Abstract
Study Objectives:
(1) To determine the efficacy of automatically adjusted positive airway pressure (APAP) with a comfort feature (A-Flex) at reducing apneas and hypopneas in participants with moderate to severe OSA. (2) To determine the relative difference between A-Flex, continuous positive airway pressure (CPAP), and APAP-derived optimal pressure for CPAP (CPAPAPAP) on adherence to treatment. (3) To determine the relative difference between APAP with A-Flex, CPAP, and CPAPAPAP on long-term change in functional outcomes.
Design:
Randomized, double-blinded, 3-arm, multicenter trial.
Setting:
University and Veterans Affairs medical centers.
Patients or Participants:
168 participants were randomized, and 140 completed the 180-day study.
Interventions:
(1) A-Flex; (2) CPAP; (3) APAP for 14 days and then switched to CPAP at a fixed pressure.
Measurements and Results:
Apnea-hypopnea indices, average and minimum oxygen saturation, time spent < 90% were significantly poorer for A-Flex vs. CPAP at the initiation of study treatment; with the exception of minimum oxygen saturation, these differences were absent at 180 days. A-Flex had lower average leak values at both 3 and 6 months. There were no significant differences between groups in major efficacy, adherence, and outcome (subjective sleepiness, objective vigilance, blood pressure, quality of life) measures. No differences between groups in attitudes toward use were observed at 3 or 6 months; participant ratings for CPAP were significantly higher than A-Flex on treatment satisfaction and benefit, but not different for sleep quality and mask comfort.
Conclusions:
We found that A-Flex shows equivalency, but non-superiority (except for average leak values), in efficacy, adherence, and functional outcomes compared to CPAP after either 3 or 6 months.
Clinical Trial Registry:
Positive Pressure Treatment of Obstructive Sleep Apnea, http://www.clinicaltrials.gov, NCT00636181.
Citation:
Kushida CA; Berry RB; Blau A; Crabtree T; Fietze I; Kryger MH; Kuna ST; Pegram GV; Penzel T. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. SLEEP 2011;34(8):1083-1092.
Keywords: A-Flex, APAP, CPAP, Auto-CPAP, automatically adjusted positive airway pressure, obstructive sleep apnea
INTRODUCTION
The most common treatment for obstructive sleep apnea (OSA) is positive airway pressure (PAP) therapy using continuous positive airway pressure (CPAP) devices. The pressure required to treat OSA can be determined during an in-laboratory titration study or the pressure can be adjusted automatically using device algorithms based on the characteristics of the airflow waveform to minimize abnormal breathing events. These automatically adjusted positive airway pressure (APAP) devices are available for both CPAP (Auto-CPAP) and bilevel positive airway pressure (Auto-BPAP). There are data comparing APAP devices to fixed pressure devices,1–12 but these studies have shown no clear evidence whether APAP yields better outcomes vs. fixed pressure devices in the average OSA patient, no difference in patient preference, and only a marginal benefit of APAP vs. fixed pressure devices in terms of subjective sleepiness and usage. However, APAP titration appears to be more cost-effective,13 though with similar adherence and outcomes compared to manual laboratory CPAP titration in determining optimal PAP pressure for long-term use.14,15 Additionally, APAP is becoming more commonly prescribed. A recent survey completed by 38 sleep center physicians in 21 countries revealed a mean ratio of 30:70 for APAP:CPAP prescriptions for those 16 countries in which both APAP and CPAP are prescribed; and in 19 of the countries APAP is used both for in-laboratory and home titration as well as for continuous treatment.16 Regardless of the device used, breathing during PAP therapy may prove to be uncomfortable to some patients, and this discomfort may present an obstacle to patient adherence to treatment. Flexible pressure relief alters the pressure profile in the transition from inhaling to exhaling to improve comfort during sleep-related breathing.
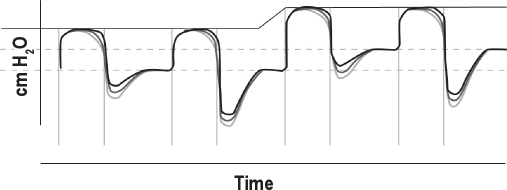
A-Flex (Philips Respironics, Murrysville, PA) is a comfort feature of PAP delivery that works with the automatically adjusting CPAP algorithm (Figure 1), using constant pressure adjustment to minimize abnormal breathing events and thus maximize therapeutic efficacy. A-Flex matches pressure delivery through the patient's entire breathing cycle. Initially, pressure is significantly reduced at the start of exhalation, with the pressure approximately 2 cm H2O less than inspiratory pressure by the end of exhalation; the pressure returns to the therapeutic level at the start of the next inspiratory phase. Similar to Bi-Flex (Philips Respironics), A-Flex softens the pressure transition from inhalation to exhalation to enhance breathing comfort, and is similar to C-Flex (Philips Respironics) by providing pressure relief during exhalation. The timing of these pressure manipulations is based on airflow measurements so the A-Flex pressure profile mirrors patient respiration.
Figure 1.
Automatically adjusted positive airway pressure (APAP) algorithm with A-Flex. Pressure (solid line in upper part of figure) is automatically adjusted by the APAP device, with the pressure reduced at the start through the end of exhalation, with the pressure returning to the therapeutic level at the start of the next inspiratory phase. The amount of pressure reduction can be adjusted (gray lines).
There is an absence of data comparing A-Flex to alternate forms of PAP therapy on adherence and outcomes. The purpose of this study was to determine if the comfort of A-Flex results in higher acceptance and adherence and improved patient outcomes compared to alternate forms of PAP therapy. More specifically, this overall hypothesis parallels the belief that if this therapy is more comfortable for the patient, he or she will be encouraged to use it more, and the greater utilization might translate to greater treatment efficacy and improvements in alertness, vigilance, and quality of life. This study was an international, multicenter, double blinded, prospective, randomized, controlled trial, in which participants were assigned to one of three groups, representing standard of care in current sleep medicine practice using CPAP, a model sometimes used in sleep centers where APAP devices are used to expedite PAP titration (CPAPAPAP), and an alternate model using A-Flex.
There were three aims for this study:
Aim 1 (Efficacy):
To determine the efficacy of APAP with A-Flex at reducing apneas and hypopneas in participants with moderate to severe OSA. Hypothesis (H)1: The groups will not differ in residual apnea and key polysomnography (PSG) variables on the first laboratory PSG night at the initiation of therapy. H2: Participants in the A-Flex group will demonstrate lower residual apnea and improved PSG variables compared to those assigned to the CPAP and CPAPAPAP groups at 180 days.
Aim 2 (Adherence):
To determine the relative difference between APAP with A-Flex and standard CPAP on adherence to treatment. H3: Participants in the A-Flex group will demonstrate the lowest dropout rate (defined as using ≤ 1 hour a night on average) at 180 days, followed by those in the CPAPAPAP group and those in the CPAP group. H4: Participants in the A-Flex group will demonstrate the greatest 180 day adherence (average nightly use), followed by those in the CPAPAPAP group and those in the CPAP group.
