Abstract
Study Objectives:
To test the hypothesis that head position, separately from trunk position, is an additionally important factor for the occurrence of apnea in obstructive sleep apnea (OSA) patients.
Design:
Prospective cohort study.
Setting:
St. Lucas Andreas Hospital, Amsterdam, the Netherlands.
Patients and Participants:
Three hundred patients referred to our department because of clinically suspected OSA.
Interventions:
N/A
Measurements and Results:
Patients underwent overnight polysomnography with 2 position sensors: one on the trunk, and one in the mid-forehead. Of the 300 subjects, 241 were diagnosed with OSA, based on an AHI > 5. Of these patients, 199 could be analyzed for position-dependent OSA based on head and trunk position sensors (AHI in supine position twice as high as AHI in non-supine positions): 41.2% of the cases were not position dependent, 52.3% were supine position dependent based on the trunk sensor, 6.5% were supine position dependent based on the head sensor alone. In 46.2% of the trunk supine position-dependent group, head position was of considerable influence on the AHI (AHI was > 5 higher when the head was also in supine position compared to when the head was turned to the side).
Conclusions:
The results of this study confirm our hypothesis that the occurrence of OSA may also be dependent on the position of the head. Therefore in patients with a suspicion of position-dependent OSA, sleep recording with dual position sensors placed on both trunk and head should be considered.
Citation:
van Kesteren ER; van Maanen JP; Hilgevoord AAJ; Laman DM; de Vries N. Quantitative effects of trunk and head position on the apnea hypopnea index in obstructive sleep apnea. SLEEP 2011;34(8):1075-1081.
Keywords: OSA, position dependence
INTRODUCTION
Several studies in the past, including a recent study from our group,1 have demonstrated the effect of body position during sleep on the severity of respiratory disturbances in patients with obstructive sleep apnea (OSA). In a large proportion of patients the apnea hypopnea index (AHI) is highest when lying on the back as compared to the other sleeping positions.2–6 In the hypnogram, this is reflected by a clustering of respiratory events correlated with the change in body position. However, in our patient population, we sometimes encountered hypnograms with this clustering of apnea/hypopnea, but without any clear relation with the recorded body position (or sleep stage). At first we suspected this to be due to a technical imperfection of the position sensor readout. Surprisingly, in the literature we found no clear established research or guidelines on how to record sleeping position most reliably. In some systems, position sensors are integrated in the portable recording device strapped to the chest of the patient. Commonly also (and used in our center) position sensors are attached to the elastic bands around either the chest or abdomen used to record respiratory movement. To rule out a sensor displacement, we taped the position sensor directly to the skin at the lower part of the sternum. In a consecutive group of patients, we verified that this gave a much more consistent relation between sensor position readout and actual trunk position and was well tolerated by the patients.
Despite this procedural refinement, the frequently observed discrepancy between apnea/hypopnea clustering and body position persisted. We hypothesized that this could be related to the position of the head relative to that of the trunk. This study was conducted to test this hypothesis by performing sleep recordings in subjects suspected of OSA with dual position sensors placed on both the chest and the head.
Patients
Patients were referred to our department because of clinically suspected OSA. In total, 300 consecutive adult patients (age > 18 years, average 50 years) who underwent overnight polysomnography in our center during the period between July 2008 and June 2009 were included in the study. There were 227 male subjects (mean age 50 years) and 73 females (mean age 49 years). The mean body mass index (BMI) was 29.5 kg/m2—28.7 for males and 32.3 for females. All patients were informed of the purpose of the double position sensor-study and gave informed consent. The study was classified locally as a technology assessment of an existing procedure without added discomfort or other direct consequences for the patient or procedure. Therefore a formal review by our local medical ethics committee was not performed nor required.
METHODS
Polysomnogram recordings were carried out using a digital polygraph system (Embla A10, Broomfield, CO, USA). This records the electroencephalogram (FP2-C4/C4-O2), electrooculogram, EKG, and submental and anterior tibial electromyogram. Nasal airflow was measured by a pressure sensor and arterial oxygen saturation by finger pulse oximetry. Thoraco-abdominal motion was monitored by straps containing piezoelectric transducers. Snoring was recorded through a piezo snoring sensor.
