Abstract
Study Objectives:
Sleep problems are a common phenomenon in most neurological and psychiatric diseases. In Parkinson disease (PD), for instance, sleep problems may be the most common and burdensome non-motor symptoms in addition to the well-described classical motor symptoms. Since sleep disturbances generally become apparent in the disease before motor symptoms emerge, they may represent early diagnostic tools and a means to investigate early mechanisms in PD onset. The sleep disturbance, REM sleep behavior disorder (RBD), precedes PD in one-third of patients. We therefore investigated sleep changes in marmoset monkeys treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP), the non-human primate model for idiopathic PD.
Design:
Mild parkinsonism was induced in 5 marmoset monkeys (3M/2F) over a 2-week period of subchronic MPTP treatment. Electroencephalograms (EEGs) and electromyograms (EMGs) were recorded weekly. Motor activity and hand-eye coordination were also measured weekly, and any signs of parkinsonism were noted each day. Sleep parameters, motor activity, and performance data before and after MPTP treatment were compared between MPTP-treated marmosets and 4 control marmosets (1M/3F).
Results:
MPTP increased the number of sleep epochs with high-amplitude EMG bouts during REM sleep relative to control animals (mean ± SEM percentage of REM 58.2 ± 9.3 vs. 29.6 ± 7.7; P < 0.05). Of all sleep parameters measured, RBD-like measures discriminated best between MPTP-treated and control animals. On the other hand, functional motor behavior, as measured by hand-eye coordination, was not affected by MPTP treatment (correct trials MPTP: 23.40 ± 3.56 vs. control: 36.13 ± 5.88 correct trials; P = 0.32).
Conclusions:
This REM sleep-specific change, in the absence of profound changes in wake motor behaviors, suggests that the MPTP marmoset model of PD could be used for further studies into the mechanisms and treatment of RBD and other sleep disorders in premotor symptom PD.
Citation:
Verhave PS; Jongsma MJ; Van den Berg RM; Vis JC; Vanwersch RAP; Smit AB; Van Someren EJW Philippens IHCHM. REM sleep behavior disorder in the marmoset MPTP model of early Parkinson disease. SLEEP 2011;34(8):1119-1125.
Keywords: Parkinson's Disease, marmoset, REM Sleep Behavior Disorder (RBD), EEG, muscle tone, atonia
INTRODUCTION
REM sleep behavior disorder (RBD) is characterized by increased muscle activity during REM sleep, which can lead to injury either to oneself or to a bed partner. The core symptom of RBD, namely the lack of normal muscle atonia during REM sleep,1,2 can emerge in 2 ways: (1) tonic muscle activity characterized by ≥ 50% muscle activity in a 30-sec REM sleep epoch or (2) phasic muscle activity and twitches within 3-sec epochs.3 RBD is a disorder of considerable interest for understanding early pathological processes in Parkinson disease (PD). At least one-third of PD patients have increased and irregular chin muscle tone during REM sleep,1,4 and many meet the criteria for RBD. While the clinical diagnosis of PD is primarily based on overt motor symptoms, these emerge relatively late in the course of the underlying neurodegenerative process. It has been estimated that ≥ 60% of the dopamine (DA) neurons are lost by the time motor symptoms emerge. In order to investigate early pathological processes of the disease and to develop early treatment approaches, the stages that precede the motor phase of PD need to be identified. Reported symptoms preceding the motor phase of PD include reduced ability to smell and taste; alterations in mood and autonomic function; and most notably, disturbed sleep.5,6 For instance, complaints of insomnia are reported in approximately 80% of all PD patients,7,8 and excessive movement during sleep frequently occurs in PD patients.9 More importantly, disturbances in sleep usually begin years before PD is diagnosed.6,10,11 RBD is a key symptom during the early phases of PD, since a third of all patients initially diagnosed with RBD are later diagnosed with PD 3 to 13 years after the initial RBD diagnosis.4,12,13 Moreover, in 40% of all RBD cases reported eventually go on to develop a neurological disorder, most notably PD.14
Because the etiology of idiopathic PD still remains largely unknown, research into the immediate pathogenic mechanisms of the disease relies heavily on appropriate animal models that mimic certain manifestations of PD. The neurotoxic compound 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP) specifically targets DA neurons of the substantia nigra via uptake by the DA transporter, where it causes cell death by compromising the mitochondrial energy supply system of these DA neurons. The marmoset MPTP model, in this regard, has been the most valuable preclinical model for mimicking core symptoms of PD. The “clinical” condition of the parkinsonian state in this model is generally based on assessments and observations while the monkey is awake, for which a large number of quantitative measures have been developed and validated.15–19 However, it is not known whether abnormalities during sleep are present prior to the pronounced wake motor disturbances in animal models of the disease. Because of the striking similarity in sleep macrostructure between marmoset monkeys and humans, a demonstration of early abnormalities during sleep in the marmoset MPTP model would be of significant value as potential biomarkers for the early stage of idiopathic PD. Therefore, we investigated the effects of MPTP treatment on sleep architecture in marmoset monkeys, with special attention to RBD-like changes in muscle tone during REM sleep. In order to evaluate these changes relative to the emergence of wake and sleep symptoms, the development of wake motor symptoms was quantified as well.