Aim 3 (Outcomes):
To determine the relative difference between APAP with A-Flex and standard CPAP on long-term change in functional outcomes. H5: Participants on APAP with A-Flex will demonstrate better improvement in functional outcomes compared to those using standard CPAP at 180 days. Secondary Hypotheses: H6: Participants on APAP with A-Flex will demonstrate superior improvements in subjective sleepiness by the Epworth Sleepiness Scale17,18 (ESS, an instrument designed to assess a subject's chance of dozing in 8 real-life situations), functional outcomes associated with sleepiness by the Functional Outcomes of Sleep Questionnaire19 (FOSQ, a sleep-related quality-of-life self-assessment questionnaire), vigilance by the Psychomotor Vigilance Task20 (PVT, a 10-min attention/vigilance test), and blood pressure. H7: Participants on APAP with A-Flex will demonstrate better attitudes toward PAP use by an Attitudes Toward Use Questionnaire (ATUQ, a self-efficacy scale based on psychological theories of behavior change and modified from one developed by Stepnowsky and Marler21) and acceptance through visual analog scales (VAS, questions assessing subjective sleep quality changes, mask comfort, treatment satisfaction and benefit) of therapy at 180 days compared to those using standard CPAP.
METHODS
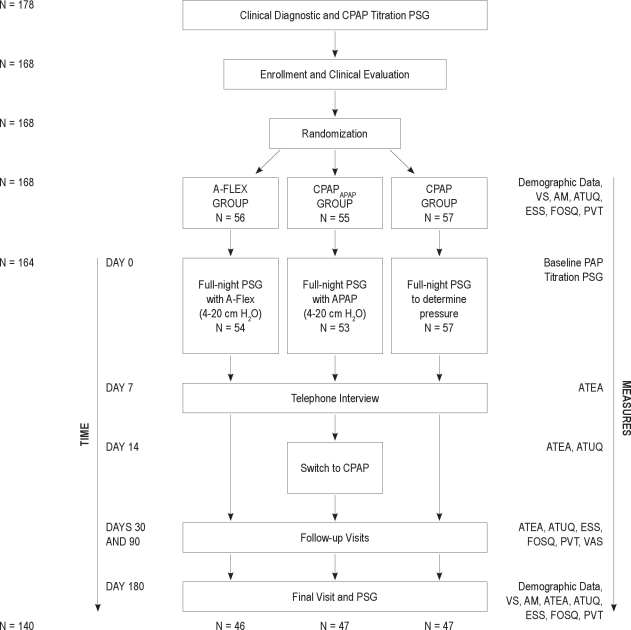
Participants (Figure 2)
Figure 2.
Participant flowchart. AM, anthropometric measurements; APAP, Auto-CPAP; ATEA, Adherence and Therapy Effectiveness Assessment; ATUQ, Attitudes Toward Use Questionnaire; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; FOSQ, Functional Outcomes of Sleep Questionnaire; PAP, positive airway pressure; PSG, polysomnography; PVT, psychomotor vigilance task; VAS, visual analog scales; VS, vital signs.
Patients who completed routine clinical diagnostic visit and CPAP titration polysomnography (PSG) at 5 international sleep centers (Stanford University, Stanford, CA; Shands and University of Florida Sleep Disorders Center, Gainesville, FL; Charite - Universitatsmedizin Berlin Interdisciplinary Center of Sleep Medicine, Berlin, Germany; Gaylord Hospital, Wallingford, CT; Sleep Disorders Center of Alabama, Birmingham, AL) were consecutively recruited for the study. These patients were approached with information on the study and were asked to consent to participate until at least 50 patients at each center were recruited and enrolled, provided they met the criteria listed below for the study. A sample size of 150 participants (50 in each arm) was derived by consensus among the study's steering committee, composed of each site's principal investigators.
The inclusion criteria were as follows: (1) age 21–75 years; (2) obstructive sleep apnea (OSA) diagnosis with a baseline apnea-hypopnea index (AHI) ≥ 15 events/h of sleep; (3) able and willing to provide written informed consent; (4) agreement to try PAP as initial treatment approach; (5) adequate clinical CPAP titration within 2 weeks of enrollment defined by these criteria (Criterion 1: The interval during which the chosen pressure is delivered contains a period of sleep ≥ 15 min that includes REM sleep that is not continuously interrupted by arousals or awakenings, and one of the following: (a) AHI ' 5, or (b) an AHI ≤ 10 in participants with moderate to severe OSA [an apnea index or AHI ≥ 20], or (c) in participants with a diagnostic AHI < 20, a reduction of the AHI ≥ 50%. Criterion 2: If REM sleep is not present during the time the selected pressure is delivered, a reduction in the AHI ≥ 75% and the sleep specialist's clinical judgment that the titration is adequate); (6) Native English speaker for participants enrolled in the US and native German speaker for participants enrolled in Germany.
The exclusion criteria were as follows: (1) participation in another interventional research study within the last 30 days; (2) the need for more than one titration PSG; (3) the use of sedatives or hypnotics during the titration PSG; (4) a major medical or psychiatric condition that would interfere with the demands of the study, adherence to PAP, or the ability to commit to follow-up assessment; (5) prior prescription for, or exposure to PAP therapy within the previous year; (6) chronic respiratory failure or insufficiency with suspected or known neuromuscular disease, moderate or severe COPD or other pulmonary disorders, or any condition with an elevation of arterial carbon dioxide levels (> 45 mm Hg) while awake or participants qualifying for oxygen therapy (arterial saturation ≤ 88% for > 5 min); (7) surgery of the upper airway, nose, sinus, or middle ear within the previous 90 days; (8) surgery at any time for the treatment of OSA; (9) presence of untreated or poorly managed (i.e., symptomatic despite treatment), non-OSA related sleep disorders; (10) use of medications with hypnotic or sedative effects or regular use of nighttime sedatives or sleeping aids ≥ 1 night per week; (11) consumption of ethanol > 4 nights per week; (12) shift workers.
The study protocol and informed consent forms were approved by the local institutional review boards at each center. After confirming eligibility, informed consent was obtained from those agreeing to participate in the trial, and a clinical evaluation including the collection of demographic data (e.g., age, gender, ethnicity, educational level), anthropometric measurements (neck circumference, height, and weight), and vital signs was conducted. The FOSQ, ESS, and ATUQ were also completed by the participants at this time.
Randomization
Those agreeing to further participate in the study were then randomized into the study. Urn randomization was used to control for the potentially confounding variables (age, gender, education, AHI, subjective sleepiness). Urn randomization is an adaptive randomization procedure that adjusts group assignment throughout the course of the study to optimize the chances of matching groups on key variables. Participants were randomized to one of the 3 study groups: A-Flex group: Automatically adjusted CPAP (APAP, pressure range between 4 and 20 cm H2O) with A-Flex for the duration of the study period; CPAPAPAP group: APAP (4-20 cm H2O) for 14 days, then switching to standard CPAP at a fixed pressure (determined from the APAP device at a level corresponding to the 90% pressure) for the remainder of the study period; CPAP group: Standard CPAP with a fixed pressure for the duration of the study period. The Principal Investigator (PI) and research staff administering questionnaires or interacting with the participant were blinded to randomization and the results of all participant evaluations. The PI was blinded to the results of all PSGs, and participants were blinded to treatment (see next section).