Determination of Body and Head Position during Sleep
Two position sensors (Sleepsense, St. Charles, IL, USA) were used to determine the position of the trunk and head. The first was placed at the lower part of the sternum, the second in the middle of the forehead just above the eyebrows. The sensors generated 5 discrete output voltage levels corresponding to the following sleep positions: upright, supine, prone, left, and right, with a threshold angle of ± 10 degrees from the 45-degree position boundary. The output of the 2 different position sensors was recorded simultaneously on 2 separate channels of the recording device.
In making inferences about body position, it is crucial that the position sensor data truly reflect the actual position of trunk and head in the patients. This was assured at different levels:
First, the position sensor readout should reliably reflect the orientation of the sensor relative to the direction of gravity. This was checked with regular intervals in a calibration setup and also during patient preparation by moving the subjects in different positions. Subjects were also requested to keep the upper part of the bed horizontal and were asked to sleep with just a single pillow.
Secondly, to accurately reflect body posture, the sensors should be secured firmly to relatively rigid parts of the trunk and head. Therefore, sensors were taped directly to the skin with self-adhesive tape on locations where under normal circumstances, with the subject lying supine, the surface is horizontal. Also, the median forehead and the lower part of the sternum are locations with the least possible amount of subcutaneous tissue. This minimizes possible movement of the sensor relative to the rigid underlying structures of the trunk and skull. The position sensor is quite flat, so there is little chance for a rotation of the sensor relative to the underlying body surface. In all subjects it was verified the following morning that the position sensors were not dislocated. In case of dislocation, subjects were excluded from further analysis in this study. In a total of 50 subjects (approximately 20 of whom were included in this study, the exact number was not recorded at the time) it was verified by overnight video-surveillance that the position sensor data corresponded well with visually observed positions.
Finally, sensor characteristics should remain stable throughout the night. Well known are slow drifts in the output of devices based on acceleration sensors. The sensor we employed is an electromechanical type, mainly based on the displacement of a small mercury droplet within the sensor in different positions. The output consists of 5 clearly separated voltage levels corresponding to those different positions. These voltage levels are digitized by the polysomnographic device and stored in a raw data file on the computer as a sequence of numbers. Numerical analysis of these data files demonstrated a good reproducibility, and did not show significant overnight drifts or possibly confounding spurious sensor output signals.
Data Analysis
All signals were analyzed offline on a PC according to the default settings of the polygraph software (Somnologica, Broomfield, CO, USA). Automated results were verified by visual inspection of the entire hypnogram and if necessary corrected if distorted (by artifacts). Sleep stages were scored according to the standard criteria of Rechtschaffen and Kales on 30-sec epochs.7 Respiratory events were scored according to the AASM 2007 criteria.8 Apnea was defined as an episode of ≥ 90% oronasal airflow reduction for ≥ 10 sec. Hypopnea was defined as an episode of > 30% reduced oronasal airflow for ≥ 10 sec accompanied by a decrease ≥ 4% of the arterial oxyhemoglobin saturation. The apnea hypopnea index (AHI) was calculated as the combined number of apnea and hypopnea episodes per hour sleep.
Despite the possibility of simultaneous recording of 2 position channels, the standard software system does not allow analysis of multiple position data channels at the same time. Therefore, 2 overnight hypnograms were constructed: one employed the data from the trunk position sensor, the other employed the data from the head position sensor. All other hypnographic data channels and parameters were the same. From both these hypnograms, the overnight AHI in different positions was determined.
To investigate the effect of trunk and head position on the AHI we employed a multi-step approach:
First, we identified subjects with OSA (AHI > 5) from our study population. Secondly, OSA patients who spent > 5% and < 95% of the total sleeping time in supine position, based on the trunk position sensor readout, were analyzed for position dependence. Position dependence was defined as an overnight AHI in supine position (determined for both the trunk position sensor and the head position sensor) at least twice as high as AHI in non-supine postions.1,5 Since we used 2 position sensors, this led to 4 possible classifications: both trunk and head supine dependent; only trunk supine dependent; only head supine dependent; and not supine dependent.
