Abstract
Study Objectives:
The objectives were (1) to assess associations between sleep consolidation at 6, 18 and 30 months and language skills at 18, 30, and 60 months; and (2) to investigate the genetic/environmental etiology of these associations.
Design:
Longitudinal study of a population-based twin cohort.
Participants:
1029 twins from the Quebec Newborn Twin Study.
Measurements and Results:
Sleep consolidation was derived from parental reports of day/night consecutive sleeping durations. Language skills were assessed with the MacArthur Communicative Development Inventory at 18 and 30 months and the Peabody Picture Vocabulary Test at 60 months. The day/night sleep ratio decreased significantly from 6 to 30 months. The 6- and 18-month ratios were negatively correlated with subsequent language skills. Children with language delays at 60 months had less mature sleep consolidation at both 6 and 18 months than children without delays and those with transient early delays. Genetic and regression analyses revealed that the sleep ratio at 6 months was highly heritable (64%) and predicted 18-month (B = −0.06) and 30-month language (B = −0.11) mainly through additive genetic influences (RGs = 0.32 and 0.33, respectively). By contrast, the sleep ratio at 18 months was mainly due to shared environment influences (58%) and predicted 60-month language (B = −0.08) through shared environment influences (RCs = 0.24).
Conclusions:
Poor sleep consolidation during the first 2 years of life may be a risk factor for language learning, whereas good sleep consolidation may foster language learning through successive genetic and environmental influences.
Citation:
Dionne G; Touchette E; Forget-Dubois N; Petit D; Tremblay RE; Montplaisir JY; Boivin M. Associations between sleep-wake consolidation and language development in early childhood: a longitudinal twin study. SLEEP 2011;34(8):987-995.
Keywords: Sleep-wake consolidation, language, early childhood, genetics
INTRODUCTION
The consolidation and maturation of sleep-wake rhythms in early childhood have been linked to a variety of early cognitive functions in premature infants,1–4 full-term infants,5–9 and a large-scale normative sample of preschoolers.10 Measures of sleep-wake cycles vary greatly in these studies, from objective measures using actigraphy or polysomnography1–5,7,8 to parental reports of sleep duration and night wakings.6,10 However, empirical results are relatively consistent: indices of more mature sleep-wake consolidation in early childhood have been associated with better cognitive outcomes.
Language acquisition seems particularly vulnerable to sleep-wake state organization.6–8,10 Two mechanisms have been evoked to explain why this may be the case. First, poor sleep consolidation may hinder memory processes11,12 required in early language learning. Second, lower-order regulatory systems, such as sleep, may have an organizing effect over higher-order systems involved in cognition.13 Therefore, as sleep-wake cycles mature, so should children's ability to regulate attention and process language during social interactions, resulting in better language skills in children whose sleep consolidation matures earlier.
Both mechanisms involve a contribution of sleep consolidation to language learning. Another possibility is that the association between sleep consolidation and language learning is due to common genetic or environmental influences. For instance, the association may stem from biological maturation governed by genetic predispositions and possibly reflect individual differences in neurological functioning. Alternately, environmental factors may affect both sleep consolidation and language learning. For example, perinatal insults may affect neurodevelopmental integrity and have an organizing effect on both sleep-wake cycles and cognitive development.14 On a more proximal level, parental practices may also be involved in the sleep-language association. Mothers affected by their infants' unconsolidated and fragmented sleep may provide less optimal language stimulation, or overall inadequate parental practices may negatively affect both sleep and language learning in infants.15 Understanding how genes and environment affect sleep consolidation may shed some light on these hypotheses and offer insight into best practices around early infant sleep.
Owing to the variety of sleep parameters in infant sleep research, there is no consensus on the best indicator of sleep maturation in early childhood. However, two recent studies suggest that indices reflecting both the gradual increase in nighttime sleep and concomitant decrease in daytime sleep offer the best measures of sleep consolidation during this period, over and above total sleep durations.
