Abstract
Study Objectives:
Sleep disordered breathing (SDB) has been associated with increased inflammatory responses. Changes in the level of pro-inflammatory leukotrienes (LTs) may initiate or exacerbate pediatric SDB and may play a major role in end-organ morbidity. The objective of the study was to investigate the relationship of LT productions with severity of SDB, obesity, and adenotonsillar hypertrophy in children.
Design/Interventions:
Prospective, observational study that included standard questionnaires, physical examinations, overnight polysomnography (PSG), and urinary leukotriene E4 (LTE4) assay.
Setting:
Sleep Center and Laboratory of Nutriology.
Patients or Participants:
282 children with SDB and 94 healthy control subjects were recruited.
Measurements and Results:
Urinary LTE4 levels were elevated in children with SDB compared to the controls, and LTE4 productions emerged disease severity- and obesity-dependent increases. In stepwise multiple regression analysis, the independent predictors of the apnea-hypopnea index (AHI) included LTE4 level and adenotonsillar-size sum score (P < 0.001 respectively; adjusted R2 = 0.318). A positive relationship between LTE4 urinary level and adenotonsillar-size sum scores was present in the underweight/normal weight SDB subjects (r = 0.276; P < 0.001), but not in the overweight/obese children (P > 0.05).
Conclusions:
Systemic inflammation mediated by LTs participates in the pathophysiological mechanisms of SDB in children. The magnitude of inflammation as reflected by urinary LTE4 is significantly related to the severity of SDB and obesity. However, a correlation between LTE4 concentration and adenotonsillar size is present only among nonobese children.
Citation:
Shen Y; Xu Z; Shen K. Urinary leukotriene E4, obesity, and adenotonsillar hypertrophy in Chinese children with sleep disordered breathing. SLEEP 2011;34(8):1135-1141.
Keywords: Sleep disordered breathing, leukotriene E4, polysomnography, systemic inflammation, obesity, adenotonsillar hypertrophy
INTRODUCTION
Pediatric sleep disordered breathing (SDB) is caused by a combination of increased upper airway resistance and repetitive pharyngeal collapsibility, resulting in intermittent hypoxemia and arousal from sleep.1 Adenotonsillar hypertrophy (ATH) is regarded as the main risk factor for SDB in children.2–4 However, strong epidemiologic evidence suggests that the prevalence of obesity in children with SDB has increased worldwide.5–7 For every increment in body mass index (BMI) of 1 kg/m2 beyond the mean BMI for age and gender, the risk of obstructive sleep apnea increased by 12%.4,7 Although obesity may affect the patency of the upper airway, it appears that the major role of obesity in the genesis of SDB is through its metabolic activity, and active visceral fat is the predominant contributor.8,9
Recently, evidence has emerged linking the presence of local airway and systemic inflammation to the pathophysiology of SDB.10–12 Among inflammatory mediators, leukotrienes (LTs) are the major arachidonic acid metabolites produced via the 5-lipoxygenase pathway. The LT family includes LTA4, LTB4, and LTC4/D4/E4 (cysteinyl leukotrienes, cysLTs). All of the compounders can modulate inflammatory responses significantly.13,14
A number of previous works have shown that LT concentration and the expression of LT receptors in upper airway lymphoid tissues of children with SDB are related to a proliferative signal pathway.15,16 And in later investigations, it was determined that LT production emerged disease severity-dependent increases in both exhaled breath condensate17 and urine18 of SDB patients. However, the relationship between obesity, ATH, and LT production has not been well examined. The patients who are most likely to benefit from antileukotriene treatments (according to disease severity and obesity degree) and how to adopt antileukotriene therapy (independent use or combined use with surgery) remain undefined.
In this study, we measured concentration of LTE4 in morning urine to evaluate systemic inflammation; our objective was to investigate the relationship of LT production with severity of SDB, obesity, and ATH in children.
MATERIALS AND METHODS
Subjects
The study was approved by the institutional ethics committee. Informed consent was obtained from the legal caretaker of each participant. Assent was also obtained from children > 6 years of age.