Thirdly, to evaluate the importance of the head position relative to the trunk position, we further analyzed all subjects with trunk supine position dependence. If the overnight AHI in head supine position exceeded the AHI in trunk supine position by ≥ 5, the patient was classified as having head position-aggravated trunk supine position dependence. If the overnight AHI in head supine position proves to be higher than in trunk supine position, this would support our initial hypothesis that in some patients the clustering of respiratory events is related more to head position than to trunk position. The dichotomy between subjects based on an AHI difference ≥ 5 between positions is in itself not sufficient to clarify the clinical importance of the head position. The relatively low cutoff value serves mainly to separate subjects in whom there is no difference between head and trunk position, or a difference possibly caused merely by chance. In the remaining group with a possible clinically significant head position-aggravated trunk supine position dependence, we can further evaluate the quantitative aspects of the interaction between the position of the head and trunk in more detail.
Therefore, finally, we combined the data from both position sensors to determine the AHI over 4 possible situations: 1 – trunk supine + head supine; 2 – trunk supine + head not supine; 3 – trunk not supine + head supine; 4 – trunk not supine + head not supine. Our standard software did not allow for this distinction to be made. Therefore we developed custom software to recode the position data of the 2 sensors into the 4 position classifications given above. In subjects with head position-aggravated trunk supine position dependence, we reanalyzed the data to examine the interplay between head and trunk position and the respiratory events. This final reanalysis was performed retrospectively in a later phase of the preparation of this manuscript, on previously archived data. Only the new combined position classifications were entered in the reanalysis. Previously scored hypnograms, i.e., sleep stages and detection of respiratory events, were retained from the archived data.
Statistics
Data were analyzed using Excel 2003 (Microsoft, Redmond, WA) and the SPSS statistical package (version 16-18, SPSS Inc, Chicago, IL).
Differences between head and trunk position based results were analyzed using the Student t-test. Polysomnographic data often show a non-strictly normal distribution. However, the Student t-test is considered quite robust to slight deviations of normality. Additional tests were performed using nonparametric statistics (Wilcoxon/Mann-Whitney). These yielded essentially similar results and are not displayed. Numerical relations between variables were evaluated through linear correlation.
RESULTS
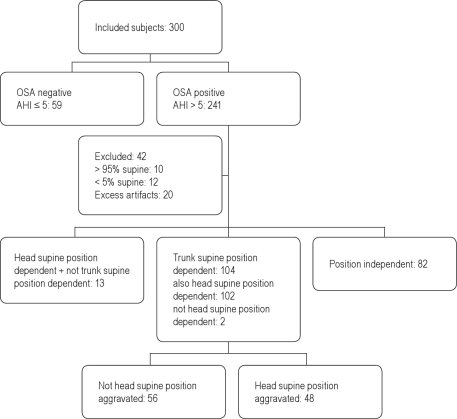
Of the 300 subjects, 241 were diagnosed with OSA based on an AHI > 5. Of these 241 patients, 42 were excluded from further evaluation. Twenty were excluded because of technically insufficient data, mostly due to excess of artifacts or sensor dislocation. Twenty-two patients were excluded because they spent > 95% (n = 10) or < 5% of the total sleep time (n = 12) in supine position.
The data from the remaining 199 patients were further analyzed for position dependence. In 82 patients (41.2%), the overnight AHI was not position dependent. One hundred four patients (52.3%) were found to be trunk supine position dependent. Of these 104 patients, 102 were both trunk and head supine position dependent. Thirteen patients (6.5%) were not position dependent based on the position sensor on the trunk and were classified as only head supine position dependent.
In the trunk supine position dependent group (104 patients), head position was of considerable influence on the AHI in 48 (46.2%) patients. These met the criteria for a head position-aggravated trunk supine position-dependent OSA. These results are depicted graphically in Figure 1 and conform to the STARD guidelines.9
Figure 1.
Flowchart of subjects included in the study.