First, using sleep measures derived from actigraphy, Acebo and colleagues16 found that the maturational changes in 24-h total sleep between 12 and 60 months of age were mostly due to the gradual decrease in daytime sleep as a function of nighttime sleep. Although total sleep declined with age, it was not the best indicator of sleep changes with age, as it masked the gradual shift from the need for daytime sleep to sleep occurring mostly at night. Indeed, sleep-wake periods are cyclical but free-running at birth, especially in premature infants,17 with more or less equal day and night sleep durations. As sleep cycles evolve with time following a normative pattern,18 total sleep decreases from 16-18 h/day (and more in premature infants prior to term date) to 13-14 h/day around 6 months. The first manifestations of a circadian rhythm appear by 2-4 months, when daytime sleep gradually represents a smaller proportion of nighttime sleep duration.19 By 6 months of age, a majority of sleep duration occurs at night: the 3 to 4 hours of average daytime nap represent about a third of nighttime sleep. By 18 months of age, daytime sleep accounts for a quarter of nighttime sleep, as most children are able to sleep 10 consecutive hours at night with one 2.5-h afternoon nap.20 Therefore, the day/night sleep ratio gradually decreases from 18 to 60 months to reach 0 once children no longer nap.16
Second, Bernier and colleagues9 recently examined the contribution of 3 parameters derived from the sleep diaries of 12 and 18 month-old-infants to later cognitive outcomes. Total sleep and sleep fragmentation did not predict cognitive outcomes, but the proportion of sleep occurring at night predicted both executive functions and language level. In fact, average total sleep did not decrease between 12 and 18 months. This may not be surprising given that individual differences in total sleep durations may partly reflect genetic tendencies21 that are distinct from maturational processes. Therefore, a ratio of day/night sleep duration should be a good indicator of the maturational changes occurring in sleep consolidation during the developmental period when most children still nap.
In the present study, we investigated the associations between sleep consolidation at 6, 18, and 30 months (corrected for gestational duration) and language outcomes at 18, 30, and 60 months in a birth cohort of 1029 twins. The first objective was to determine if day/night sleep ratios at 6, 18, and 30 months were associated with short-term and long-term language outcomes. First, we looked at associations between sleep consolidation and language skills with correlations and regression models. Second, we compared the sleep consolidation of children with differing trajectories of language development between 18 and 60 months, based on the presence or absence of language delays. One central question in language development concerns outcomes of early delays. Some children with early language delays catch up with the language skills of their peers, usually before the end of the preschool years, whereas for other children, language delays persist or develop over time.22,23 It remains unclear why this is the case. If sleep consolidation affects language learning, we hypothesized that children with persistent or late onset language delays would have shown less mature sleep consolidation at earlier ages. To test this hypothesis, we compared the sleep ratio of 3 groups from a subsample of 618 children with complete language assessments at both 18 months and 60 months: children without delays, children with transient delays, and children with persistent or late onset delays.
The second objective was to investigate the etiology of the sleep-language associations using multivariate genetic modeling. If the association is largely due to genetic factors, then we may conclude that genetically driven maturational processes explain why language outcomes are linked to sleep maturation in early childhood. If the association is largely due to environmental factors, then we may look to environmental processes to understand why sleep consolidation and language learning, or parents' report of them, are linked during development.
METHODS
Participants and Procedures
Data were extracted from the Quebec Newborn Twin Study (QNTS) which conducted annual assessments of physiological, behavioral, and cognitive development in a population-based sample of 1430 twins (715 families) from the greater Montreal area. All twin births between November 1995 and July 1998 were recruited at delivery. To be included in the sample, infants had to be born without major medical conditions, have available birth records to derive perinatal data (available for 1182 individual twins), and be fluent in either French or English. To be included in the analyses, children had to have at least one sleep and one language assessment between 6 and 60 months (5 years). This was the case for 1029 twins, 506 boys and 523 girls, 419 MZ twins and 610 DZ twins, including 192 opposite-sex pairs. Attrition averaged 9% per year, yielding smaller sample sizes for later sleep and language assessments. Mean corrected gestational ages at the targeted 6, 18, 30, and 60-month assessments included in this study were: 5.39 months (SD 0.51), 18.61 months (SD 0.60), 30.95 months (SD 0.81), and 62.64 months (SD 3.03). For all parent reports, a 2-week interval separated the twins' assessments to avoid inflated familial aggregation. Ethics approval and informed parental consent were obtained prior to each data collection; non-nominal data only were available for analyses.