MATERIAL AND METHODS
Animals
Common marmoset monkeys (Callithrix jacchus) (5M/4F) between 2 and 3 years of age were obtained from the Biomedical Primate Research Centre (BPRC), Rijswijk, the Netherlands. They were housed in individual primate cages (61 × 61 × 41 cm) under controlled humidity (60%), temperature (23-25°C) and lighting (12-h light/dark cycles). Marmosets were fed daily with pellet chow. Diet was enriched with peanuts, fruit and vegetables, raisins, sunflower seeds and an occasional grasshopper. Water was available ad libitum. All marmosets were provided with enriched-varying cage environments. Protocols were reviewed by the Ethical Review Committee on Experimental Animals at TNO prior to the start of experiments. All animals were observed closely throughout the course of the experiments. General welfare and changes in body weight were monitored twice a day.
Surgical Procedures
Two stainless steel electroencephalogram (EEG) electrodes were placed into the skull, one above and the other 5 mm anterior to the intra-aural line, and both were placed on the right hemisphere, 2 mm from the sutura sagittalis, leaving the dura mater intact. Surgery was performed under isoflurane/O2 anesthesia combined with the local anesthetic lidocaine. To measure muscle activity, one flexible electromyogram (EMG) electrode was attached with a single stitch to the chin muscle (trigonum submandibularis) and a second flexible EMG electrode was attached to the neck muscle (trapezius). Both EEG and EMG electrodes were fixed to the skull with dental cement. For sleep measurements, the electrodes were connected to a 2-channel telemetric transmitter (F20-EET, Data Sciences International, a division of Transoma Medical, Arden Hills, USA) for wireless recording of endogenous brain signals.
Drug Treatment
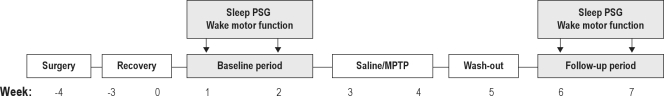
The MPTP group (n = 5) received subcutaneous (SC) MPTP (Sigma Aldrich, St. Louis, USA) injections into the abdominal area. The neurotoxin was administered on Mondays and Thursdays (in the first week 2 mg/kg, and in the second week 1.5 mg/kg). The vehicle group (n = 4), received time and volume-matched SC 0.9% saline injections. After a one-week washout period, the 2-week experimental phase, followed under otherwise identical conditions to the baseline period, (see follow-up period, Figure 1). Behavior was observed twice daily. Sleep recording and wake motor function tests were recorded weekly during both the baseline and the follow-up period. Data from each animal were combined to give an average value for either the baseline or the follow-up period.
Figure 1.
Schematic diagram depicting the experimental design. Surgery: placing EEG and EMG electrodes; Recovery: 4 weeks recovery from surgery; Baseline period: baseline measurements (sleep and motor function) before treatment; Saline/MPTP: Saline (n = 4) or MPTP (n = 5) treatment; Washout: period to allow recovery from direct MPTP effects; and Follow-up period: all measurements (sleep and motor function) after treatment.