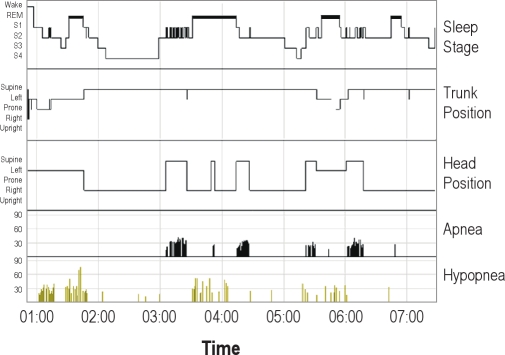
Figure 2 shows the hypnogram in one individual in whom the AHI in trunk supine position (21.4/h, sleeping in trunk supine position 79.9% of the total sleeping time) is almost the same as the AHI calculated over the total sleeping time (23.6). Based on our predefined criteria, this patient does not have a supine position-dependent OSA (AHI not in trunk supine position = 30.8). However, the overnight AHI in head supine position is much higher (76.8, during 16.6% of the total sleeping time), indicating a head supine position-dependent OSA. The clustering of apnea in this patient is clearly more related to supine position of the head than to trunk position (no evidence was found suggesting dislocation or malfunction of the position sensors). A general picture was seen in the majority of head supine position-dependent subjects but not trunk supine position-dependent OSA subjects (n = 13) with visual analysis of the hypnograms. These subjects slept a relatively long proportion of total sleep time in trunk supine position. During the largest part of sleep in trunk supine position, the head was turned sideways. Respiratory events clustered during only a small part of the time spent in trunk supine position. Because of this, these patients did not meet our predefined criteria for a trunk supine position dependent OSA.
Figure 2.
Overnight hypnogram in a single subject with respiratory events and trunk/head position indicated. Normally, there is only a single position channel displayed. To show the effect of head position, the head position channel recording was inserted into the standard hypnogram report by means of a picture editor program. Notice that in this subject apneas are primarily related to the position of the head, not to the position of the trunk. Hypopneas are more apparent in the remainder of the night.
Table 1 summarizes the demographic and polysomnographic characteristics of the 199 patients diagnosed with OSA. In our inclusion criteria we made no distinction between men and women. The men outnumbered the women in a proportion similar to that in our general OSA population. The proportion of women in the head position-aggravated trunk supine position-dependent group (10/48) did not differ significantly from the proportion of women in the total OSA group (38/199, χ2 test). Although the average BMI in women was higher than in men and women spent more time in supine position, position dependence of the overnight AHI was more pronounced in men.
Table 1.
Demographic and polysomnographic characteristics* of OSA-positive-patients (average [ ± SD])
| Characteristic | Male | Female | Total |
|---|---|---|---|
| Patients (n) | 161 | 38 | 199 |
| Age (years) | 47.7 (10.5) | 49.4 (13.6) | 48.0 (11.1) |
| BMI (kg/m2)# | 28.7 (4.6) | 32.4 (9.4) | 29.4 (6.0) |
| AHI (events/h) | |||
| Total | 28.6 (21.3) | 23.4 (18.8) | 27.6 (20.9) |
| Trunk supine# | 40.1 (24.0) | 25.6 (19.8) | 37.3 (23.9) |
| Head supine# | 44.9 (26.9) | 33.3 (26.5) | 42.7 (27.1) |
| % time in tsp# | 44.2 (20.7) | 53.3 (22.9) | 45.9 (21.4) |
| % time in hsp | 31.6 (20.4) | 31.1 (24.2) | 31.5 (21.1) |
| AHI hsp minus AHI tsp | 4.8 (16.8) | 7.7 (13.7) | 5.3 (16.2) |
| AHI hsp / AHI tsp ratio (%) | 131 (106) | 134 (93) | 132 (103) |
BMI, body mass index; AHI, apnea hypopnea index; OSA, obstructive sleep apnea; SD, standard deviation. hsp (head supine position), tsp (trunk supine position) = values in supine position based on head and trunk position sensor respectively.
statistically significant difference for males and females (t-test and Mann-Whitney: P < 0.05)
The average AHI in trunk supine position in the OSA positive subjects was higher than the average AHI during total sleep time. Also, the average overnight AHI over the entire group was higher in head supine position than in trunk supine position.
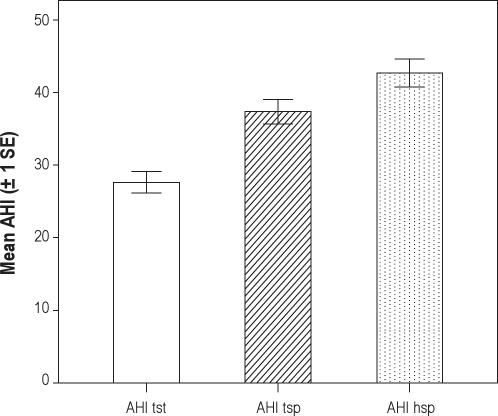
Figure 3 graphically summarizes the AHI values determined over the different time periods in OSA subjects (n = 199). The average AHI over the entire night was 28 (SD 21, median 21). In the trunk supine position, this was 37 (SD 24, median 32). The average AHI in supine position based on the head position sensor was 43 (SD 27, median 41). The differences between the 3 different AHI values are all significant at the level of P < 0.001 with both parametric and nonparametric statistics (paired Student t- and Wilcoxon signed rank test).