PAP Titration
Participants randomized to the CPAP group underwent a full-night PSG to document the efficacy of the standard, fixed pressure CPAP determined from their prior clinical CPAP titration PSG. Participants in the CPAPAPAP group underwent full-night PSG on conventional APAP. Participants in the A-Flex group underwent full-night PSG on APAP with A-Flex. For all groups, these PSGs were scheduled as quickly as possible after consent, and heated humidification was provided and used as needed. The participant performed the PVT once before (evening) and once after (morning) the PSG. Sleep technologists at each site fitted and adjusted the PAP masks prior to the PSG, and the PAP devices and supplies were dispensed by the site personnel following instruction about PAP therapy and the health consequences of OSA. All three groups received treatment with the same PAP device (REMstar Auto M-Series, Philips Respironics, Murrysville PA) set to deliver the appropriate mode. The standard display on the device was altered to not reveal therapy mode information (e.g., A-Flex options) so that participants could be blinded to treatment. The PSG data were reviewed by the sites to verify efficacy of treatment, and were then scored by an independent, centralized PSG Reading Center at the University of Pennsylvania using 2007 AASM scoring rules.22
Testing and Follow-Up
Following the PSG, participants were contacted by telephone at 7 days of treatment and had a follow-up visit at 14 days of treatment to assess adherence and identify and manage any treatment-related problems. For the CPAPAPAP group only, the therapy mode was changed from APAP to conventional, fixed pressure CPAP (based on the 90% pressure determined from the device), so the participants in this group did not remain on APAP. Outcomes and adherence data were collected at 3 more follow-up visits at 30, 90, and 180 days after initiating treatment. If the participant reported snoring while on treatment, the minimum pressure (A-Flex group) or therapy pressure (CPAP or CPAPAPAP groups) could be adjusted. The maximum pressure (A-Flex group) or therapy pressure (CPAP or CPAPAPAP groups) could be reduced for pressure intolerance. Additionally, during these visits, objective PAP adherence and efficacy data were collected from the PAP device, and the participant completed the ATUQ, FOSQ, ESS, PVT, and VAS. Participants were also instructed to contact study personnel if they experienced any therapy problems or side effects, felt they were not using PAP sufficiently, experienced residual sleepiness, or had questions related to their equipment or treatment of OSA for the duration of their study period. At the final follow-up visit at 180 days, a final full-night PSG using the assigned therapy was conducted, and demographic, anthropometric, and vital signs (heart rate and blood pressure measured after sitting quietly for 10 min) data were collected. After the final PSG, participants were unblinded, adherence was reviewed, participants had the opportunity to ask questions, and participants returned their PAP devices (unless they did not have other means for continuing treatment).
Statistical Methods
Analyses were performed on an intention-to-treat basis. All participants who were randomized and received any amount of study therapy were included in the analysis, in the treatment group to which they were randomized. Appropriate post hoc tests that account for the multiplicity of comparisons were used to identify pair-wise differences where the main effect of treatment group was significant. P-values for the main effect of treatment group membership are reported along with the pairs that are significantly different. No adjustments were planned or made for testing of hypotheses for multiple endpoints. A two-sided P-value of 0.05 was used to determine statistical significance.
Aim 1 (Efficacy)
Hypothesis tests for PSG outcomes employed analysis of variance for approximately normally distributed outcomes (or outcomes that could be transformed to an approximately normal distribution), and the Kruskal-Wallis test for non-normal outcomes. Where significant differences across groups were found, pairwise differences in groups were determined using the Tukey-Kramer test (where ANOVA was applied), or by performing multiple Mann-Whitney-Wilcoxon summed rank tests and applying a Bonferroni adjustment. Separate analyses were run on each of the key sleep variables (AHI, oxygen saturation, arousal index, sleep efficiency, % slow wave sleep, and % REM sleep) from the first PSG night (H1) and the last PSG night (H2).
Aim 2 (Adherence)
H3: Three definitions were considered for “dropout.” On a per patient basis, dropout was defined as < 1 h use per night on average, < 2 h per night on average, and a median of < 2 h per night. Difference in dropout rates across groups was tested using either the χ2 test or Fisher exact test, where ≥ 50% of expected cell counts were < 5. This analysis was performed separately for results through 90 days and results through 180 days. H4: The average daily hours of use, computed by participant across available days, was analyzed using analysis of variance to assess differences across treatment groups. The analysis was performed for results through 90 and through 180 days.
Aim 3 (Outcomes)
H5 and Secondary Hypotheses H6 and H7: Difference across groups, in changes from baseline (PAP Titration PSG), were tested for H5 using a mixed model approach, in order to account for the repeated measures within participants, where outcomes were approximately normally distributed (or outcomes that could be transformed to an approximately normal distribution). Outcomes that significantly departed from normality and did not fit a known distribution were analyzed using the Kruskal-Wallis test. Separate analyses were run for each variable of interest. Dependent variables to test H6 included: subjective sleepiness (Epworth), functional outcomes associated with sleepiness (FOSQ), vigilance (PVT), and blood pressure; and dependent variables to test H7 included measures of attitudes towards use (ATU), and visual analogue ratings of therapy. Appropriate post hoc tests were used to assess pair-wise difference where the main effect of treatment group was significant.
RESULTS
There were 168 randomized participants and 4 participants who were excluded from the intention-to-treat analysis since they withdrew from the trial prior to any study procedures. The demographic characteristics, including the diagnostic polysomnography data, of the 164 participants are depicted in Table 1; importantly, there were no significant differences among the 3 groups. Fourteen participants did not receive the therapy to which they were randomized, but were included in the intention-to-treat analysis. One participant among the 14 randomization errors received CPAPAPAP instead of A-Flex, and this participant was treated as a CPAPAPAP participant. The treatment received by the remaining 13 participants included multiple therapy modes. A total of 140 participants completed the study. Of those dropped or disqualified, the majority were dropped from the study per their request, with the remainder disqualified for various reasons (3 lost to follow-up, 1 due to shift work, 1 due to surgery for aortic insufficiency, and 1 for inability to adhere to the protocol).
Table 1.