Sleep Analysis
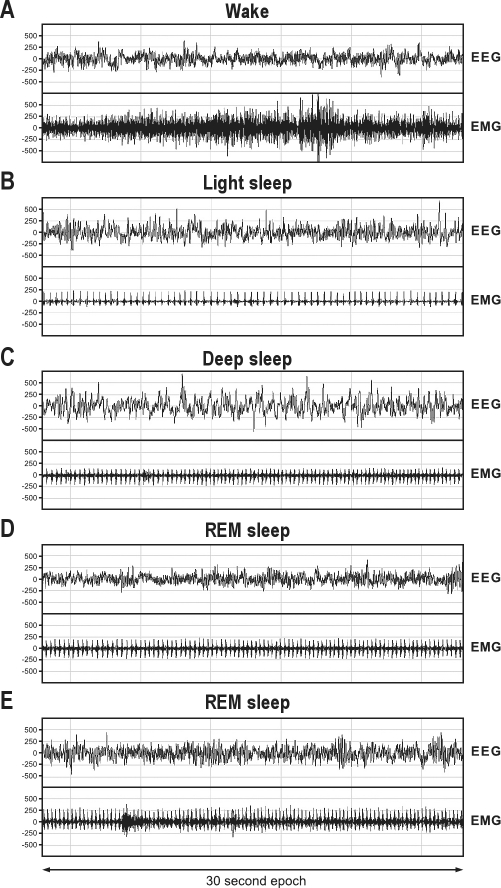
Animals were placed in special sleep cages (40 × 20 × 30 cm) and provided with bedding material, food and ad libitum water access. The marmosets could move freely around the sleep cages while having olfactory, visual and auditory contact with other marmosets. Climate and light/dark cycles were kept constant throughout the experiment. Sleep recordings started 1 h before lights were switched off and lasted until 1 h after they came back on again. The endogenous brain signals, recorded with a sample frequency of 100 Hz, were transferred to a receiver (RPC-1, Data Sciences International) and then to an acquisition program (Dataquest A.R.T., Data Sciences International, a division of Transoma Medical, Arden Hills, USA) and stored in European Data Format (EDF). Software Somnologica (Embla Inc, Broomfield, USA) was used for sleep staging to obtain hypnograms. Without knowing the treatment received by an individual animal, an experienced sleep technologist classified each 30-sec EEG epoch into sleep stages according to the criteria of Rechtschaffen and Kales20 and adapted for marmosets.21,22 Sleep macrostructure was quantified into sleep efficiency, total sleep time, number of wake bouts, wake time, number of sleep stage transitions, and the duration of 3 different sleep stages. Duration of sleep stages 1 and 2 were combined into the variable “light sleep”, and duration of sleep stages 3 and 4 were combined into a variable “deep sleep” (Figure 2A-C). Four categories of muscle tone were distinguished for the 30-sec REM epochs: (1) complete atonia (Figure 2D), (2) up to 3 twitches, (3) increased tone for 10% to 50% of the epoch (Figure 2E), and (4) increased tone for > 50% of the epoch. Outcome variables were calculated as the percentage occurrence of the category relative to the total number of REM epochs.
Figure 2.
Recordings of sleep stages in marmoset monkeys. Images represent examples of 30-sec epochs of (A) wake, (B) light sleep, (C) deep sleep, (D) REM sleep with complete atonia, and (E) REM sleep with > 50% of the time muscle activity.
Behavioral Observations
Marmoset monkeys were scored at 08:00 and 18:00, using the dedicated clinical observation scale.16 This is a clinical condition-scoring list specified for parkinsonian signs, which is used to monitor the pathological condition of MPTP-treated animals. Apathy, tremors, immobility, and muscle rigidity were scored on a scale form zero (normal/healthy) to 4 (severely affected). The sum of these separate observations together formed a clinical score outcome.
Locomotor Activity
An automated activity test was used to quantify locomotor behavior.23,24 The apparatus consisted of 4 equal compartments (23 × 23 × 23 cm) connected to each other by 6 non-transparent PVC tubes. The compartments were closed off on all sides except for a wire mesh roof. The animals were always placed in the same compartment before the start of a session and could thereafter move freely from one compartment to another during a 20-min period. A video tracking system registered the movements and position of the animal within the apparatus. Locomotor activity was quantified as the number of compartment changes during this 20-min period.
Hand-Eye Coordination
Reward-related hand-eye coordination performance was tested with an automated robot-guided test setup.16,24 In brief, the marmoset was placed in a test cage in front of a black plastic panel with a small window (5 × 8 cm). On the other side of the panel a robot arm presented 42 small marshmallow rewards, which the marmosets could reach through the window. The rewards were offered at 3 different rates: non-moving (0.0 m/s for a maximum of 30 sec), slow moving (0.04 m/s), and fast moving (0.08 m/s). Each test session consisted of 14 trials at each rate. A brief sound signal was used to alert the animal before each trial and in between each trial the window was closed by a sliding door. The percentage of correct hits was used as a criterion to judge the performance of each animal. Training started 3 months before the start of the study, and all animals were trained to take > 80% of the presented rewards.