The mean gestation duration was 36 weeks (SD 2.57; median 37), based on a full-term criterion of 37 weeks; 470 twins (45.7%) were born premature; mean birth weight was 2454 g (SD 546 g), mean 1/5 minute Apgar scores were 8.5/10 (SD 1.2; median 9), and the mean number of days spent in the hospital after birth were 9.5 (SD 13; median 5). Forty-seven percent of twins were born by caesarean section. Mean age of mothers at birth was 30 years (SD 4.7 years); 17% had no high school diploma, and 31% had a university degree; average family income was between 40K and 50K/year CAD; 91.7% of mothers and 91% of fathers described themselves as Caucasians; and 5% of twins were born to single mothers.
French was the first language for 85% of infants and English for 15% of infants, although 12% of mothers had a first language other than French or English. Children identified as using both French and English at time of assessment (6%) were assessed using the language they use most often based on parent indication.
Measures
Perinatal data were derived from medical records. Data on cigarette use (average number of cigarettes smoked per day throughout pregnancy), maternal education and first language (French/English or other), and family income and structure (1- or 2-parent families) were obtained via self-report in interviews with mothers at the 6-month assessment. At 18 months, zygosity was assessed for same-sex twin pairs (n = 237) based on physical resemblance using the Zygosity Questionnaire for Young Twins24 and confirmed using a DNA analysis of 8-10 highly polymorphous genetic markers on half the sample. Twin concordance was 94%, which is similar to rates in older twin samples.25
Infant sleep
When children were approximately 6, 18, and 30 months old, mothers were questioned on their infants' sleep and asked to use the week prior to the interview as a reference. At 6 months, the duration of consecutive nighttime sleep was assessed in rounded hours ranging from < 4 consecutive hours, 5, 6, 7, 8, or > 8 consecutive hours. Mothers were also asked if their twins slept in the same bed at night at this age to assess possible confounding effects of twin cosleeping. At 18 and 30 months, the duration of consecutive nighttime sleep was assessed in rounded hours ranging from < 4 consecutive hours, 4, 5, 6, 7, 8, 9, and 10 to > 10 consecutive hours. At all ages, the duration of consecutive daytime sleep was assessed in rounded hours ranging from does not nap (0), ≤ 1 consecutive hour, 2, 3, 4, to > 4 consecutive hours. The “less than” and “more than” categories were rounded to the nearest whole value to compute a sleep ratio of day/night consecutive sleep duration. Consecutive sleep takes into account the maturational changes that lead to less fragmented sleep and longer sleep bouts with age during this developmental period. Descriptive statistics are given on raw data. Data were then corrected for gestational age and transformed to Z scores for subsequent analyses.
Language skills
At 18 and 30 months, language was measured using parent reports on the McArthur Communicative Development Inventory-Short Form (MCDI-SF)26 expressive and receptive vocabularies using a 77-word checklist at 18 months and a 100-word checklist at 30 months, from which parents indicated words the child could say (expressive) and words the child only understood (receptive). The French versions of the MCDI-SF at 18 and 30 months were adaptations of the American short forms used for English-speaking children. French and English raw means were not significantly different; therefore, they were analyzed together.
At 60 months, receptive vocabulary was assessed with French or English versions of the Peabody Picture Vocabulary Test (PPVT).27 Expressive vocabulary was assessed with an adapted version of the PPVT in which children were first asked to name designated objects. There were language-based mean differences on the normative scores; therefore, raw scores were retained for analyses. Twins from the same family were assessed by separate examiners.
Both the MCDI-SF and PPVT are widely used and have excellent psychometric properties.26,27 All language measures were adjusted for corrected gestational age and converted to Z scores.
Preliminary analyses (not shown) showed that the pattern of results did not differ for expressive and receptive language scores; therefore they were combined to yield a total language score at each age. For analyses based on the presence or absence of delays, we created 3 language groups from a subsample of 618 children with complete assessments at both 18 and 60 months: children without delays (N = 385), children with transient delays (N = 93; delay at 18 months but not at 60 months), and children with persistent or late onset delays (N = 140; delays at both 18 and 60 months or no delay at 18 months, but delayed at 60 months). Language delay was determined based on the total language score being one standard deviation (SD) below the mean.