Participant characteristics
| Characteristic | A-Flex (N = 54) | CPAPAPAP (N = 53) | CPAP (N = 57) | P |
|---|---|---|---|---|
| Age: mean ± SD | 49.1 ± 11.6 | 48.3 ± 10.0 | 48.8 ± 12.0 | 0.9 |
| (range) | (29-77) | (25-70) | (27-77) | |
| Gender: % Female (#) | 24.1% (13) | 24.5% (13) | 24.6% (14) | > 0.9 |
| Ethnicity: % (#) | 0.4 | |||
| Asian | 5.6% (3) | 1.9% (1) | 0% | |
| Black | 5.6% (3) | 15.1% (8) | 14.0% (8) | |
| Hispanic or Latino | 3.7% (2) | 1.9% (1) | 1.8% (1) | |
| White | 85.2% (46) | 79.2% (42) | 82.5% (47) | |
| Other | 0% | 1.9% (1) | 1.8% (1) | |
| Body Mass Index: kg/m2, mean ± SD | 33.0 ± 6.6 | 35.6 ± 8.3 | 34.9 ± 8.0 | 0.2 |
| (range) | (20.3-57.2) | (21.0-65.0) | (20.3-54.9) | |
| Neck Circumference: inches, mean ± SD | 16.5 ± 1.7 | 17.1 ± 3.9 | 16.6 ± 2.3 | 0.8 |
| (range) | (12.2-21.0) | (12.2-42.0) | (6.9-20.5) | |
| Systolic Blood Pressure: mm Hg, mean ± SD | 128.1 ± 14.0 | 127.9 ± 14.0 | 127.7 ± 16.0 | 0.9 |
| (range) | (100-180) | (100-160) | (84-165) | |
| Diastolic Blood Pressure: mm Hg, mean ± SD | 80.6 ± 9.3 | 79.1 ± 9.4 | 77.3 ± 9.8 | 0.2 |
| (range) | (60-100) | (56-103) | (51-107) | |
| Snoring: % (#) | 96.3% (52) | 100% (53) | 98.2% (56) | 0.5 |
| Nasal congestion: % (#) | 22.2% (12) | 28.3% (15) | 24.6% (14) | 0.7 |
| Nasal allergies: % (#) | 35.2% (19) | 35.8% (19) | 47% (27) | 0.3 |
| Bed partner: % (#) | 84.6% (44) | 82.7% (43) | 85.5% (47) | 0.9 |
| Education level: % (#) | > 0.9 | |||
| High school | 22.2% (12) | 18.9% (10) | 29.8% (17) | |
| College | 61.1% (33) | 67.9% (36) | 47.4% (27) | |
| Post-graduate | 16.7% (9) | 13.2% (7) | 22.8% (13) | |
| Apnea-Hypopnea Index*: mean ± SD | 36.87 ± 30.00 | 37.29 ± 31.10 | 41.08 ± 31.57 | 0.6 |
| (range) | (0.2-112) | (0.1-109.9) | (0.5-137.6) | |
| Apnea Index*: mean ± SD | 21.39 ± 23.34 | 22.90 ± 25.20 | 23.31 ± 22.79 | 0.8 |
| (range) | (0-106.1) | (0-89.7) | (0-86.3) | |
| Hypopnea Index*: mean ± SD | 15.45 ± 20.49 | 14.30 ± 18.07 | 17.73 ± 18.52 | 0.5 |
| (range) | (0-99.1) | (0-109.1) | (0-76) | |
| Average Oxygen Saturation*: %, mean ± SD | 93.70 ± 3.08 | 93.72 ± 3.02 | 93.82 ± 2.65 | 0.9 |
| (range) | (79-97) | (81-97) | (85-99) | |
| Arousal Index*: mean ± SD | 39.39 ± 24.33 | 40.65 ± 28.28 | 39.58 ± 22.12 | 0.9 |
| (range) | (9-107.4) | (1.9-123) | (1.9-123) | |
| Sleep Efficiency*: %, mean ± SD | 83.46 ± 12.65 | 79.89 ± 13.88 | 79.18 ± 13.57 | 0.2 |
| (range) | (46-98) | (43-96) | (42-98) | |
| % Slow Wave Sleep*: mean ± SD | 6.30 ± 8.08 | 7.28 ± 9.82 | 6.47 ± 9.42 | 0.8 |
| (range) | (0-29) | (0-45) | (0-38) | |
| % REM Sleep*: mean ± SD | 14.48 ± 9.62 | 14.79 ± 10.20 | 13.05 ± 8.95 | 0.7 |
| (range) | (0-40) | (0-37) | (0-31) |
Diagnostic polysomnography data.
Aim 1 (Efficacy)
H1: There were no significant differences between treatment groups for key PSG variables on the first laboratory PSG night at the initiation of therapy, with the exception of AHI and average oxygen saturation (Table 2). For AHI, the values for the A-Flex group were significantly higher than those for CPAP and CPAPAPAP groups; however, there were no significant differences in AHI between CPAP and CPAPAPAP groups. For average oxygen saturation, the values for the CPAP group were significantly higher than those for A-Flex and CPAPAPAP groups; however, there were no significant differences in average oxygen saturation between A-Flex and CPAPAPAP groups.
Table 2.
Polysomnography data at PAP titration PSG and at 180 days*
| Measure | PAP Titration PSG |
180 Days |
||||||
|---|---|---|---|---|---|---|---|---|
| A-Flex (N = 54) | CPAPAPAP(N = 53) | CPAP (N = 57) | P | A-Flex (N = 46) | CPAPAPAP(N = 47) | CPAP (N = 47) | P | |
| Apnea-hypopnea index | 6.09 ± 7.20 (0-39.6) | 3.54 ± 4.39 (0-24.8) | 3.24 ± 3.46 (0-14.9) | 0.02 | 1.26 ± 2.92 (0-19.3) | 0.67 ± 0.93 (0-4.3) | 1.04 ± 1.28 (0-5.2) | 0.3 |
| Average oxygen saturation, % | 95.70 ± 1.38 (92-98) | 95.74 ± 1.35 (92-98) | 96.46 ± 1.52 (93-100) | 0.01 | 95.65 ± 1.34 (92-98) | 96.21 ± 1.32 (92-98) | 96.09 ± 1.60 (92-98) | 0.08 |
| Arousal index | 18.05 ± 8.59 (5-41.4) | 16.57 ± 8.05 (5.4-36.4) | 17.61 ± -9.72 (3-55) | 0.7 | 20.43 ± 10.31 (3.5-50.6) | 17.43 ± 7.94 (5.2-38) | 17.18 ± 10.22 (5-53) | 0.2 |
| Sleep efficiency, % | 83.19 ± 10.65 (51-98) | 83.98 ± 10.64 (52-100) | 83.18 ± 11.80 (44-98) | 0.9 | 85.26 ± 9.59 (57-98) | 86.89 ± 10.17 (57-99) | 87.49 ± 9.78 (52-98) | 0.3 |
| % Slow wave sleep | 8.98 ± 10.41 (0-43) | 9.87 ± 9.49 (0-34) | 10.14 ± 9.63 (0-41) | 0.7 | 8.41 ± 7.12 (0-27) | 8.87 ± 9.48 (0-35) | 7.83 ± 8.90 (0-41) | 0.7 |
| % REM sleep | 20.65 ± 8.31 (1-42) | 23.40 ± 7.99 (7-45) | 22.84 ± 8.41 (3-43) | 0.2 | 20.70 ± 6.71 (3-37) | 22.15 ± 6.38 (8-32) | 20.87 ± 8.22 (0-39) | 0.6 |
Mean ± standard deviation (range).
H2: Those in the A-Flex group did not demonstrate significantly lower residual apnea, higher oxygen saturation, or improvement in key PSG variables compared to those assigned to the CPAP and CPAPAPAP groups at 180 days (Table 2).
Aim 2 (Adherence)
There were no significant differences found between treatment groups.
H3: There were no significant differences in the dropout rate between treatment groups at 90 or 180 days (Table 3).
Table 3.
