognised sleep-disordered breathing in the elderly. Eur Respir J. 2009;33:797–803. doi: 10.1183/09031936.00023208. [DOI] [PubMed] [Google Scholar]
- 31.Roche F, Sforza E, Pichot V, et al. Obstructive sleep apnoea/hypopnoea influences highdensity lipoprotein cholesterol in the elderly. Sleep Med. 2009;10:882–6. doi: 10.1016/j.sleep.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Pitson DJ, Sandell A, van den Hout R, Stradling JR. Use of pulse transit time as a measure of inspiratory effort in patients with obstructive sleep apnoea. Eur Respir J. 1995;8:1669–74. doi: 10.1183/09031936.95.08101669. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 34.Hanley JA, Mc Neil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 35.Mc Neil BJ, Keeler E, Adelstein J. Primer on certain elements of medical decision making. New Engl J Med. 1975;293:211–5. doi: 10.1056/NEJM197507312930501. [DOI] [PubMed] [Google Scholar]
- 36.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 37.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 38.Pennestri MH, Whittom S, Adam B, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: prevalence and interval distribution. Sleep. 2006;29:1183–7. doi: 10.1093/sleep/29.9.1183. [DOI] [PubMed] [Google Scholar]
- 39.Goff EA, O'Driscoll DM, Simonds AK, Trinder J, Morrell MJ. The cardiovascular response to arousal from sleep decreases with age in healthy adults. Sleep. 2008;31:1009–17. [PMC free article] [PubMed] [Google Scholar]
- 40.Gosselin N, Michaud M, Carrier J, Lavigne G, Montplaisir J. Age difference in heart rate changes associated with micro-arousals in humans. Clin Neurophysiol. 2002;113:1517–21. doi: 10.1016/s1388-2457(02)00189-x. [DOI] [PubMed] [Google Scholar]
- 41.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–7. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ancoli-Israel S, Ayalon L, Salzman C. Sleep in the elderly: normal variations and common sleep disorders. Harv Rev Psychiatry. 2008;16:279–86. doi: 10.1080/10673220802432210. [DOI] [PubMed] [Google Scholar]