Statistical Analysis
Data were assembled in Excel spreadsheets (Microsoft Corporation, Redmond, USA) and averaged to obtain a single value for both the baseline and the follow-up period for each animal. These averages were transferred to SPSS (version 15.0, SPSS Inc, Chicago, USA). After testing for normality (Shapiro-Wilk), analyses consisted of either repeated measures ANOVA for data with normal distributions or Friedman tests for non-normal data to determine variation across time for each group separately, followed by a Mann-Whitney test for within or between group differences. Additionally, a stepwise discriminant analysis was performed to elucidate the sleep variable(s) that best discriminated between MPTP-treated and vehicle-treated animals. The level of probability for statistical significance was set at 0.05. Data are presented as means ± SEM.
RESULTS
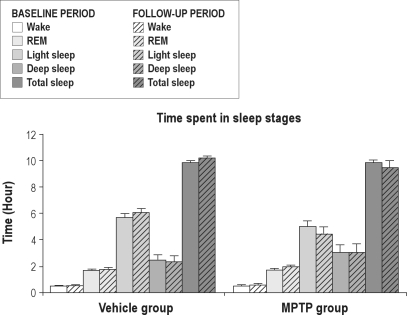
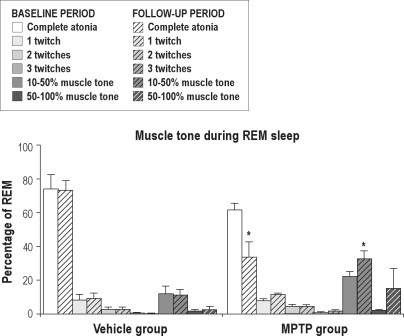
The experimental timeline consisted of (1) surgical preparation followed by (2) a 4-week recovery period and then by (3) 7 weeks of experimental testing (Figure 1). The first 2 experimental weeks consisted of baseline measurements. After this baseline period a 2-week parkinsonism induction period started in the MPTP group. We first measured the average sleep macrostructure variables and wake motor function variables before and after saline or MPTP treatment (Table 1). Subsequently, average values of baseline and follow-up period measurements of time spent in different sleep stages were determined (Figure 3). Repeated measures ANOVA showed no treatment or treatment-time effects in the macrostructure of sleep between the 2 experimental groups (P > 0.05). MPTP-treated marmosets showed no reduction in total sleep time, time spent in REM sleep, light sleep, or deep sleep. Further, wake time after sleep onset was not significantly affected by the MPTP treatment. However, MPTP significantly increased endogenous muscle tone during REM sleep (P < 0.05). We also determined the distribution of muscle tone as a percentage of the total time in REM sleep (Figure 4). REM epochs were categorized according to muscle tone as either being absent or present in one of 3 predefined levels. For both the baseline and the follow-up period, epochs with muscle tone > 10% of the time were scored most often. Epochs with muscle tone > 50% of the time were found to be rare; however, they were more frequently scored in animals treated with MPTP. Friedman analysis showed a significant time effect within the MPTP group for the occurrence of epochs with muscle tone > 10% of the time. MPTP, but not saline, increased the occurrence of epochs with muscle tone > 10% of the time (32.8% ± 4.5% vs. 22.4% ± 2.9% of REM epochs; P < 0.05). This repeated measures analysis also revealed an effect in the epochs with complete atonia within the MPTP group, but not within the control group. There were significantly less epochs without atonia in the nights after MPTP treatment than in those before MPTP treatment (66.3 ± 9.1 vs. 38.3 ± 4.1; P < 0.05). Discriminant analysis showed that, in the follow-up period, the number of epochs with 10% muscle tone during REM sleep classified 80% of the animals correctly, and epochs with ≥ 3 twitches added the remaining 20% of this classification. These 2 variables together classified the MPTP treatment up to a maximum of 100% (P < 0.05).
Table 1.