Consecutive children referred to the Sleep Center for suspected SDB from August 2009 to June 2010 were recruited in the study. Age-, sex-, and weight-matched control subjects were healthy volunteers without a history of snoring, who were recruited from a community-based physical check-up activity. Inclusion criteria were the presence of habitual snoring (snoring as reported by parents > 3 nights/week) and age between 2 and 12 years. Exclusion criteria included the presence of cardiovascular, neuromuscular, craniofacial, or genetic disorders; acute or chronic inflammation; asthma, allergic rhinitis, or other allergies; pharmacologic treatment including antibiotics, aspirin, nonsteroidal anti-inflammatory drugs, corticosteroids, and LT receptor antagonists in the previous month. In addition, any children who already had undergone tonsillectomy and adenoidectomy (T&A) or had oral appliances or CPAP treatment were not considered eligible.
Anthropometry and Clinical Evaluation
Weight, height and waist circumference were measured. BMI was calculated as weight (in kilograms)/height (in square meters). The waist height ratio (WHtR) was applied as an abdominal fat index. To adjust BMI for the effect of age and gender, BMI z-score was further analyzed based on Chinese growth curves.19 Values equal to −1.645, 0, and 1.036 correspond to the 5th, 50th, and 85th percentiles of the growth curves for BMI, respectively. Overweight/obese children were classified as those with BMI z-score ≥ 1.036; normal weight children were those with BMI z-score ≥ −1.645 and < 1.306; and underweight children were those with BMI z-score < −1.645.
A detailed questionnaire and physical examination were performed. The questions referred to symptoms and duration of SDB, presence of comorbidity, past medical history, medication use, and family history. Tonsil size was graded from 1 to 4 by direct inspection of the oropharynx.20 Adenoid size, determined by flexible fiberoptic nasopharyngoscopy, was graded from 1 to 4 in accordance with the criteria of Modrzynski et al.21 The sum of the adenoid and tonsil scores was used as the global estimate of adenotonsillar size.7
Polysomnographic Assessment
Polysomnography (PSG) (Compumedics E-series; Compumedics Inc; Abbotsford, VIC, Australia) was performed on all SDB children except the controls for evaluation. No sleep deprivation or sedation was used. Patients were studied for up to 8 h in a dedicated, quiet, dark room in the company of one of their parents. The following parameters were measured: 4-channel electroencephalogram with bilateral central and occipital leads, electrooculogram, submental electromyogram, electrocardiogram, and body position. Respiratory variables included thoracic and abdominal wall movement, nasal airflow, and oxygen saturation. The sleep staging was assessed accorded to the criteria of Rechtschaffen and Kales.22 Sleep efficiency (SE) referred to the percentage of the total recording time that the patient was asleep. Obstructive apnea was defined as the cessation of airflow with persistent chest wall and abdominal movement for > 2 respiratory cycles.23,24 Hypopnea was defined as a reduction in oronasal flow ≥ 50% compared to baseline with a corresponding decrease in pulse oximetry saturation (SpO2) ≥ 4% and/or arousal.24 The apnea-hypopnea index (AHI) was defined as the average number of apneas and hypopnea episodes per hour of total sleep time (TST). The obstructive apnea index (OAI) was defined as the average number of apnea episodes per hour of TST. The oxygen desaturation index (ODI) was defined as the number of oxyhemoglobin desaturation events ≥ 4% from baseline per hour of TST. Sleep staging and respiratory events analysis were summarized by computer software (ProFusion 2; Compumedics Inc). SDB severity was classified by AHI. Briefly, mild SDB was defined as AHI < 5 episodes/h and ≥ 1 episodes/h, moderate SDB was AHI < 20 episodes/h and ≥ 5 episodes/h, and severe SDB was AHI ≥ 20 episodes/h.
Measurement of Urinary LTE4
First urine specimens were provided by SDB children and controls immediately after awakening in the morning. After collection, samples were centrifuged at 10,000 × g for 8 min at 4°C; the supernatant was then transferred to clean test tubes. Approximately 10 mL of each sample was used for measurement of urinary creatinine, and the remainders were coded and frozen at −80°C until assayed. Urinary LTE4 levels were measured using commercially available ACE enzyme-linked immunoassay kits (Leukotriene E4 EIA kit; Cayman Chemical Company; Ann Arbor, MI, USA) following the manufacturer's instructions. All samples were loaded in duplicates and assayed in ≥ 2 dilutions, and plate reader absorbance results were analyzed with a 4-parameter logistic curve fit. The sensitivity was 8.29 pg/mL. The intraassay and interassay variability for the assays were all < 10%, and the specificity was 100%. LTE4 levels were expressed as ng/mM of urinary creatinine to adjust for the renal concentrating effect.