Figure 3.
Mean AHI values determined over the total sleep time (TST) and the different time periods spent in supine position based on the trunk position sensor (tsp) and head position sensor (hsp) in OSA patients (n = 199).
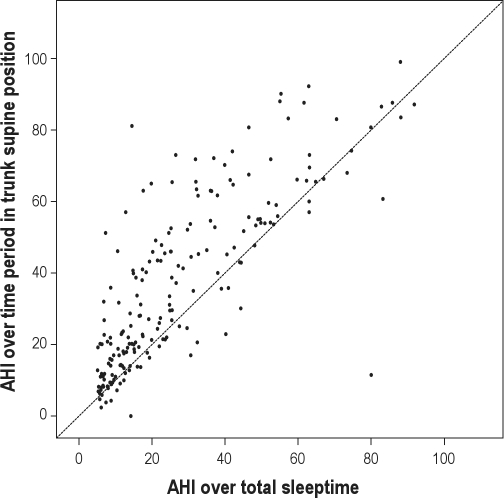
Figure 4 shows the correlation of the AHI determined over the total sleep time with the AHI during trunk-supine position for the entire OSA positive study population (n = 199). Observe the extensive spread of the points lying above the unity line (x = y), indicating a higher AHI in trunk supine position in a considerable proportion of subjects. This is in line with previous findings in literature on position dependent OSA.
Figure 4.
Correlation of the AHI determined over the total sleeping time with the AHI during trunk-supine position for OSA positive subjects (n = 199, using the standard trunk position sensor). The line indicates the unity line (x = y).
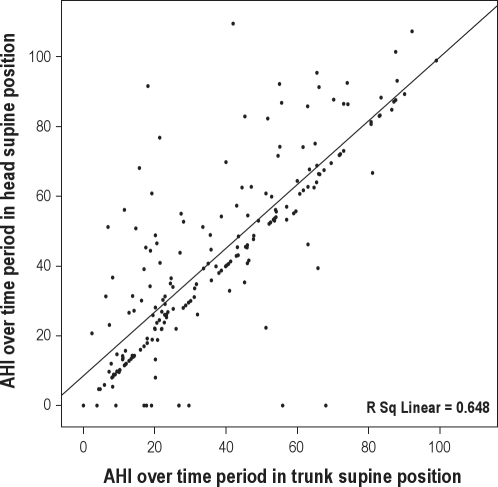
Figure 5 shows that there is a clear correlation between the overnight supine AHI based on the trunk and head position sensor (r2 = 0.65). Most points fall on the unity line, indicating an equal AHI with trunk and head supine position. However, again there are a marked number of outliers, mainly above the unity line. This is reflected in the trajectory of the linear regression line. (The straight line in the graph indicates the linear regression line. The unity line is not drawn, but clearly visible by the clustering of points on the x = y line.) The figure illustrates that the higher average AHI in head supine position is not a structurally present factor in all subjects but only seen in a subgroup of subjects. Thus, the effect of position on the AHI may be undervalued based on the AHI in trunk supine position. A number of subjects, with various trunk supine position AHI values demonstrated a head supine position AHI of zero. On review, these were patients in whom the head was always turned sideways while sleeping on the back.
Figure 5.
Correlation of the AHI over the time period spent in supine position based on the trunk position sensor (x-axis) and head position sensor (y-axis). The line indicates the linear regression line of the correlation between the 2 AHI values in our OSA positive study group (n = 199).