Adherence: cumulative dropout rate*
| A-Flex (N = 54) | CPAPAPAP(N = 53) | CPAP (N = 57) | P Value | |
|---|---|---|---|---|
| Adherence at 90 Days | ||||
| Mean < 2 h per night | 11% (6) | 8% (4) | 13% (7) | 0.7 |
| Median < 2 h per night | 11% (6) | 8% (4) | 13% (7) | 0.7 |
| Mean < 1 h per night | 0% | 2% (1) | 5% (3) | - |
| Adherence at 180 Days | ||||
| Mean < 2 h per night | 15% (8) | 8% (4) | 16% (9) | 0.4 |
| Median < 2 h per night | 17% (9) | 9% (5) | 20% (11) | 0.3 |
| Mean < 1 h per night | 2% (1) | 2% (1) | 7% (4) | 0.4 |
Percent (number).
H4: There were no significant differences in PAP adherence between treatment groups at 90 or 180 days (Table 4).
Table 4.
Adherence: mean hours of PAP use per night*
| Duration | A-Flex (N = 54) | CPAPAPAP(N = 53) | CPAP (N = 57) | P |
|---|---|---|---|---|
| 90 Days† | 4.64 ± 1.89 (1.5-7.5) | 4.72 ± 1.64 (0.03-7.4) | 4.63 ± 1.88 (0.4-8.3) | > 0.9 |
| 180 Days | 4.44 ± 1.98 (0.9-7.3) | 4.63 ± 1.75 (0.03-7.85) | 4.40 ± 2.02 (0.4-8.2) | 0.8 |
Mean ± standard deviation (range).
90 days includes days 2 to 90 and 180 days includes days 2 to 180.
Aim 3 (Outcomes)
H5 and H6:
Epworth Sleepiness Scale (ESS): There were no significant differences across treatment groups in change of ESS scores from baseline (PAP Titration PSG) at 30, 90, and 180 days, with the following mean (± SD) scores at baseline and at 180 days: A-Flex (baseline = 10.43 ± 5.25, 180 days = 6.93 ± 4.30), CPAPAPAP (baseline = 10.49 ± 4.59, 180 days = 7.02 ± 4.66), and CPAP (baseline = 12.27 ± 5.94, 180 days = 7.87 ± 5.35), P = 0.8.
Functional Outcomes of Sleep Questionnaire (FOSQ) Global: There were no significant differences across treatment groups in change of FOSQ Global Scores from baseline (PAP Titration PSG) at 30, 90, and 180 days, with the following mean (± SD) scores at baseline and at 180 days: A-Flex (baseline = 15.17 ± 3.13, 180 days = 17.11 ± 2.22), CPAPAPAP (baseline = 15.85 ± 2.94, 180 days = 17.78 ± 2.06), and CPAP (baseline = 14.57 ± 3.97, 180 days = 16.74 ± 3.26), P = 0.9. Similarly, there were no significant treatment group differences in the change from baseline at 30, 90, and 180 days for FOSQ Activity, Vigilance, Relationships, Productivity, or Social Scores.
Psychomotor Vigilance Task (PVT): There were no significant differences across treatment groups in change of PVT mean reaction time from baseline (PAP Titration PSG) at 30, 90, and 180 days. The following mean (± SD) times were obtained for baseline and at 180 days: A-Flex (baseline = 276.75 ± 48.81 msec, 180 days = 263.09 ± 41.33 msec), CPAPAPAP (baseline = 285.70 ± 89.66 msec, 180 days = 257.46 ± 40.64 msec), and CPAP (baseline = 287.59 ± 68.25 msec, 180 days = 256.67 ± 30.07 msec), P = 0.5. Similarly, there were no significant differences across treatment groups in change of PVT lapses from baseline at 30, 90, and 180 days. The following mean (± SD) number of lapses were obtained for baseline and at 180 days: A-Flex (baseline = 2.15 ± 3.74, 180 days = 0.98 ± 2.14), CPAPAPAP (baseline = 2.15 ± 4.10, 180 days = 1.30 ± 3.32), and CPAP (baseline = 3.41 ± 7.75, 180 days = 0.96 ± 1.32), P = 0.6. The data presented in this section are for the evening PVT administration; however, there were similarly no significant differences for mean reaction time or number of lapses across treatment groups collected during the morning PVT administration.
Blood Pressure: There were no significant differences across treatment groups in change of systolic or diastolic blood pressure from baseline (PAP Titration PSG) at 30, 90, and 180 days, with the following mean (± SD) systolic/diastolic pressures (mm Hg) at baseline and at 180 days: A-Flex (baseline = 128.1 ± 14.0/80.6 ± 9.3, 180 days = 127.5 ± 13.4/78.4 ± 9.4), CPAPAPAP (baseline = 127.9 ± 14.0/79.1 ± 9.4, 180 days = 128.3 ± 12.9/80.8 ± 8.2), and CPAP (baseline = 127.7 ± 16.0/77.3 ± 9.8, 180 days = 130.2 ± 14.9/80.6 ± 9.6), Psystolic = 0.7, Pdiastolic = 0.07.
H7: The dependent variables evaluated were ATU A (Attitudes Towards Use − Confidence) and ATU B+C (Attitudes Towards Use − Expectations and Importance). There were statistically significant differences in ATU A; at baseline (PAP Titration PSG), the CPAP group exhibited significantly better ATU A values than the A-Flex group (22.30 ± 2.80 vs. 20.54 ± 3.60, P = 0.02). At the 30-day visit, the CPAPAPAP group exhibited significantly better ATU A values than the A-Flex group (22.41 ± 2.63 vs. 20.64 ± 4.24, P = 0.04). These differences disappeared at the 90 and 180 day visits. There were no significant differences between groups for ATU B+C at any time point.
We compared subjective ratings for sleep quality, mask comfort, and treatment satisfaction and benefit for participants across treatment groups (Table 5). There were no significant differences between groups for ratings of sleep quality and mask comfort at 30, 90, and 180 days. However, for subjective ratings of treatment satisfaction, A-Flex scores were significantly worse than CPAPAPAP and CPAP scores at 30 and 180 days. For subjective ratings of treatment benefit, at 90 and 180 days, A-Flex scores were significantly worse than CPAPAPAP.
Table 5.
Visual analog scale (VAS) ratings for sleep quality, mask comfort, and treatment satisfaction and benefit
| Visit Interval | A-Flex |
CPAPAPAP |
CPAP |
P | |||
|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
| Sleep Quality1 | |||||||
| 30 Days | 53 | 60.4 ± 21.1 | 51 | 63.9 ± 17.3 | 53 | 66.6 ± 16.7 | 0.2 |
| 90 Days | 51 | 61.2 ± 23.1 | 46 | 70.2 ± 16.1 | 51 | 64.3 ± 18.7 | 0.053 |
| 180 Days | 46 | 64.7 ± 18.7 | 47 | 72.8 ± 15.1 | 47 | 66.5 ± 21.1 | 0.056 |
| Satisfaction2 | |||||||
| 30 Days | 53 | 60.1 ± 19.7 | 51 | 67.5 ± 18.4 | 53 | 69.4 ± 16.5 | 0.03 |
| 90 Days | 51 | 65.4 ± 22.0 | 46 | 69.8 ± 17.1 | 51 | 69.7 ± 17.4 | 0.3 |
| 180 Days | 46 | 63.5 ± 22.0 | 47 | 71.0 ± 16.2 | 47 | 71.6 ± 16.1 | 0.03 |
| Comfort3 | |||||||
| 30 Days | 53 | 50.8 ± 21.5 | 51 | 57.9 ± 20.5 | 53 | 53.9 ± 18.9 | 0.2 |
| 90 Days | 51 | 53.8 ± 25.1 | 46 | 61.0 ± 15.0 | 51 | 59.2 ± 18.3 | 0.2 |
| 180 Days | 46 | 54.7 ± 21.7 | 47 | 60.1 ± 14.7 | 47 | 60.6 ± 17.9 | 0.1 |
| Benefit4 | |||||||
| 30 Days | 53 | 66.3 ± 24.6 | 51 | 70.4 ± 16.9 | 53 | 73.1 ± 16.2 | 0.2 |
| 90 Days | 51 | 68.0 ± 24.5 | 46 | 77.0 ± 13.2 | 51 | 71.9 ± 16.2 | 0.046 |
| 180 Days | 46 | 70.8 ± 24.2 | 47 | 79.3 ± 10.5 | 47 | 77.4 ± 15.6 | 0.02 |
No VAS was obtained at baseline (PAP Titration PSG).