Statistical Analysis
All statistical analyses were conducted using statistical software (version 16.0; SPSS, Chicago, IL, USA). Data were presented as mean ± SD or median (interquartile range, IQR) depending on the distribution unless stated otherwise. PSG indices and LTE4 concentrations were log-transformed (natural logarithm) to correct for skewed distribution. Comparisons according to group assignment were made with a Kruskal-Wallis test followed by nonparametric Bonferroni multiple comparison tests for continuous variables and χ2 test (Yates correction) for categorical characteristics. Correlations were analyzed without adjustment by using a Spearman rank test. Stepwise multiple linear regressions were performed to identify independent predictors of log-transformed AHI. A 2-sided P value of < 0.05 was used to define statistical significance.
RESULTS
Cohort Selection
The 368 children who fulfilled the inclusion criteria were recruited, of which 86 subjects were excluded because of incomplete clinical data (n = 35), primary snoring (subjects with snoring but AHI < 1; n = 10), or refusal to supply morning urinary samples (n = 41). Ultimately, a total of 282 subjects were included in the analysis.
LTE4 Concentration and SDB Severity
The characteristics of the 94 non-snoring control subjects and 282 patients stratified by SDB severity, matched for age, sex, and weight, are described in Table 1.
Table 1.
Characteristics of recruited subjects by SDB severity*
| Variables | Control Subjects (n = 94) | Subjects With Mild SDB (n = 109) | Subjects With Moderate SDB (n = 118) | Subjects With Severe SDB (n = 55) |
|---|---|---|---|---|
| Age, y | 5.12 ± 1.65 | 5.31 ± 2.29 | 4.98 ± 1.82 | 5.32 ± 1.73 |
| Sex, male/female | 63:31 | 70:39 | 85:33 | 39:16 |
| WHtR | - | 0.49 ± 0.05 | 0.50 ± 0.05 | 0.51 ± 0.06 |
| BMI z-score | 0.49 ± 1.31 | 0.36 ± 1.29 | 0.36 ± 1.53 | 0.92 ± 1.77 |
| Clinical data | ||||
| Disease duration, y | - | 1.0 (0.5,2.0) | 1.0 (0.5,2.0) | 2.0 (1.0,2.5) |
| Adenotonsillar size sum score | 5.0 (4.0,6.0)† | 6.0 (5.0,6.0)‡ | 6.0 (6.0,7.0)‡ | |
| Polysomnographic Parameters | ||||
| SE, % | - | 84.13 ± 7.58 | 82.49 ± 8.55 | 82.59 ± 11.21 |
| AHI, episodes/h | - | 2.50 (1.50,3.35)† | 9.80 (7.50,13.90)† | 28.50 (24.00,35.90)† |
| OAI, episodes/h | - | 1.10 (0.55,1.40)† | 3.80 (2.35,5.93)† | 10.1 (7.30,18.10)† |
| ODI, episodes/h | - | 0.80 (0.15,1.70)† | 6.00 (2.90,10.03)† | 20.70 (16.50,34.90)† |
| Mean SpO2, % | - | 98 (97,98)† | 97 (95,97)† | 96 (94,97)† |
| Minimal SpO2, % | - | 92 (88,93)† | 86 (80,89)† | 74 (61,81)† |
| SLT90%, %TST | - | 0.00 (0.00,0.00) † | 0.11 (0.00,0.40) † | 2.30 (1.10,7.20) † |
| LTE4, ng/mM creatinine | 64.35 (47.70,82.23)† | 99.74 (70.25,133.75)† | 117.41 (92.23,154.57)† | 194.15 (150.24,272.16)† |
Values represent mean ± SD or median (IQR) depending on the data distribution.
P < 0.05 vs. other groups.
P < 0.05 vs. mild group.
As expected, the sleep respiratory-disturbance parameters values were significantly different among the 4 groups. There were no significant differences in terms of age, gender, disease course, or BMI z-score. Conversely, the adenotonsillar-size sum score was slightly higher in the moderate and severe SDB group than the mild SDB group.