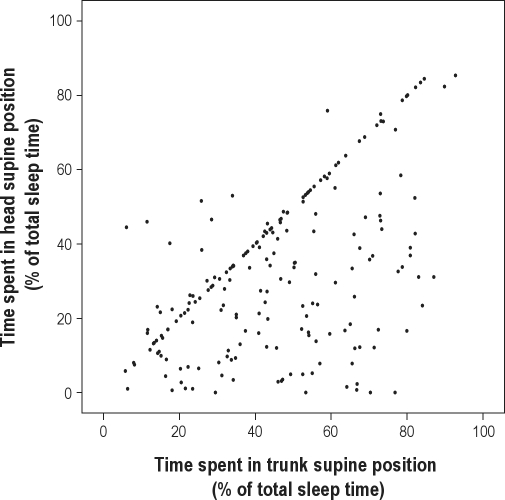
Figure 6 shows the correlation between the relative time spent in different positions. The average duration of trunk supine position was 46% of total sleep time (SD 21, median 46%). It is apparent that in the majority of subjects, trunk supine position time was equal to head supine position time (while lying on the back, the head was also in the supine position). However, in a considerable number of subjects, head supine position time was shorter than trunk supine position time. This indicates that during part of the time spent in the trunk supine position, the head was turned sideways. On average, the duration of the time spent in head supine position was 77% of trunk supine position time. In a few subjects, head supine position time was longer than trunk supine position time. This suggests that these subjects were sleeping with the trunk slightly turned sideways while the head remained in the supine position.
Figure 6.
Correlation between the relative time spent in supine position based on the trunk and head sensor in OSA positive subjects (n = 199).
In subjects with a head position-aggravated trunk supine position-dependent OSA, we additionally examined the interaction between head and trunk position and the effect this had on the AHI. The stored data for one subject could not be fully recovered due to a storage failure. Therefore data from 47 subjects were available for analysis (37 male, 10 female).
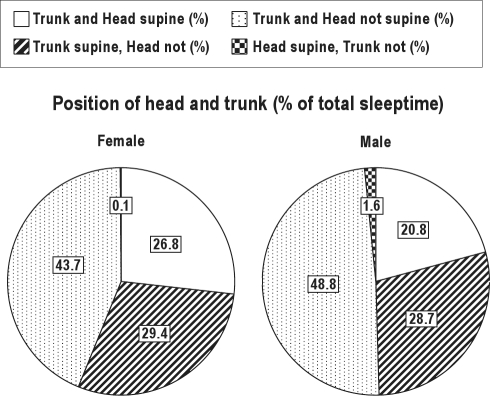
Figure 7 shows the relative time spent in the different positions of head and trunk for men and women. Three conclusions can be made based on these graphs. First, approximately, on average, half of the total sleep time in this subgroup was spent in trunk supine position. Secondly, in more than half of the time spent in trunk supine position the head was turned sideways. Thirdly, the percentage of time spent in trunk supine position was somewhat higher for women than for men.
Figure 7.
Relative time spent in the different positions of head and trunk for men and women in patients with head position-aggravated trunk supine position-dependent OSA (n = 47).
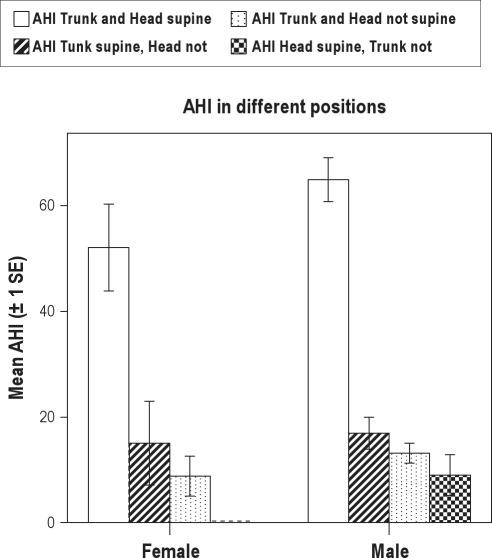
Figure 8 displays the AHI calculated over the time spent in the different positions. The AHI was highest over the time period in which both trunk and head were in supine position. There is a significant difference between this value and the AHI over the time period spent in other positions (paired Student t-test, P < 0.001) for both men and women. The effect of sideways rotation of the head when lying in trunk supine position was larger for men than for women (men: mean AHI 65 > 17, difference = 48; female: mean AHI 52 > 15, difference = 37, Student t-test, P < 0.001).
Figure 8.