Child control variables
Child sex, birth weight, gestation duration, average 1 and 5 min Apgar score at birth, number of days spent in hospital after birth, and difficult temperament were used as control variables. Difficult temperament was assessed by mothers using 7 items from the Infant Characteristics Questionnaire (ICQ)28 when children were 6 and 18 months of age. Parents rated items on a 7-point scale asking them how difficult their child was, in comparison to other children (Cronbach α at 6 and 18 months = 0.77 and 0.89). Six- and 18-month scores were averaged (r = 0.36) to yield a general difficultness score allowing one missing value to maximize sample size.
Maternal/family control variables
Maternal education, family income, average number of cigarettes smoked per day throughout pregnancy, maternal depressive symptoms at 6 and 18 months, mothers' perceived parental impact, and maternal overprotection were used as maternal and family covariables. Maternal education was assessed on a 3-point scale, (0) no high school diploma, (1) high school or technical diploma, and (2) university diploma. Family income at birth of twins was rated on a 0-10 scale, starting with (0) no income with 5,000 CAD increments up to (10) more than 80,000 CAD. The average number of cigarettes smoked per day throughout pregnancy was assessed at 6 months in a maternal self-report questionnaire. Maternal depressive symptoms were assessed at both 6 and 18 months on the 12-item abbreviated version of the 20-item Center for Epidemiological Studies Depression Scale (CES-D)29 designed for the National Institute of Mental Health to measure depressive symptomatology in the general population. Mothers were asked how often in the previous week they experienced various symptoms of depression, on a 4-point scale. Items were averaged and converted to a 0-10 scale (Cronbach α = 0.80). Six and 18-month scores were averaged to yield a general depression score (r = 0.48) allowing one missing value to minimize missing data. Mothers' perceived parental impact and maternal overprotection were assessed at 6, 18 and 30 months post-term with 5 and 4 items respectively on a 0-10 scale from the Parental Cognitions and Conduct Toward the Infant Scale (PACOTIS).30 For items of the perceived parental impact scale, mothers were asked how much they felt they could influence the course of their child's development. For items of the overprotection scale, mothers were asked if they felt the need to keep their babies close at all times and/or felt at ease to leave babies with a sitter. Scores were averaged by age (Cronbach α > 0.75 for all age-specific scales) to yield 6-, 18-, and 30-month scores that were then averaged allowing for 2 missing values (rs ranging from 0.48 to 0.67 for perceived parental impact, and 0.65 to 0.75 for maternal overprotection).
Analyses
After an initial look at the correlations between sleep and language measures, structural equation regressions using the AMOS 5 package31 were used to predict language skills at 18, 30, and 60 months from sleep measures. AMOS 5 was preferred to other statistical tools for 2 reasons. First, it allows a multilevel approach (family level, child level) correcting estimates for the non-independence of twin data when analyses require considering twins as individuals. Second, it allows the treatment of missing data with the Full Information Maximum Likelihood (FIML) estimation procedure reducing bias due to selective attrition. The FIML estimation procedure, contrary to multiple imputations, is implemented directly in the process of fitting a model; it treats missing data by fitting the model to all non-missing data for each observation.32 We chose FIML over multiple imputations because there is evidence that FIML has more power unless a great number of imputations are done.33
A stepwise procedure was adopted for the regression models. Models were first tested with zygosity, child sex, other child covariables (factor scores including gestation duration, birth weight, mean 1/5 minute Apgar score, child difficult temperament), maternal covariables (factor scores including smoking during pregnancy, maternal education, family income, depression symptoms, perceived parental impact and maternal overprotection), and maternal first language (dichotomized into French/English or other) as predictors. The sleep ratio of day/night consecutive hours was added in successive models by age. The α threshold was set at 0.05. Finally, models predicting language skills at 30 and 60 months were tested adjusting for previous language outcome to test the contribution of the ratio of day/night consecutive sleeping duration to language development between time-points.