Sleep characteristics and wake motor function measurements before and after vehicle (saline) or MPTP treatments
| Vehicle group | MPTP group | |||||
|---|---|---|---|---|---|---|
| Treatment | Baseline ± SEM | Saline ± SEM | Baseline ± SEM | MPTP ± SEM | F statistic/ | P value |
| Sex (male/female) | 3/1 | 3/1 | 2/3 | 2/3 | χ2 | |
| Sleep efficiency (% of sleep time) | 87.54 ± 1.31 | 89.46 ± 1.46 | 88.09 ± 1.65 | 83.22 ± 4.37 | 2.74 | 0.14a |
| Total Sleep time (minutes) | 594.84 ± 10.09 | 615.19 ± 10.48 | 593.75 ± 13.47 | 570.95 ± 31.67 | 2.13 | 0.19a |
| Transitions (#) | 259.63 ± 6.77 | 259.88 ± 11.65 | 258.00 ± 23.54 | 253.70 ± 10.66 | 0.04 | 0.85a |
| Wake bouts (#) | 30.00 ± 4.25 | 32.38 ± 4.55 | 30.60 ± 5.695 | 35.30 ± 7.65 | 0.10 | 0.77a |
| Wake time (minutes) | 66.09 ± 2.60 | 72.5 ± 8.75 | 67.98 ± 4.16 | 144.25 ± 29.68 | 2.78 | 0.14a |
| Time in REM (minutes) | 102.19 ± 5.60 | 107.13 ± 8.72 | 105.35 ± 5.98 | 118.35 ± 7.73 | 0.70 | 0.43a |
| Time in light sleep (minutes) | 343.13 ± 20.93 | 366.50 ± 20.48 | 303.30 ± 24.68 | 268.50 ± 31.77 | 2.79 | 0.14a |
| Time in deep sleep (minutes) | 149.63 ± 28.57 | 141.56 ± 31.53 | 185.10 ± 33.69 | 184.10 ± 39.57 | 0.63 | 0.81a |
| Latency to first REM (minutes) | 82.00 ± 5.18 | 60.56 ± 21.42 | 58.75 ± 13.89 | 32.40 ± 10.70 | 0.03 | 0.87a |
| Clinical Score (cumulative score) | 0.20 ± 0.29 | 0.21 ± 0.35 | 0.13 ± 0.08 | 8.03 ± 1.60 | 5.0 | 0.025b |
| Activity (# compartment changes) | 85.75 ± 16.79 | 94.88 ± 9.74 | 64.40 ± 15.80 | 23.70 ± 8.80 | 7.67 | 0.028a |
| Hand-eye coordination (# trials) | 34.13 ± 4.12 | 36.13 ± 5.88 | 33.80 ± 3.79 | 23.40 ± 3.56 | 1.0 | 0.32b |
Repeated-measurements ANOVA interaction effect for parametric comparisons.
Friedman test for nonparametric MPTP versus Saline comparisons.
Figure 3.
Sleep stages, wake time after sleep onset and total sleep time in the vehicle group and the MPTP group, before (baseline, solid bars) and after saline (n = 4) or MPTP (n = 5) treatment (follow-up period, striped bars). No differences were found between baseline and follow-up period.
Figure 4.
REM sleep epochs with tone as a percentage of REM sleep in the vehicle group and the MPTP group before (baseline, solid bars) and after saline (n = 4) or MPTP (n = 5) treatment (follow-up period, striped bars). Asterisks indicate significant differences between baseline and follow-up period (*P < 0.05 Friedman)
The PD-induction protocol also affected the motor behavior of the MPTP-treated marmosets while they were awake to a moderate extent. This protocol could therefore be described as a model of mildly parkinsonian signs. Repeated measures analysis showed that the MPTP-treated marmosets' wake motor function was affected in terms of both clinical score and activity (Table 1). Friedman analysis showed that the clinical score was significantly increased within the MPTP-treated marmosets (cumulative score 8.0 ± 1.6 vs. 0.13 ± 0.1; P < 0.05). Repeated measures ANOVA revealed a significant treatment effect and a significant treatment-time interaction in spontaneous locomotor activity for the MPTP-treated group. In addition, the number of compartment changes was decreased after MPTP treatment (23.7 ± 8.8 vs. 64.4 ± 15.8; P < 0.05). In contrast to the spontaneous activity and rating scales, Friedman analysis showed that the specific motor function, in the hand-eye coordination task, was not significantly affected after MPTP treatment in the last 2 weeks of the experiment relative to the baseline values (number of correct trials, 23.40 ± 3.56 vs. 33.80 ± 3.79; P = 0.22).