Mean AHI over the time spent in the different positions of head and trunk in patients with a head position-aggravated trunk supine position-dependent OSA (n = 47).
DISCUSSION
The results from this study confirm our hypothesis that head position, separately from trunk position, is an additional important factor in the occurrence of apnea/hypopnea in a subpopulation of OSA patients. Unfortunately, routine PSG is most often limited to recording of trunk position solely. While preparing this study we were confronted with the apparent lack of standardization of position recording during sleep studies. Our results indicate that these technical factors deserve more attention in the development of future clinical standards.
Overnight results based on the two different sensor positions show that the AHI calculated over the total sleep period with the head lying supine frequently was higher than the AHI calculated over the total period with the trunk in supine position. This occurred in a considerable proportion of OSA subjects (48 head position-aggravated trunk supine-position dependent + 13 solely head supine position dependent out of 199 = 30.7%). In the patients with an overnight head position-aggravated trunk supine position-dependent OSA (n = 48), the effect of head rotation was further confirmed. Over the time period when lying on the back with the head also supine, the AHI was significantly higher than during the time period with the head turned sideways.
Overnight position dependence was defined as an AHI in supine position at least twice as high as the AHI in the other positions. The same definition has been used in previous studies.1,5 As with every cutoff value, this distinction is somewhat arbitrary. However, with this criterion, we think we select only those subjects in whom there is a relevant (and likely etiological) difference between supine position and other positions. Head position dependence was defined as an AHI difference of 5 or more between head and trunk position recordings. This low threshold was chosen to select subjects with a possible clinically relevant head position dependence for further analysis. If we would use a higher cutoff value we would have increased the clinical relevance of the distinction. However, we would also introduce a larger selection bias which would hamper further quantitative analysis and interpretation of the results in this subgroup.
For the detection of rotation, we used the same sensor as commonly is used for detection of trunk rotation in many commercially available systems. The use of a sensor with a threshold for rotation detection of 45 degrees could lead to an underestimation of rotation of the head when this is only a slight rotation. Review of the data and our previous observations indicate that in the majority of subjects there are frequent head turns which exceed this 45-degree detection threshold. We rarely saw quick fluctuations in position sensor readout, which would indicate a rotation in an angle around the detection threshold. The 45-degree threshold seems also justified because smaller rotations would have less pronounced mechanical effects on the anatomical configuration of the upper airway. Although this study was not aimed at unravelling this underlying pathophysiological basis, a change in the local anatomical configuration of the upper airway during head rotation seems a likely underlying factor. With the head in supine position, the tongue and to a lesser extent the soft palate, in accordance with gravity, fall backward due to the physiological muscle relaxation. In the trunk supine position with the head turned to either the left or right side, this effect of gravity on the tongue and soft palate would presumably be smaller, causing fewer obstructive events in the upper airway. Another possibility is that rotation of the head exerts a stretching force on the wall of the upper airway, which decreases the susceptibility to airway collapse during sleep.
We mainly focused our investigation on the effect of lateral rotation of the head. Of course, flexion and extension of the neck could have a similar effect on the AHI. However, with the patient lying on a horizontal bed with the head supported by a single pillow, movement freedom to flexion and extension is more limited than lateral rotation. If lateral rotation was associated with flexion of the neck, one would expect an increase of the AHI with sideways rotation due to increased mechanical compression. On the contrary, the AHI decreases with lateral rotation in a subgroup of patients. Lateral rotation associated with hyperextension of the neck could possibly reduce the tendency for airway collapse. However, during video-surveillance in our center, we rarely observed either major flexion or extension movements of the head during sleep that could have a distinct influence on respiratory events. Flexion-extension of more than 45 degrees would also be detected by the head position sensor. This almost never occurred in our study population. Small flexion and extension movements could potentially already have a major effect on the anatomical configuration of the upper airway, and as such affect the AHI. However, if this was relevant, small flexion-extension movements should cause major fluctuations in flow and clustering of apneas in time periods over which the recorded position of the head (and sleep stage) remained constant. This was not observed in our data. Clustering of apneas seemed primarily related to the recorded supine position of the head, with an overall reduction of apneas during recorded lateral rotation. Thus, although we cannot disprove conclusively that head flexion-extension may partly explain our results, we believe this is less likely, but further research is needed to rule out or support this possibility more specifically.