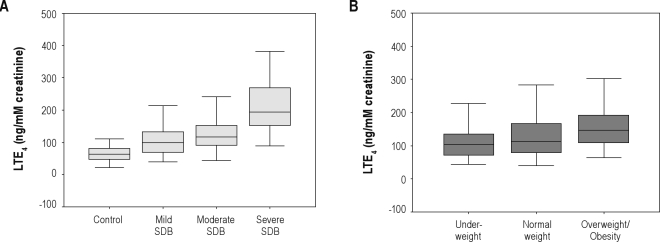
Urinary LTE4 concentrations increased significantly more in patients with SDB than the control subjects, and LT production emerged disease severity-dependent increases (controls vs. mild SDB vs. moderate SDB vs. severe SDB: media [IQR] 64.35 [47.70, 82.23] vs. 99.74 [70.25, 133.75] vs. 117.41 [92.23, 154.57] vs. 194.15 [150.24, 272.16] ng/mM creatinine, P < 0.001) (Table 1, Figure 1A).
Figure 1.
(A) LTE4 levels in morning urine obtained from children with severe/moderate/mild SDB and control subjects (P < 0.001). (B) LTE4 levels in morning urine obtained from 282 children with SDB stratified by obesity (P < 0.001). Boxes represent values within the interquartile rage; whiskers represent the data range; and the line across the boxes, median values.
LTE4 Concentration and Obesity
The characteristics of the 282 SDB subjects stratified by obesity are described in Table 2. There were no significant differences with respect to age, gender, disease course, adenotonsillar size, or sleep respiratory-disturbance parameter values.
Table 2.
Characteristics of 282 SDB subjects stratified by obesity*
| Variables | Underweight (n = 29) | Normal weight (n = 165) | Overweight/Obesity (n = 88) |
|---|---|---|---|
| Age, y | 5.11 ± 1.81 | 5.01 ± 1.88 | 5.51 ± 2.23 |
| Male/female ratio | 19:10 | 113:52 | 62:26 |
| WHtR | 0.45 ± 0.04† | 0.48 ± 0.04† | 0.54 ± 0.05† |
| BMI z-score | −1.98 ± 0.43† | −0.03 ± 0.67† | 2.21 ± 0.94† |
| Disease duration, y | 1.0 (0.5,2.0) | 1.0 (0.5,2.0) | 1.0 (0.5,2.0) |
| Adenotonsillar size sum score | 6.0 (5.0,7.0) | 6.0 (5.0,6.0) | 6.0 (4.0,6.0) |
| SE, % | 83.24 ± 8.59 | 83.98 ± 8.07 | 81.54 ± 9.97 |
| AHI, episodes/h | 9.80 (5.05,17.00) | 6.40 (2.80,13.90) | 8.95 (3.23,19.98) |
| OAI, episodes/h | 3.80 (1.45,7.40) | 2.10 (1.10,5.75) | 2.80 (1.10,6.18) |
| ODI, episodes/h | 7.80 (2.65,13.20) | 3.10 (0.90,10.80) | 3.15 (1.30,15.78) |
| Mean SpO2, % | 96 (94,97) | 97 (95,98) | 97 (96,98) |
| Minimal SpO2, % | 84 (79,91) | 88 (82,92) | 85 (75,91) |
| SLT90%, %TST | 0.16 (0.00,1.15) | 0.00 (0.00,0.40) | 0.10 (0.00,1.35) |
| LTE4, ng/mM creatinine | 103.41 (70.57,138.66) | 113.85 (80.38,166.89) | 146.85 (109.45,193.02)† |
Values represent mean ± SD or median (IQR) depending on the data distribution.
P < 0.05 vs. other groups.
Urinary LTE4 concentrations increased significantly more in the overweight/obese SDB group than either the normal-weight or underweight patients (146.85 [109.45, 193.02] vs. 113.85 [80.38, 166.89] vs. 103.41 [70.57, 138.66] ng/mM creatinine, P < 0.001), however there was no statistical differences between the normal-weight and underweight group (Table 2, Figure 1B).
Effects of Confounders on LTE4 Concentration
There was a significant correlation between LTE4 concentration and BMI z-score, WHtR, adenotonsillar size, and PSG parameters (AHI, OAI, ODI, percentage of time spent with saturation < 90% [SLT90%], and mean and minimal pulse oximetry saturation [SpO2]), but not with age, gender, disease course, or SE (Table 3).
Table 3.