DISCUSSION
The aim of the present study was to develop and to evaluate an animal model for RBD, in order to facilitate studies into the mechanisms and treatment of RBD and other sleep disturbances in the premotor stage of PD. Because of the direct link between RBD and PD,4,10–13,25 we evaluated certain sleep components, particularly REM sleep chin muscle disturbances in an experimental model for PD, the marmoset MPTP model. Previous work using this preclinical animal model for idiopathic PD has focused mainly on motor behaviors. Since the focus in PD research has recently shifted from the motor phase to the premotor phase of the disease,5,6,26–28 it is of particular interest to investigate further the validity of the classic MPTP model for PD for the study of premotor symptoms. To achieve this focus, we investigated general sleep characteristics and the EMG changes during REM sleep in the marmoset MPTP model of PD.
Rodent studies are frequently used for PD research. However, the nocturnal nature of these animals' behavior and their short sleep bouts29,30 make them less suitable as models for sleep in PD. Sleep changes have been reported in the rat,31 cat,32 and cynomolgous macaque22 immediately following MPTP treatment. In rats, MPTP induces a temporary reduction of REM sleep and increases sleep efficiency.31 MPTP also induces a reduction in REM sleep in cats and monkeys.22,32 The temporary reduction of REM sleep after MPTP in these mammalian species can be interpreted as a sign of general discomfort. On the other hand, reductions in REM sleep appeared to remain in rhesus monkeys in a longitudinal follow-up study.33 However, possible changes in muscle tone during REM sleep were not addressed in any of these studies. In our study, we induced a mild parkinsonian state that did not significantly affect the sleep macrostructure and only mildly affected motor function, resembling an early phase of PD in humans. The animals were less active in a daytime locomotor activity test and they were mildly parkinsonian, based on clinical scores in their home cages. Although the MPTP-treated animals were less active than control animals, they were not incapacitated, as their hand-eye coordination was not affected.
The present study is the first report of selective abnormalities in REM muscle tone in non-human primates. The MPTP-treated marmoset model can be used for further studies into the mechanisms of RBD and sleep disturbances in the premotor symptom phase of PD (i.e., when patients can be diagnosed with RBD but not with PD).4,12,13 However, the stringent criteria for RBD described in human studies (50%-100% muscle tone per epoch) were not met by the parkinsonian marmosets in the present study. The International Classification of Sleep Disorders34 describes RBD as the presence of REM sleep without atonia, and disruptive behavior during sleep. The atonia, normally observed during REM sleep, is interrupted by either short (phasic, 2-3 sec) or long (tonic, 20-30 sec) episodes of EMG activity.3,4,10 Then again, a significant change in tonic activity is definitely apparent in our experimental animals, which suggests an RBD-like phenomenon. Indeed, an alternative and more suitable measure of RBD may be muscle activity as a percentage of REM sleep, given the variable outcome of polysomnogram measurements from 62 diagnosed patients.35 This interpretation would be supported by the parameters proposed by Mayer and colleagues.35 The marmosets show a slight increase in phasic activity and a definite increase in tonic activity as a percentage of total REM sleep. The slight increase in phasic activity of EMG bursts or twitches (0.1-5 sec) was seen in the epochs with one single twitch, and the increase in tonic EMG was seen in epochs with more than 10% tone. This corresponds with EMG activity in at least one-third of the 30-sec epochs.
The MPTP induced changes in REM sleep muscle tone are presumably due to changes in DA neurotransmission. Reduction in DA neurons in the substantia nigra caused by MPTP exposure results in a decrease DA signaling to its receptors localized within the striatum.36 In this regard, knocking out the DA transporter37 or reducing DA signaling in the brain results in sleep changes in mice.29 Similarly, a loss of DA neurons in the substantia nigra underlies some of the sleep changes in rats. Clinical conditions affecting DA, such as those seen in PD, also alter sleep architecture1 which result in changes in REM sleep38 and muscle tone during REM sleep.39,40 Therefore, nigrostriatal neurons whose axons are located in the striatum are assumed to play a major role in the regulation of REM sleep. Additionally, a relatively small nigral lesion is enough to produce sleep changes31 On the other hand, it has been suggested that the degenerative process in PD is initiated in the medulla, advances to the pons, and subsequently targets the midbrain.41 Thus, the presence of RBD might also reflect early involvement of non-DA medullary and/or pontine REM sleep related structures.10 Therefore, it is suggested that these structures, which are closely connected to the substantia nigra pathways, are affected by an imbalance of DA levels,42 which would precede the actual neurodegeneration process.