To look at longitudinal language outcomes based on the presence or absence of delays at 18 and 60 months, ANOVAs were performed to compare the 3 longitudinal language groups on sleep ratios at 6, 18, and 30 months. Individual ANOVAs were preferred to a MANOVA to maximize sample size. T-tests were chosen for post hoc comparisons.
Finally, genetic models for associated sleep-language measures were fitted to raw data using structural equations with the Mx package.34 The relative contribution of additive genes (A), shared environment (C) and nonshared environment (E) to variation is estimated by comparing within and across family similarity of twin pairs based on their genetic similarity. This is possible because monozygotic twins (MZ) share 100% of their genes, whereas dizygotic twins share 50% of their genes. The extents to which identical twins are more similar on an outcome translate into a larger contribution of additive genes to this outcome. This reflects the heritability of the measures. The extent to which twin pairs are similar, regardless of their genetic similarity, indicates an effect of shared environmental influences, usually associated with family factors making siblings more similar. The extent to which identical twins are dissimilar may reflect nonshared environmental influences, factors making siblings different, or measurement error.
Child sex and maternal first language being other than French or English were entered as covariables in the genetic models. Figure 1 shows the path model for the basic bivariate Cholesky decomposition. Variance components are derived from genetic and environmental contributions to each measure whereas covariance components are derived from paths across measures. Using the bivariate Cholesky decomposition, the covariance between sleep and language can be divided into its genetic and environmental components by calculating a genetic correlation (RG), a shared environment correlation (RC), and a unique environment correlation (RE) reflecting the extent to which the same genetic and/or environmental factors are influencing both sleep and language variances. Nested models were compared to saturated models to test path significance. Best fitting models are reported.
Figure 1.
Path diagram depicting the genetic and environmental model of the correlation between sleep consolidation and language. A, C, and E refer to additive genetic, shared environmental, and nonshared environmental influences, respectively. RG, RC, and RE refer to the genetic, shared environmental, and nonshared environmental correlations derived from covariance estimates.
RESULTS
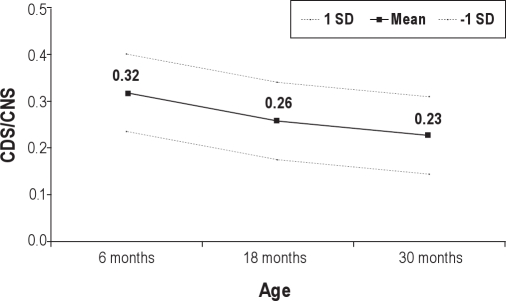
Table 1 presents descriptive statistics for consecutive daytime sleeping durations, and consecutive nighttime sleeping durations at 6, 18, and 30 months. Repeated measures analyses indicated that consecutive nighttime sleeping increased with age (F2,591 = 551.99, P < 0.001), whereas consecutive daytime sleeping decreased with age (F2,565 = 49.18, P < 0.001) during this period. Figure 2 depicts the mean, range, and standard deviation for the ratio of day/night consecutive sleeping durations at 6, 18, and 30 months. At 6 months, the consecutive daytime sleeping duration represented on average one-third of the consecutive nighttime sleeping duration, indicating that most infants are sleeping through the night at this age and take shorter naps during the day. The sleep ratio of day/night consecutive hours decreases significantly with age between 6 and 30 months (F2,533 = 280.27, P < 0.001). By 30 months of age, some children no longer slept during the day (minimum = 0), but the consecutive daytime sleeping duration still represented, on average, a little less than a quarter of the duration of consecutive nighttime sleep (23%).
Table 1.
Means (M) and standard deviation (SD) for sleep measures at 6, 18, and 30 months
| 6 months | 18 months | 30 months | |
|---|---|---|---|
| n = 977 | n = 881 | n = 797 | |
| Sleep measures | |||
| CNS | 8h06 (1h26) | 10h06 (1h28) | 10h12 (1h31) |
| CDS | 2h30 (0h48) | 2h30 (0h38) | 2h12 (0h39) |
CNS, consecutive nighttime sleep duration; CDS, consecutive daytime sleep duration.
Figure 2.