Correlation coefficients between LTE4 production/adenotonsillar size/AHI and confounders in the cohort of 282 children with SDB
| Variables | LTE4/creatinine |
Adenotonsillar-size sum score |
AHI |
|||
|---|---|---|---|---|---|---|
| R | P | r | P | r | P | |
| Age | −0.079 | NS | −0.132 | 0.027 | 0.017 | NS |
| Gender | 0.078 | NS | 0.06 | NS | −0.078 | NS |
| Disease duration | 0.052 | NS | −0.035 | NS | 0.078 | NS |
| BMI z-score | 0.328 | < 0.001 | −0.111 | NS | 0.092 | NS |
| WHtR | 0.322 | < 0.001 | 0.093 | NS | 0.125 | 0.036 |
| Adenotonsillar size sum score | 0.166 | 0.005 | - | - | 0.371 | < 0.001 |
| SE | −0.040 | NS | −0.012 | NS | −0.064 | NS |
| AHI | 0.469 | < 0.001 | 0.371 | < 0.001 | - | - |
| OAI | 0.372 | < 0.001 | 0.322 | < 0.001 | 0.849 | < 0.001 |
| ODI | 0.400 | < 0.001 | 0.405 | < 0.001 | 0.917 | < 0.001 |
| Mean SpO2 | −0.195 | < 0.001 | −0.319 | < 0.001 | −0.533 | < 0.001 |
| Minimal SpO2 | −0.320 | < 0.001 | −0.366 | < 0.001 | −0.750 | < 0.001 |
| SLT90% | 0.358 | < 0.001 | 0.365 | < 0.001 | 0.740 | < 0.001 |
| LTE4/creatinine | - | - | 0.166 | 0.005 | 0.469 | < 0.001 |
Effects of Confounders on Respiratory Disturbance Parameters
AHI correlated significantly with BMI z-score, WHtR, adenotonsillar size, and PSG indices (OAI, ODI, SLT90%, mean and minimal SpO2), but not with age, gender, disease course, or SE (Table 3).
When multiple linear regression analysis was performed, log-transformed LTE4 and adenotonsillar-size sum score were significant predictors of ln(AHI) (P < 0.001 respectively; adjusted R2 = 0.310).
ln(AHI) = −4.182 + 0.941 × ln(LTE4/creatinine) + 0.298 × adenotonsillar-size sum score (Table 4)
Table 4.
Stepwise multiple regression models of ln(AHI)*
| Constant |
Ln(LTE4/creatinine) |
Adenotonsillar size sum score |
Ajusted R2 | ||||
|---|---|---|---|---|---|---|---|
| B | P | B | P | B | P | ||
| Ln(AHI) | −4.182 | < 0.001 | 0.941 | < 0.001 | 0.298 | < 0.001 | 0.318 |
Independent variables considered: ln(age), gender, adenotonsillar-size sum score, BMI z-score, and ln(LTE4/creatinine).
Association of Adenotonsillar Size with Respiratory Disturbance, Adiposity, and LTE4
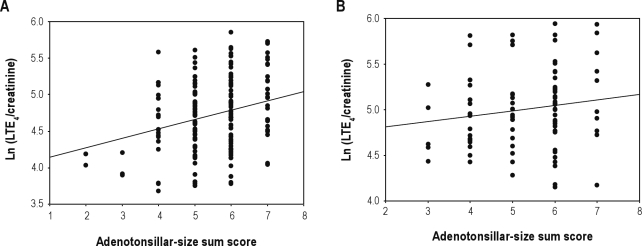
There was a significant correlation of adenotonsillar size with PSG parameters (AHI, OAI, ODI, SLT90%, mean and minimal SpO2), but not with BMI z-score, or WHtR (Table 3). A positive, albeit modest, relationship between LTE4 urinary level and adenotonsillar-size sum score was present among the underweight/normal weight SDB subjects (r = 0.276; P < 0.001), but not among the overweight/obese children (P > 0.05) (Figure 2).
Figure 2.
(A) Scatterplot between log-transformed LTE4 concentration and adenotonsillar-size sum score in 194 nonobese children (r = 0.276; P < 0.001). (B) Scatterplot between log-transformed LTE4 concentration and adenotonsillar-size sum score in 88 overweight/obese children (difference not significant).
DISCUSSION
This study conclusively demonstrates that pediatric SDB is associated with increased chronic systemic inflammation, as assessed by the higher than control LTE4 level detected in the morning urine of children with SDB. Furthermore, the magnitude of inflammation is in relation to SDB severity, the adipose level, and ATH.