Question: “In the last month, how well did you sleep?” 0 = poorly to 100 = very well.
Question: “How do you like your therapy?” 0 = very unlikable to 100 = very likable.
Question: “In the last month, how do you rate the overall comfort of the mask?” 0 = very uncomfortable to 100 = very comfortable.
Question: “How do you rate the benefit of your treatment?” 0 = no benefit to 100 = large benefit.
Post Hoc Analyses
Poor PAP adherers
Participants who had poor adherence soon after starting treatment, (< 5 h of PAP use in the first 7 days) were analyzed to assess changes in the dropout rate and mean hours of adherence at 90 and 180 days between treatment arms. No significant differences were observed between groups for these measures (Table 6).
Table 6.
Post hoc adherence data excluding good early adherers (≥ 5 hours in week 1)
| Adherence Excluding Good Early Adherers | A-Flex | CPAPAPAP | CPAP | P Value |
|---|---|---|---|---|
| Number (% Total) for Those < 5 hours in Week 1 | 26 (48%) | 20 (41%) | 22 (41%) | |
| Adherence: Mean Hours of PAP Use per Night, Mean ± SD | ||||
| 90 Days* | 3.21 ± 1.39 | 3.46 ± 1.34 | 3.49 ± 1.85 | 0.8 |
| 180 Days* | 3.05 ± 1.55 | 3.48 ± 1.46 | 3.18 ± 2.00 | 0.7 |
| Adherence: Dropout Rate 90 days, Percent (Number) | ||||
| Mean < 2 h per night | 23% (6) | 15% (3) | 23% (5) | 0.8 |
| Median < 2 h per night | 23% (6) | 15% (3) | 27% (6) | 0.7 |
| Mean < 1 h per night | 0% | 5% (1) | 14% (3) | 0.1 |
| Adherence: Dropout Rate 180 days, Percent (Number) | ||||
| Mean < 2 h per night | 31% (8) | 15% (3) | 32% (7) | 0.4 |
| Median < 2 h per night | 35% (9) | 15% (3) | 41% (9) | 0.2 |
| Mean < 1 h per night | 4% (1) | 5% (1) | 18% (4) | 0.3 |
90 days includes days 2-90 and 180 days includes days 2–180.
Oxygen saturation
We evaluated the effects of the treatments on other measures of oxygen saturation: time spent below 90% and minimum oxygen saturation. At baseline (PAP Titration PSG), the A-Flex group had a significantly (P = 0.0001) longer time below 90% (1.55 ± 3.70 min) than the CPAPAPAP (0.55 ± 1.72 min) and CPAP (0.37 ± 1.10 min) groups; however, there were no significant differences between groups at 180 days. At the baseline visit, the A-Flex group had a significantly (P < 0.0001) lower oxygen saturation (87.41% ± 4.15%) than the CPAPAPAP (89.38% ± 3.69%) and CPAP (90.86% ± 3.45%). At 180 days, the A-Flex group had a significantly (P = 0.03) lower oxygen saturation (89.13% ± 3.41%) than the CPAPAPAP (90.57% ± 3.92%) group, but not the CPAP (90.06% ± 3.82%) group.
Pressure
Average pressures were obtained for each participant; for CPAPAPAP and A-Flex groups the 90% pressure was used to calculate average pressure. There were no significant differences in pressure between groups, with the exception of the CPAP and CPAPAPAP groups at 180 days (Table 7). It is important to note that the pressure in the CPAPAPAP group was derived from the APAP titration study at 14 days; however, the difference between the CPAP and CPAPAPAP groups was not apparent at either 14 or 90 days. As discussed in the Methods section, during the follow-up visits, the pressure could be increased for snoring or reduced for pressure intolerance; the pressure was unchanged for A-Flex group participants, but was reduced for 1 participant in each of the CPAPAPAP and CPAP groups.
Table 7.
Pressure and leak data for treatment groups
| A-Flex | CPAPAPAP | CPAP | P Value | |
|---|---|---|---|---|
| Pressure | ||||
| Titration | 10.7 ± 2.8 (54) | 10.6 ± 3.0 (53) | 10.8 ± 2.7 (57) | 0.8 |
| 14 Days | 10.8 ± 3.0 (54) | 9.7 ± 3.0 (52) | 10.6 ± 2.7 (55) | 0.06 |
| 90 Days | 10.2 ± 2.9 (49) | 9.7 ± 2.8 (46) | 10.4 ± 2.6 (51) | 0.2 |
| 180 Days | 9.9 ± 3.0 (44) | 9.6 ± 2.8 (46) | 10.7 ± 2.3 (44) | 0.04* |
| Average Leak Values (l/min) | ||||
| 90 Days | 32.0 ± 10.4 (54) | 36.8 ± 7.2 (53) | 38.3 ± 12.6 (56) | 0.005† |
| 180 Days | 30.3 ± 10.8 (54) | 35.4 ± 7.8 (53) | 36.5 ± 12.8 (56) | 0.007† |
| Average Minutes of Large Leak (Per Day) | ||||
| 90 Days | 4.8 ± 8.5 (54) | 5.5 ± 7.9 (53) | 13.2 ± 29.3 (56) | 0.2 |
| 180 Days | 5.1 ± 8.6 (54) | 5.0 ± 7.4 (53) | 13.5 ± 30.4 (56) | 0.3 |
Numbers in parentheses indicate numbers of participants.
CPAPAPAP and CPAP are significantly different.
A-Flex shows significantly lower average leak values as compared to both CPAPAPAP and CPAP at 90 and 180 days.
Leak values
Average leak values were first calculated per participant over all days of data collection; participants in the A-Flex group showed less average leak at 90 and 180 days compared to those in the CPAP and CPAPAPAP groups (Table 7). However, there were no significant differences in minutes of large leak, which were collected and recorded daily, and then calculated by average value over the time period within participant (Table 7).
Safety Analyses
There were 22 adverse events (AEs) in the A-Flex group, 12 in the CPAPAPAP group, and 14 in the CPAP group, all of which were self-limited and resolved by the end of the study. There were no serious AEs (SAEs), and the number (and incidence rates) of the most commonly reported AEs were cold/flu = 6 (0.046), sinus infection = 4 (0.031), and headache/migraine = 3 (0.023). No adverse events appeared to be device related.