Changes in means and standard deviations (SD) for the ratio of consecutive daytime sleep/consecutive nighttime sleep (CDS/CNS) between 6, 18 and 30 months of age adjusted for gestation duration; F2,533 = 280.27, P < 0.001. N = 977 at 6 months, 881 at 18 months, and 797 at 30 months.
There were differences in the consecutive nighttime sleeping duration at 6 months associated with twin cosleeping. Twins sleeping in the same bed (N = 45 twin pairs) slept on average 1 h less at night than twins not sleeping together (N = 440 twin pairs) (7h07[1h52] versus 8h12[1h21], t953 = 5.35, P < 0.001). Therefore, the consecutive nighttime sleeping duration and the ratio of day/night sleep at 6 months were corrected for twin cosleeping in subsequent analyses. There were also some sex differences on daytime sleep durations. Boys slept significantly longer consecutive durations than girls during the daytime at 18 months (2h35[0h44] versus 2h30[0h37], t 880 = 2.18, P = 0.03) and at 30 months (2h16[0h40] versus 2h10[0h38], t 747 = 2.11, P = 0.04]), but not at 6 months. There were no differences between boys and girls or between MZ and DZ twins on consecutive nighttime sleeping durations or sleep ratios.
Table 2 presents Spearman correlations between sleep measures within and across ages. Within-age correlations are highlighted in bold below the diagonal. At all ages, the sleep ratio was positively correlated with the consecutive daytime sleeping duration and negatively correlated with the consecutive nighttime sleeping duration. At 6 months, the consecutive daytime sleeping duration and the consecutive nighttime sleeping duration were modestly correlated (r = 0.13) indicating that infants who slept more consecutive hours at this age tended to do so both at night and during the day. This could reflect individual differences in total sleep duration rather than maturational processes. Therefore, the sleep ratio may be a better indicator of sleep consolidation than 2 individual measures of consecutive sleep duration. By 18 months, the consecutive daytime and nighttime sleeping durations were no longer correlated. The cross-age correlations are shown above the diagonal in Table 2. Overall, cross-age correlations were modest but increased with time indicating early intra-individual change and progressive stability of individual differences.
Table 2.
Within- and across-age Spearman correlations between sleep measures
| 6 months |
18 months |
30 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CNS | CDS | Ratio | CNS | CDS | Ratio | CNS | CDS | Ratio | |
| 6M CNS | 0.36*** | 0.26*** | |||||||
| 6M CDS | 0.13*** | 0.20*** | 0.12** | ||||||
| 6M Sleep ratio | −0.53*** | 0.74*** | 0.26*** | 0.21*** | |||||
| 18M CNS | 0.05 | −0.16*** | 0.40*** | ||||||
| 18M CDS | 0.04 | 0.17*** | 0.01 | 0.25*** | |||||
| 18M Sleep ratio | −0.21*** | 0.13*** | −0.64*** | 0.72*** | 0.31*** | ||||
| 30M CNS | 0.01 | −0.11** | 0.06 | −0.21*** | |||||
| 30M CDS | −0.02 | 0.08* | −0.01 | 0.21*** | 0.05 | ||||
| 30M Sleep ratio | −0.17*** | 0.12** | −0.23*** | 0.18*** | −0.56*** | 0.76*** | |||
Within-age correlations are in bold text. Across-age correlations within measures are shown above the diagonal.
CNS, consecutive nighttime sleep duration; CDS, consecutive daytime sleep duration.
Sleep ratio is the CDS divided by CNS.
P <0.05,
P < 0.01,
P < 0.001.
Are Sleep Measures Associated with Language Outcomes?
Table 3 shows within- and cross-age Spearman correlations between sleep measures and language outcomes. Correlations were modest, but those involving the sleep ratios were generally higher than the 2 individual measures of consecutive sleep duration. As larger sleep ratios reflect less mature sleep consolidation, sleep ratios at 6 and 18 months were both negatively associated with subsequent language measures. This indicates that children with more consolidated sleep at 6 and 18 months had better language skills up to 3 and a half years later. By contrast, same-age sleep ratios and language outcomes were not correlated. The time delay suggests that sleep consolidation may have a causal effect on the language learning process.