In conclusion, the MPTP-treated marmoset provides a new opportunity for quantitative studies on the mechanisms and intervention strategies of RBD and the premotor phase of PD. Unlike the nocturnal preference and fragmented pattern of sleep in mice and rats, the architecture of marmoset monkeys' sleep resembles that of humans. Marmosets are diurnal, and, as in humans, their night sleep architecture consists of a recurring pattern of cycles with light, deep, and REM sleep. Further, quantifying the different stages in marmoset monkeys can be performed with the classical sleep scoring system directly adapted from human scoring.20 The observed increase in muscle tone during REM sleep is typical for RBD and one of the major symptoms preceding the classic motor problems in many PD patients. The fact that MPTP affects REM sleep atonia suggests a direct role for DA depletion as a cause for the increased REM sleep muscle tone in PD.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank H.P.M. van Helden, D. Davis, and G Torres for critical reading of the manuscript. This study was performed at BU CBRN Protection, TNO Defence, Security and Safety. This study was supported by the U.S. Army Medical Research and Materiel Command, Award No. W81XH-05-1-0517.
REFERENCES
- 1.Comella CL, Tanner CM, Ristanovic RK. Polysomnographic sleep measures in Parkinson's disease patients with treatment-induced hallucinations. Ann Neurol. 1993;34:710–4. doi: 10.1002/ana.410340514. [DOI] [PubMed] [Google Scholar]
- 2.Ondo WG, Dat Vuong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology. 2001;57:1392–6. doi: 10.1212/wnl.57.8.1392. [DOI] [PubMed] [Google Scholar]
- 3.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 4.Gagnon JF, Bedard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–9. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 5.Berg D. Biomarkers for the early detection of Parkinson's and Alzheimer's disease. Neurodegener Dis. 2008;5:133–6. doi: 10.1159/000113682. [DOI] [PubMed] [Google Scholar]
- 6.Tolosa E, Gaig C, Santamaria J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72:S12–20. doi: 10.1212/WNL.0b013e318198db11. [DOI] [PubMed] [Google Scholar]
- 7.Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord. 1998;13:895–9. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- 8.Oerlemans WG, de Weerd AW. The prevalence of sleep disorders in patients with Parkinson's disease. A self-reported, community-based survey. Sleep Med. 2002;3:147–9. doi: 10.1016/s1389-9457(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 9.van Hilten B, Hoff JI, Middelkoop HA, et al. Sleep disruption in Parkinson's disease. Assessment by continuous activity monitoring. Arch Neurol. 1994;51:922–8. doi: 10.1001/archneur.1994.00540210094018. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–52. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 11.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–51. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 12.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 13.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 14.Ferini-Strambi L, Zucconi M. REM sleep behavior disorder. Clin Neurophysiol. 2000;111:S136–40. doi: 10.1016/s1388-2457(00)00414-4. [DOI] [PubMed] [Google Scholar]
- 15.Jenner P. The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology. 2003;61:S4–11. doi: 10.1212/wnl.61.6_suppl_3.s4. [DOI] [PubMed] [Google Scholar]
- 16.van Vliet SA, Vanwersch RA, Jongsma MJ, van der Gugten J, Olivier B, Philippens IH. Neuroprotective effects of modafinil in a marmoset Parkinson model: behavioral and neurochemical aspects. Behav Pharmacol. 2006;17:453–62. doi: 10.1097/00008877-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Verhave PS, Vanwersch RA, van Helden HP, Smit AB, Philippens IH. Two new test methods to quantify motor deficits in a marmoset model for Parkinson's disease. Behav Brain Res. 2009;200:214–9. doi: 10.1016/j.bbr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Pearce RK, Collins P, Jenner P, Emmett C, Marsden CD. Intraventricular infusion of basic fibroblast growth factor (bFGF) in the MPTP-treated common marmoset. Synapse. 