Using the urinary LTE4 level, we confirmed the systemic inflammation in SDB reported in previous studies using other techniques.9,11,25–27 LTE4, the major urinary metabolite of cysLTs, is considered as the most reliable analytic parameter for monitoring the endogenous synthesis of cysLTs.28,29 First, morning urine is the most concentrated because the components are less affected by movement, dehydration, and other artificial factors. Moreover, the collection of the urine specimen is easy, noninvasive, and accepted by most of parents. By using this biomarker, previous researchers have confirmed an activation of the 5-lipoxygenase pathway in patients with asthma, allergic rhinitis,30 and respiratory syncytial virus bronchiolitis.31
Our finding of the elevation of LTE4 levels in children with more severe SDB suggests that sleep fragmentation and intermittent hypoxia might play major roles in the activation of systemic inflammation. The reoccurring desaturation-reoxygenation process has been shown to induce oxidative stress and promote the formation of reactive oxygen species,3,32,33 which are the greatest contributors to the generation of adhesion molecules, the production of leukocytes and the activation of the LT pathway.34 Interestingly, we found that even children with mild SDB had significantly elevated LTE4 levels, suggesting that even relatively mild alterations in gas homeostasis during sleep and disturbances in sleep continuity may profoundly affect the activation of the LT pathway, and ultimately induce a low-grade inflammatory state.
In particular, obesity, especially visceral obesity, is one of the major confounders in the analysis of the association between SDB and inflammation.9 Indeed, obesity directly induces a low-grade inflammatory state because adipocytes can produce numerous cytokines.35 After adjustment for adenotonsillar size, sleep respiratory disturbance parameters and other confounders, urinary LTE4 concentrations increased significantly more in the overweight/obese SDB group than the normal weight or underweight patients (Table 2). Two other investigators18,36 have discussed the association between urinary LT concentration and obesity, but the results are inconsistent. Our current finding is consistent with that of Stanke-Labesque adults36 but differs from that of Kaditis in children.18 Conflicting results between two pediatric studies might be because of the different ethnicity (Greek vs. Chinese) and/or the different urinary LTs measured (cysLTs vs. LTE4). Limited sample size may be an additional reason the negative findings by Kaditis. Even when compared with the study by Stanke-Labesque et al., the extent that obesity influences LTE4 production differs. Middle-aged adult patients tend to have more chances to be exposed to metabolic syndrome, so obesity affects LTE4 production to a larger extent than hypoxia; whereas in the current pediatric study, the impact of the two factors (BMI z-score and SLT90%) are almost the same.
Although inflammation plays a significant role in the pathophysiology of SDB, it cannot be determined whether the inflammatory mechanism is a cause, a consequence, or both in the disorder.11,18 No data confirm preexisting inflammation in children with newly diagnosed SDB. Nevertheless, the improved understanding of the relationship between inflammation and SDB may shed light on an alternative therapy for treating SDB with anti-inflammatory medications.
Although ATH is regarded as the main contributing factor for the onset and development of SDB in pediatric patients, the impact of adenotonsillar size on the severity of the disease has been unclear. Lam et al. reported in a large study of 482 children, that simple clinical staging of the tonsil size was correlated with AHI.37 Dayyat et al. reported that adenotonsillar size correlated with AHI only among nonobese children.7 However, most of the previous studies failed to establish a relationship between tonsil size by clinical inspection and AHI.38,39 The conflicting results might be because of the different patient groups and different criteria used for assessing adenotonsillar size. The current work confirms that AHI was independently predicted by both LTE4 and adenotonsillar size (Table 4), which indicates that combined management of both anti-inflammation and anti-lymphoid proliferation could improve the respiratory disturbance radically. And it also explains why only T&A without anti-inflammatory therapy was not always efficacious for some patients.40 Since even mild SDB presents an elevated level of LTs, anti-inflammatory therapy applied at an early stage of the disease may be beneficial. In the published research of Goldbart and colleagues, once-daily oral administration of montelukast, a cysLT-receptor antagonist, could serve as an effective therapeutic approach for mild cases to shrink the size of upper-airway lymphoid tissues and to normalize sleep respiratory parameters, and finally to avoid T&A.15 However, that study did not take into account the influence of obesity on therapeutic effect. Of interest, in the current study, a positive relationship between the adenotonsillar-size sum score and LTE4 urinary level was found among the nonobese SDB subjects, but not among the overweight/obese children (Figure 2). This finding may be important for the management of mild SDB in children with different obesity levels, and it is possible that antileukotriene therapy could be used independently for underweight/normal weight and mild cases; not merely to reduce inflammation but to abrogate the lymphoid proliferative signals. In overweight/obese and mild cases, however, a weight reduction strategy combined with antileukotriene therapy may be preferred. T&A still needs to be considered if the weight loss strategy is not carried out by obese patients. Since a long-lasting inflammatory state could be an important contributor to a high frequency of residual mild SDB after T&A as well as recurrence at a later age, antileukotriene medications are also suggested pre- and postoperatively to improve surgical outcome for moderate to severe cases. Nevertheless, more longitudinal studies are needed to clarify the efficacy of antileukotriene medications before wide clinical application.