Looking at the demographic characteristics there are some clear gender differences. In the present study, position dependence of the AHI for both trunk and head supine position was more pronounced in men, despite the fact that women had a higher average BMI and spent more time in supine position.
We did not investigate the relation between AHI parameters based on trunk and head position and the different sleep stages. Respiratory events are known to be more prevalent in sleep stage 2 and in REM sleep. Sleep architecture is heavily influenced by the severity of OSA. One could, for example, hypothesize that head supine position is more often associated with REM sleep or sleep stage 2. However, visual review of the hypnograms in our study group gives no support for this hypothesis. Full quantitative analysis of the interaction between sleep stages, position, and respiratory events would require a more elaborate study design, possibly in a larger patient cohort.
The data reported here are not only interesting from a scientific point of view; they have clinical relevance as well. Two recent studies on the role of sleep position have shown a remarkably consistent 56% of OSA to be position dependent (defined as having a AHI at least twice as high in a certain sleep position than in another position).1,6 The worst position is usually supine, but not always. Another 30% of patients do worse in a certain (usually supine) position but not with an AHI twice as high as in another position. So in roughly 80% of patients, sleep position is reported to play an important role in the severity of OSA. Note that results in literature all seem based on recording the trunk position. Our results indicate that trunk position-based data may underestimate the effects of position on respiratory events.
The percentage of sleeping in the worst sleeping position may very well be the most important factor in night to night variability in sleep studies.10,11 Given these facts, it is remarkable that in sleep studies, both primary as well as in reporting results of therapeutical interventions (surgery, oral appliance therapy, etc.), the severity of the OSA and the percentage of sleeping time in the various positions is not routinely reported. For a meticulous reporting of interventions, ideally the severity and the amount of sleeping in different positions should be reported as well.
Apart from diagnostic accuracy, our results may also have therapeutic consequences, both in surgical and nonsurgical strategies. For example, position therapy of OSA now commonly aims to arouse the patient when lying on the back so the subject rotates the body on his/her side to alleviate respiratory obstructions.12,13 In head position-aggravated trunk supine position-dependent OSA, it may be sufficient to stimulate the subject to rotate only the head sideways, based on a position sensor monitoring the orientation of the head. It can be expected that this would have a much less profound negative effect on sleep quality.
In conclusion: We have provided evidence that in a significant proportion of patients, trunk and head position during sleep are not the same. In addition, this may have clinical relevance. OSA is almost per definition caused by obstruction in the upper airway, and it makes sense to analyze head position in addition to trunk position. This study warrants further research in the underlying pathophysiology and clinical consequences.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 985.
REFERENCES
- 1.Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263:946–50. doi: 10.1007/s00405-006-0090-2. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–4. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 3.Kavey NB, Blitzer A, Gidro-Frank S, Korstanje K. Sleeping position and sleep apnea syndrome. Am J Otolaryngol. 1985;6:373–7. doi: 10.1016/s0196-0709(85)80015-6. [DOI] [PubMed] [Google Scholar]
- 4.George CF, Millar TW, Kryger MH. Sleep apnea and body position during sleep. Sleep. 1988;11:90–9. doi: 10.1093/sleep/11.1.90. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright RD, Diaz F, Lloyd S. The effects of sleep posture and sleep stage on apnea frequency. Sleep. 1991;14:351–3. doi: 10.1093/sleep/14.4.351. [DOI] [PubMed] [Google Scholar]
- 6.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–39. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Kales A. Los Angeles: UCLA, Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology: techniques and scoring systems for sleep stages of human subjects; pp. 1–15. [Google Scholar]
- 8.American Academy of Sleep Medicine. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders. [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–4. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosko SS, Dickel MJ, Ashurst J. Night-to-night variability in sleep apnea and sleep-related periodic leg movements in the elderly. Sleep. 1988;11:340–8. [PubMed] [Google Scholar]
- 11.Fietze I, Dingli K, Diefenbach K, et al. Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur Respir J. 2004;24:987–93. doi: 10.1183/09031936.04.00100203. [DOI] [PubMed] [Google Scholar]
- 12.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: A 6-month follow-up study. Laryngoscope. 2006;116:1995–2000. doi: 10.1097/01.mlg.0000237674.66716.a7. [DOI] [PubMed] [Google Scholar]