DISCUSSION
This is the first study to compare CPAP and A-Flex in a systematic and controlled manner. However, several studies have compared CPAP vs. variable pressure (APAP),1–12 including a meta-analysis,23 and these studies have not shown significant advantages or benefits of APAP over CPAP, except for less subjective sleepiness and greater PAP use on APAP observed in one study.12 Some investigators have shown that for CPAP > 10 cm H2O24 and for those with high within-night pressure variability,7 variable pressure devices may deliver better patient outcomes.24 Additionally, one study showed significantly improved adherence, excessive daytime sleepiness, and several FOSQ domains in patients after 15 days and 10 weeks of Auto-BPAP use (with expiratory pressure relief) as a rescue therapy for optimally treated OSA patients with poor adherence to CPAP; however, there were no differences in respiratory or sleep parameters between groups.25
Additionally, there have been studies that have compared CPAP and C-Flex (Philips Respironics, Murrysville, PA), which is a comfort feature of PAP delivery, similar to that of A-Flex, in that it reduces the pressure at the start of exhalation and increases the pressure to therapeutic level for the latter part of exhalation and subsequent inhalation.26 C-Flex did not show significant advantages or benefits over CPAP,26–32 except for higher PAP adherence in one study.27 In a recently completed three-month, double-blinded, parallel-arm randomized controlled trial comparing CPAP vs. C-Flex in 76 patients with severe OSA, the use of C-Flex did not result in greater adherence, and neither treatment appeared superior. However, both CPAP and C-Flex resulted in substantial improvements in sleepiness, vigilance, and quality of life.26
In the present study, indices of sleep disordered breathing (AHI, average and minimum oxygen saturation, time spent below 90%) were significantly worse for A-Flex compared to CPAP at baseline (PAP Titration PSG); however, after 180 days of use, there were no significant differences between A-Flex and CPAP groups on these measures (except for minimum oxygen saturation) as well as other indices of sleep quality and quantity. This indicates that long-term use of A-Flex is comparable to that of CPAP with respect to control of sleep disordered breathing and improvement in measures of sleep. The higher AHI reported in this study at baseline for A-Flex vs. CPAP was also observed after 6 weeks of APAP vs. CPAP use in a separate study.12 Contrary to our study, APAP was found to deliver a significantly lower 95th percentile pressure than CPAP; however, the patient population differed from ours in that they were morbidly obese.33 A-Flex did have a significant advantage over CPAP with respect to average leak values (but not in the average of minutes of large leak), after both 3 months and 6 months. Paradoxically, this decrease in leak values was not reflected in measures of differences between groups in severity of sleep disordered breathing.
There were no significant differences between improvement in subjective sleepiness, objective vigilance, blood pressure, and quality of life during the study for participants in A-Flex vs. the CPAP groups (i.e., CPAP and CPAPAPAP groups). These results are similar to those reported in prior studies comparing APAP and CPAP,1–12 though some endpoints like blood pressure have not been comprehensively studied.34 Besides these outcomes, there were no major efficacy, adherence, and other outcome differences between A-Flex and the CPAP groups. The main protocol difference between the CPAP and CPAPAPAP groups was that the CPAPAPAP group was switched to CPAP 14 days following the APAP titration study. Given that the participants in the CPAPAPAP group spent the majority of their time on a single fixed pressure, it is not surprising that there were no major differences in the study results between the CPAP and CPAPAPAP groups. Nevertheless, this study reveals that comparable data from efficacy, adherence, and outcome measures can be obtained regardless if patients are titrated by either APAP or CPAP titration PSG.
Initially, participants using CPAP had significantly more positive attitudes toward their treatment compared to those using A-Flex at baseline (PAP Titration PSG) and at 30 days, but these differences were not apparent at later visits, and there were no significant differences for attitudes toward use (expectations and importance) between the groups. Participant ratings for A-Flex on treatment satisfaction and benefit were significantly worse compared to those for CPAP, but there were no significant differences between groups for sleep quality and mask comfort. The reasons for these differences are unclear, and contrary to expectations.
The level of treatment adherence is an important factor for studies on CPAP; other studies have demonstrated that CPAP-related education and close monitoring of participants with OSA significantly improved CPAP adherence.35 Use of A-Flex did not convey advantages over CPAP in adherence; participants were equally adherent to A-Flex and CPAP devices with respect to mean hours of PAP use per night and dropout rate throughout the study. No significant changes were observed when poor, early adherers were compared between groups for these measures of adherence. There were no differences between groups for adherence outcomes, and the close monitoring of participants throughout the study may have played a major role in this level of adherence among all participants.
Limitations of this study included the relatively restricted battery of tests that could be accommodated during the participant visits as a tradeoff to minimize participant burden. Besides the psychomotor vigilance task (PVT), other objective measures of vigilance/alertness (e.g., maintenance of wakefulness test [MWT]) could have been assessed; however, studies comparing CPAP and C-Flex failed to find a difference between groups for both the PVT and MWT. Lastly, this study does not necessarily reflect routine clinical practice due to the close monitoring and comprehensive nature of the follow-up of participants in this study; these factors may have played important roles in the lack of differences between groups.
In conclusion, this randomized controlled trial demonstrated APAP with A-Flex did not show differences in efficacy, adherence, or functional outcomes, with the exception of average leak values, compared to CPAP for up to 6 months. Overall, these findings are rather perplexing in light of anecdotal clinical experience. Given this era of emphasis on comparative effectiveness research, these findings nonetheless question the use of the newer, potentially more expensive A-Flex device over regular CPAP. However, there does exist evidence that selected populations might benefit from A-Flex who were not tested in the current study7,24,33,36; in the future, populations that might be considered for further study include those who develop early pressure intolerance, have more severe OSA,36 are morbidly obese,33 require higher pressures,24 or have high within-night pressure variability.7 Until that time, since there was no indication that either treatment was superior, patient and prescribing physician preference will undoubtedly continue to dictate choice of use for these devices.