Several limitations of this study deserve comment. First, PSG and nasopharyngoscopy were not performed in the control subjects because of objections by the parents. Second, the evaluation of adenoid and tonsil size was conducted by multiple clinicians rather than by a designated investigator, which undoubtedly could cause large inter-individual variability. Third, children with sleep apnea have a high prevalence of sensitization to allergens.41,42 Although in the cohort selection, the exclusion criteria included asthma, allergic rhinitis and other allergies, those were excluded only through a detailed questionnaire given to parents, without any allergen detection in the blood. Finally, children with primary snoring were not enrolled in our study because of the small sample size.
In summary, this investigation demonstrates an increased urinary level of LTE4 in pediatric SDB, providing further evidence that systemic inflammatory response participated in the pathophysiological mechanisms of SDB in childhood. And LT production emerges SDB severity and obesity-dependent increases. AHI, a sensitive index of SDB severity, can be predicted by both LTE4 concentration and adenotonsillar size, and LTE4 level significantly correlates with adenotonsillar size in underweight/normal weight SDB subjects but not in overweight/obese children, indicating that LTs modification therapy, adiposity loss strategy, and T&A should be applied appropriately in the treatment of pediatric patients with differing degrees of SDB severity and obesity.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the children and parents who participated in this study as controls, the staff at the Laboratory of Virology and Nutriology who provided expert technical assistance and the technical staff who performed the polysomnograms.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ATH
adenotonsillar hypertrophy
- BMI
body mass index
- CysLT
cysteinyl leukotriene
- IQR
interquartile range
- LTs
leukotrienes
- LTE4
leukotriene E4
- OAI
obstructive apnea index
- ODI
oxygen desaturation index
- PSG
polysomnography
- SDB
sleep disordered breathing
- SLT90%
percentage of time spend saturation lower than 90%
- SpO2
pulse oximetry saturation
- SE
sleep efficiency
- T&A
tonsillectomy and adenoidectomy
- TST
total sleep time
- WHtR
waist height ratio
REFERENCES
- 1.Marcus CL, McColley SA, Carroll JL, et al. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 2.Arens R, McDonough JM, Corbin AM, et al. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2003;167:65–70. doi: 10.1164/rccm.200206-613OC. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Kheirandish L. Oxidant stress and inflammation in the snoring child: confluent pathways to upper airway pathogenesis and end-organ morbidity. Sleep Med Rev. 2006;10:83–96. doi: 10.1016/j.smrv.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med Clin. 2007;2:433–44. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–13. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 6.Kohler MJ, Thormaehlen S, Kennedy JD, et al. Differences in the association between obesity and obstructive sleep apnea among children and adolescents. J Clin Sleep Med. 2009;5:506–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, et al. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137–44. doi: 10.1378/chest.08-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaditis AG, Alexopoulos EI, Hatzi F, et al. Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath. 2008;12:25–31. doi: 10.1007/s11325-007-0132-z. [DOI] [PubMed] [Google Scholar]
- 9.Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–6. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 10.Capdevila OS, Kheirandish-Gozal L, Dayyat E, et al. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5:274–82. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldbart AD, Tal A. Inflammation and sleep disordered breathing in children: a state-of-the-art review. Pediatr Pulmonol. 2008;43:1151–60. doi: 10.1002/ppul.20943. [DOI] [PubMed] [Google Scholar]