Table 3.
Within- and across-age Spearman correlations between 6-, 18-, and 30-month sleep measures and 18-, 30-, and 60-month language outcomes
| Language outcomes |
|||
|---|---|---|---|
| Sleep measures | 18 months | 30 months | 60 months |
| 6 months | |||
| CNS | −0.04 | 0.03 | 0.05 |
| CDS | −0.14*** | −0.17*** | −0.12*** |
| Sleep ratio | −0.15** | −0.16*** | −0.14*** |
| 18 months | |||
| CNS | −0.05 | 0.06 | 0.12** |
| CDS | −0.03 | −0.11* | −0.08 |
| Sleep ratio | 0 | −0.14*** | −0.16*** |
| 30 months | |||
| CNS | 0.05 | 0.05 | 0.05 |
| CDS | −0.04 | −0.05 | 0 |
| Sleep ratio | −0.07 | −0.08 | −0.05 |
CNS, consecutive nighttime sleep duration; CDS, consecutive daytime sleep duration.
Sleep ratio is the CDS divided by CNS.
P < 0.05,
P < 0.01,
P < 0.001.
Does Early Sleep Consolidation Predict Language Outcomes after Controlling for Confounding Factors?
Table 4 shows the results of structural equation regression models including confounding variables. The model predicting language at 18 months revealed a significant contribution of the 6-month (B = −0.06) sleep ratio after control variables were taken into account. Zygosity, sex and maternal first language were also significant predictors in the model. The model predicting language at 30 months also showed a significant contribution of the 6-month sleep ratio (B = −0.11). Child sex, maternal covariables, and first language were also significant predictors in the model. Once 30-month language was adjusted for 18-month language, the 6-month sleep ratio still predicted the outcome (B = −0.09). This suggests that the 6-month sleep ratio influences not only the language outcome at 30 months, but also language development between 18 and 30 months. Finally, the model predicting language at 60 months revealed a significant contribution of the 18-month sleep ratio (B = −0.08) after covariables were taken into account. In addition, the contribution of the 18-month sleep ratio remained significant (B = −0.07) after controlling for language at 30 months suggesting again that sleep consolidation has a continuing contribution to language development during this period.
Table 4.
Structural equation regression models predicting language outcomes at 18, 30, and 60 months from sleep ratios at 6 and 18 months
| Model – Outcome | Step | Predictor | B (SE) | P | b | c2 | df | P |
|---|---|---|---|---|---|---|---|---|
| 18-month Language | 1 | Zygosity | 0.19 (0.07) | 0.008 | 0.11 | 19.54 | 15 | 0.19 |
| Sex | 0.15 (0.04) | < 0.001 | 0.09 | |||||
| Child covariables | −0.07 (0.05) | 0.16 | −0.04 | |||||
| Mother covariables | 0.11 (0.05) | 0.03 | 0.09 | |||||
| Language status | −0.22 (0.10) | 0.02 | −0.10 | |||||
| 2 | 6-month sleep ratio | −0.06 (0.02) | 0.01 | -0.07 | 23.85 | 19 | 0.20 | |
| 30-month Language | 1 | Zygosity | 0.00 (0.01) | 0.83 | 0.01 | 16.36 | 15 | 0.36 |
| Sex | 0.02 (0.01) | 0.01 | 0.08 | |||||
| Child covariables | 0.00 (0.01) | 0.89 | 0.00 | |||||
| Mother covariables | 0.03 (0.01) | 0.004 | 0.12 | |||||
| Language status | −0.07 (0.02) | < 0.001 | −0.19 | |||||
| 2 | 6-month sleep ratio | −0.02 (0.01) | 0.002 | −0.11 | 19.85 | 19 | 0.40 | |
| 3 | 18-month sleep ratio | −0.00 (0.01) | 0.84 | −0.01 | 35.77 | 23 | 0.04 | |
| Adjusted for 18-month language | 4 | 6-month sleep ratio | −0.01 (0.01) | 0.01 | −0.09 | 32.83 | 23 | 0.08 |
| 5 | 18-month sleep ratio | −0.01 (0.01) | 0.12 | −0.06 | 50.68 | 27 | 0.004 | |