1996;23:192–200. doi: 10.1002/(SICI)1098-2396(199607)23:3<192::AID-SYN8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Iravani MM, Jackson MJ, Kuoppamaki M, Smith LA, Jenner P. 3,4-methylenedioxymethamphetamine (ecstasy) inhibits dyskinesia expression and normalizes motor activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci. 2003;23:9107–15. doi: 10.1523/JNEUROSCI.23-27-09107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A, editors. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 21.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almirall H, Pigarev I, de la Calzada MD, Pigareva M, Herrero MT, Sagales T. Nocturnal sleep structure and temperature slope in MPTP treated monkeys. J Neural Transm. 1999;106:1125–34. doi: 10.1007/s007020050228. [DOI] [PubMed] [Google Scholar]
- 23.Wolthuis OL, Groen B, Philippens IH. A simple automated test to measure exploratory and motor activity of marmosets. Pharmacol Biochem Behav. 1994;47:879–81. doi: 10.1016/0091-3057(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 24.Philippens IH, Melchers BP, Roeling TA, Bruijnzeel PL. Behavioral test systems in marmoset monkeys. Behav Res Methods Instrum Comput. 2000;32:173–9. doi: 10.3758/bf03200799. [DOI] [PubMed] [Google Scholar]
- 25.Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–37. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 26.Marek K, Jennings D. Can we image premotor Parkinson disease? Neurology. 2009;72:S21–6. doi: 10.1212/WNL.0b013e318198df97. [DOI] [PubMed] [Google Scholar]
- 27.Tolosa E, Poewe W. Premotor Parkinson disease. Neurology. 2009;72:S1. doi: 10.1212/wnl.0b013e318198dace. [DOI] [PubMed] [Google Scholar]
- 28.Stephenson R, Siderowf A, Stern MB. Premotor Parkinson's disease: clinical features and detection strategies. Mov Disord. 2009;24(Suppl 2):S665–70. doi: 10.1002/mds.22403. [DOI] [PubMed] [Google Scholar]
- 29.Monaca C, Laloux C, Jacquesson JM, et al. Vigilance states in a parkinsonian model, the MPTP mouse. Eur J Neurosci. 2004;20:2474–8. doi: 10.1111/j.1460-9568.2004.03694.x. [DOI] [PubMed] [Google Scholar]
- 30.Yi PL, Tsai CH, Lu MK, Liu HJ, Chen YC, Chang FC. Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep. 2007;30:413–25. doi: 10.1093/sleep/30.4.413. [DOI] [PubMed] [Google Scholar]
- 31.Lima MM, Andersen ML, Reksidler AB, Vital MA, Tufik S. The role of the substantia nigra pars compacta in regulating sleep patterns in rats. PLoS ONE. 2007;2:e513. doi: 10.1371/journal.pone.0000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pungor K, Papp M, Kekesi K, Juhasz G. A novel effect of MPTP: the selective suppression of paradoxical sleep in cats. Brain Res. 1990;525:310–4. doi: 10.1016/0006-8993(90)90880-k. [DOI] [PubMed] [Google Scholar]
- 33.Barraud Q, Lambrecq V, Forni C, et al. Sleep disorders in Parkinson's disease: the contribution of the MPTP non-human primate model. Exp Neurol. 2009;219:574–82. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Sleep Medicine. International classification of sleep disorders: Diagnostic and coding manual. 2nd ed. Westchester IL: American Academy of Sleep Medicine; 2005. pp. 148–52. [Google Scholar]
- 35.Mayer G, Kesper K, Ploch T, et al. Quantification of tonic and phasic muscle activity in REM sleep behavior disorder. J Clin Neurophysiol. 2008;25:48–55. doi: 10.1097/WNP.0b013e318162acd7. [DOI] [PubMed] [Google Scholar]
- 36.Levey AI, Hersch SM, Rye DB, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A. 1993;90:8861–5. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dzirasa K, Ribeiro S, Costa R, et al. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26:10577–89. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–41. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Borreguero D, Caminero AB, De La Llave Y, et al. Decreased phasic EMG activity during rapid eye movement sleep in treatment-naive Parkinson's disease: effects of treatment with levodopa and progression of illness. Mov Disord. 2002;17:934–41. doi: 10.1002/mds.10233. [DOI] [PubMed] [Google Scholar]
- 40.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–20. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 41.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–36. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 42.Lai YY, Siegel JM. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci. 1990;10:2727–34. doi: 10.1523/JNEUROSCI.10-08-02727.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]