- 12.Gozal D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009;10(Suppl 1):S12–6. doi: 10.1016/j.sleep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 14.Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–95. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 15.Goldbart AD, Goldman JL, Veling MC, et al. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172:364–70. doi: 10.1164/rccm.200408-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayyat E, Serpero LD, Kheirandish-Gozal L, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135:1142–9. doi: 10.1378/chest.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldbart AD, Krishna J, Li RC, et al. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest. 2006;130:143–8. doi: 10.1378/chest.130.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Kaditis AG, Alexopoulos E, Chaidas K, et al. Urine concentrations of cysteinyl leukotrienes in children with obstructive sleep-disordered breathing. Chest. 2009;135:1496–501. doi: 10.1378/chest.08-2295. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Ji CY, Zong XN, et al. Body mass index growth curves for Chinese children and adolescents aged 0 to18 years. Zhonghua Er Ke Za Zhi. 2009;47:493–8. [PubMed] [Google Scholar]
- 20.Wang JH, Chung YS, Cho YW, et al. Palatine tonsil size in obese, overweight, and normal-weight children with sleep-disordered breathing. Otolaryngol Head Neck Surg. 2010;142:516–9. doi: 10.1016/j.otohns.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Modrzynski M, Zawisza E. An analysis of the incidence of adenoid hypertrophy in allergic children. Int J Pediatr Otorhinolaryngol. 2007;71:713–9. doi: 10.1016/j.ijporl.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A, editors. Washington, DC: US Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, National Institute of Neurological Disorders; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 23.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery-Downs HE, O'Brien LM, Gulliver TE, et al. Polysomnographic characteristics in normal preschool and early school-age children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 25.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 26.Waters KA, Mast BT, Vella S, et al. Structural equation modeling of sleep apnea, inflammation, and metabolic dysfunction in children. J Sleep Res. 2007;16:388–95. doi: 10.1111/j.1365-2869.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 27.Gozal D, Serpero LD, Sans Capdevila O, et al. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9:254–9. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi M, Higashi N, Ono E, et al. Hyperleukotrieneuria in patients with allergic and inflammatory disease. Allergol Int. 2008;57:313–20. doi: 10.2332/allergolint.08-RAI-0040. [DOI] [PubMed] [Google Scholar]
- 30.Rabinovitch N. Urinary leukotriene E4. Immunol Allergy Clin North Am. 2007;27:651–64. doi: 10.1016/j.iac.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Piedimonte G, Renzetti G, Auais A, et al. Leukotriene synthesis during respiratory syncytial virus bronchiolitis: influence of age and atopy. Pediatr Pulmonol. 2005;40:285–91. doi: 10.1002/ppul.20285. [DOI] [PubMed] [Google Scholar]
- 32.Lavie L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 33.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–75. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werz O, Szellas D, Steinhilber D. Reactive oxygen species released from granulocytes stimulate 5-lipoxygenase activity in a B-lymphocytic cell line. Eur J Biochem. 2000;267:1263–9. doi: 10.1046/j.1432-1327.2000.01000.x. [DOI] [PubMed] [Google Scholar]
- 35.Visser M, Bouter LM, McQuillan GM, et al. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107:E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 36.Stanke-Labesque F, Bäck M, Lefebvre B, et al. Increased urinary leukotriene E4 excretion in obstructive sleep apnea: Effects of obesity and hypoxia. J Allergy Clin Immunol. 2009;124:364–70. doi: 10.1016/j.jaci.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 37.Lam YY, Chan EY, Ng DK, et al. The correlation among obesity, apnea-hypopnea index, and tonsil size in children. Chest. 2006;130:1751–6. doi: 10.1378/chest.130.6.1751. [DOI] [PubMed] [Google Scholar]
- 38.Li AM, Wong E, Kew J, et al. Use of tonsil size in the evaluation of obstructive sleep apnoea. Arch Dis Child. 2002;87:156–9. doi: 10.1136/adc.87.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard NS, Brietzke SE. Pediatric tonsil size: objective vs subjective measurements correlated to overnight polysomnogram. Otolaryngol Head Neck Surg. 2009;140:675–81. doi: 10.1016/j.otohns.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Brietzke SE, Gallagher D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:979–84. doi: 10.1016/j.otohns.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 41.Kalra M, Lemasters G, Bernstein D, et al. Atopy as a risk factor for habitual snoring at age 1 year. Chest. 2006;129:942–6. doi: 10.1378/chest.129.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chng SY, Goh DY, Wang XS, et al. Snoring and atopic disease: a strong association. Pediatr Pulmonol. 2004;38:210–6. doi: 10.1002/ppul.20075. [DOI] [PubMed] [Google Scholar]