DISCLOSURE STATEMENT
Philips Respironics provided funding for this study; Drs. Kushida, Berry, Blau, Fietze, Kryger, Kuna, Pegram, and Penzel received research support for the conduct of this study through contracts between Philips Respironics and their respective institutions. Ms. Crabtree received consulting fees for statistical data analysis from Philips Respironics. Dr. Kushida has received research support from Philips Respironics, ResMed, Ventus Medical, and Pacific Medico. Dr. Berry has received research support from Philips Respironics, ResMed, and Ventus Medical. Dr. Blau has received research support from Philips Respironics, Breas, and Hoffrichter) Dr. Fietze has received research support from Philips Respironics, ResMed, Advanced Sleep Research, Breas, Hoffrichter, and Weinmann. Dr. Kryger has received research support from Ventus, and ResMed. Dr. Penzel has received research support from Philips Respironics, ResMed, Advanced Sleep Research, Breas, Hoffrichter, Somnomedics, and Weinmann.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of Mark Aloia, PhD, Bill Hardy, Donna O'Malley, Kelli Moffa, and Susan Corgnati from Philips Respironics. The following research staff worked diligently to ensure the successful completion of the study: Chia-Yu Cardell (Stanford); Susan Purdy (University of Florida); Polina Dimitrova, Sandra Zimmermann, and Beate Diecker (Charité); Val Assalone, Gary Lavalette, and Laurie Skinger (Gaylord Hospital); Bethany Staley and Haideliza Soto-Calderon (University of Pennsylvania); Krager Vaughn, Wes Booth, and Chris Jeter (Sleep Disorders Center of Alabama)
REFERENCES
- 1.Hudgel DW, Fung C. A long-term randomized, cross-over comparison of auto-titrating and standard nasal continuous airway pressure. Sleep. 2000;23:645–8. [PubMed] [Google Scholar]
- 2.Hukins C. Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep. 2004;27:1512–7. doi: 10.1093/sleep/27.8.1512. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SF, Love L, Burt H, Fleetham JA. A randomized trial of auto-titrating CPAP and fixed CPAP in the treatment of obstructive sleep apnea-hypopnea. Respir Med. 2004;98:330–3. doi: 10.1016/j.rmed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Marrone O, Resta O, Salvaggio A, Giliberti T, Stefano A, Insalaco G. Preference for fixed or automatic CPAP in patients with obstructive sleep apnea syndrome. Sleep Med. 2004;5:247–51. doi: 10.1016/j.sleep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Mulgrew AT, Cheema R, Fleetham J, Ryan CF, Ayas NT. Efficacy and patient satisfaction with autoadjusting CPAP with variable expiratory pressure vs standard CPAP: a two-night randomized crossover trial. Sleep Breath. 2007;11:31–7. doi: 10.1007/s11325-006-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolan GM, Doherty LS, Mc Nicholas WT. Auto-adjusting versus fixed positive pressure therapy in mild to moderate obstructive sleep apnoea. Sleep. 2007;30:189–94. doi: 10.1093/sleep/30.2.189. [DOI] [PubMed] [Google Scholar]
- 7.Noseda A, Kempenaers C, Kerkhofs M, Braun S, Linkowski P, Jann E. Constant vs auto-continuous positive airway pressure in patients with sleep apnea hypopnea syndrome and a high variability in pressure requirement. Chest. 2004;126:31–7. doi: 10.1378/chest.126.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Nussbaumer Y, Bloch KE, Genser T, Thurnheer R. Equivalence of autoadjusted and constant continuous positive airway pressure in home treatment of sleep apnea. Chest. 2006;129:638–43. doi: 10.1378/chest.129.3.638. [DOI] [PubMed] [Google Scholar]
- 9.Randerath WJ, Schraeder O, Galetke W, Feldmeyer F, Ruhle KH. Autoadjusting CPAP therapy based on impedance efficacy, compliance and acceptance. Am J Respir Crit Care Med. 2001;163:652–7. doi: 10.1164/ajrccm.163.3.2006168. [DOI] [PubMed] [Google Scholar]
- 10.Resta O, Carratu P, Depalo A, et al. Effects of fixed compared to automatic CPAP on sleep in obstructive sleep apnoea syndrome. Monaldi Arch Chest Dis. 2004;61:153–6. doi: 10.4081/monaldi.2004.694. [DOI] [PubMed] [Google Scholar]
- 11.Senn O, Brack T, Matthews F, Russi EW, Bloch KE. Randomized short-term trial of two autoCPAP devices versus fixed continuous positive airway pressure for the treatment of sleep apnea. Am J Respir Crit Care Med. 2003;168:1506–11. doi: 10.1164/rccm.200304-542OC. [DOI] [PubMed] [Google Scholar]
- 12.Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS) Sleep. 2010;33:267–71. doi: 10.1093/sleep/33.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McArdle N, Singh B, Murphy M, et al. Continuous positive airway pressure titration for obstructive sleep apnoea: automatic versus manual titration. Thorax. 2010;65:606–11. doi: 10.1136/thx.2009.116756. [DOI] [PubMed] [Google Scholar]
- 14.Fietze I, Glos M, Moebus I, Witt C, Penzel T, Baumann G. Automatic pressure titration with APAP is as effective as manual titration with CPAP in patients with obstructive sleep apnea. Respiration. 2007;74:279–86. doi: 10.1159/000100364. [DOI] [PubMed] [Google Scholar]
- 15.Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Fietze I, Penzel T, Alonderis A, et al. Management of obstructive sleep apnea in Europe. Sleep Med. 2011;12:190–197. doi: 10.1016/j.sleep.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 20.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. [Google Scholar]
- 21.Stepnowsky CJ, Jr, Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med. 2002;3:239–47. doi: 10.1016/s1389-9457(01)00162-9. [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan SF American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 23.Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep. 2004;27:249–53. doi: 10.1093/sleep/27.2.249. [DOI] [PubMed] [Google Scholar]
- 24.Massie CA, McArdle N, Hart RW, et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167:20–3. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 25.Gentina T, Fortin F, Douay B, et al. Auto bi-level with pressure relief during exhalation as a rescue therapy for optimally treated obstructive sleep apnoea patients with poor compliance to continuous positive airways pressure therapy-a pilot study. Sleep Breath. 2010 doi: 10.1007/s11325-009-0322-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 26.Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep. 2010;33:523–9. doi: 10.1093/sleep/33.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130:1018–24. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 29.Wenzel M, Kerl J, Dellweg D, Barchfeld T, Wenzel G, Kohler D. [Expiratory pressure reduction (C-Flex Method) versus fix CPAP in the therapy for obstructive sleep apnoea] Pneumologie. 2007;61:692–5. doi: 10.1055/s-2007-980075. [DOI] [PubMed] [Google Scholar]
- 30.Dolan DC, Okonkwo R, Gfullner F, Hansbrough JR, Strobel RJ, Rosenthal L. Longitudinal comparison study of pressure relief (C-Flex) vs. CPAP in OSA patients. Sleep Breath. 2009;13:73–7. doi: 10.1007/s11325-008-0199-1. [DOI] [PubMed] [Google Scholar]
- 31.Marshall NS, Neill AM, Campbell AJ. Randomised trial of compliance with flexible (C-Flex) and standard continuous positive airway pressure for severe obstructive sleep apnea. Sleep Breath. 2008;12:393–6. doi: 10.1007/s11325-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 32.Leidag M, Hader C, Keller T, Meyer Y, Rasche K. Mask leakage in continuous positive airway pressure and C-Flex. J Physiol Pharmacol. 2008;59(Supp6):401–6. [PubMed] [Google Scholar]
- 33.Bakker J, Campbell A, Neill A. Randomised controlled trial of auto-adjusting positive airway pressure in morbidly obese patients requiring high therapeutic pressure delivery. J Sleep Res. 2011;20:233–40. doi: 10.1111/j.1365-2869.2010.00846.x. [DOI] [PubMed] [Google Scholar]
- 34.Patruno V, Aiolfi S, Costantino G, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131:1393–9. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 35.Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep. 1997;20:284–9. doi: 10.1093/sleep/20.4.284. [DOI] [PubMed] [Google Scholar]
- 36.So YK, Dhong HJ, Kim HY, Chung SK, Jang JY. Initial adherence to autotitrating positive airway pressure therapy: influence of upper airway narrowing. Clin Exp Otorhinolaryngol. 2009;2:181–5. doi: 10.3342/ceo.2009.2.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]