| 60-month Language | 1 | Zygosity | 0.06 (0.08) | 0.81 | 0.03 | 12.70 | 15 | 0.63 |
| Sex | −0.06 (0.05) | 0.30 | −0.03 | |||||
| Child covariables | −0.08 (0.06) | 0.17 | −0.04 | |||||
| Mother covariables | 0.49 (0.06) | < 0.001 | 0.34 | |||||
| Language status | −0.85 (0.10) | < 0.001 | −0.34 | |||||
| 2 | 6-month sleep ratio | −0.05 (0.03) | 0.09 | −0.06 | 21.39 | 19 | 0.32 | |
| 3 | 18-month sleep ratio | −0.07 (0.03) | 0.05 | −0.07 | 24.05 | 23 | 0.40 | |
| Adjusted for 30-month language | 4 | 6-month sleep ratio | −0.04 (0.03) | 0.22 | −0.04 | 47.67 | 23 | 0.002 |
| 5 | 18-month sleep ratio | −0.06 (0.03) | 0.06 | −0.07 | 49.80 | 27 | 0.005 |
The factor score for child covariables includes gestation duration, birth weight, Apgar score, number of days in the hospital after birth, and child difficult temperament. The factor score for maternal covariables includes education, family income, depressive symptoms, overprotectiveness, and impact perception.
Does Sleep Consolidation Vary in Groups with Differing Longitudinal Outcomes Based on the Presence or Absence of Language Delays?
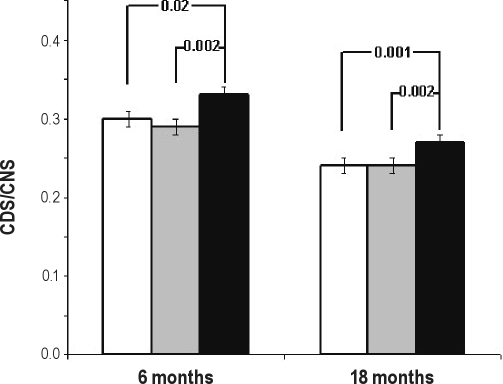
ANOVAs performed to compare longitudinal language groups on the sleep ratio, show significant differences between groups at 6 months (F2,514 = 4.667, P = 0.01) and 18 months (F2,581 = 10.292, P < 0.001), but not at 30 months (F2,486 = 0.065, P = 0.94). Figure 3 shows the means and standard errors (SEs) of the sleep ratio at 6 and 18 months for longitudinal language groups. Post hoc t-tests revealed that children with persistent or late onset delays had less mature sleep consolidation (higher ratios) not only compared to children without delays (t433 = −2.446, P = 0.02; t161.86 = −3.318, P = 0.001 at 6 and 18 months, respectively), but also compared to children with transient delays (t197 = −2.790, P = 0.006; t212.94 = −3.168, P = 0.002 at 6 and 18 months, respectively). Children with transient delays did not differ from children without delays on the sleep ratio at any age. In fact, 90% of children with transient language delays had sleep ratios at or below the mean at both 6 months and 18 months. By contrast, 62% and 57% of children with persistent or late onset language delays had sleep ratios above the mean at 6 and 18 months, respectively.
Figure 3.
Longitudinal language groups as a function of the ratio of consecutive daytime sleep/consecutive nighttime sleep (CDS/CNS) at 6 and 18 months. Children with persistent or late language delays (■ N = 140) differed significantly from children without language delays (☐ N = 385) and from children with transient delays ( N = 93) on both the 6- and 18-month sleep ratio. Sleep ratio was adjusted for gestation duration corrected age.
N = 93) on both the 6- and 18-month sleep ratio. Sleep ratio was adjusted for gestation duration corrected age.
How are Sleep and Language Associated?
Table 5 shows the results from the genetic modeling which was applied only to 6- and 18-month sleep ratios and associated language skills to determine the genetic and/or environmental etiology of their association. Best fitting models are shown after the significance of parameters was tested in nested models. All models presented good fit to the data (not shown). The top part of the panel shows variance estimates for sleep and language measures. Results indicate that heritability explains the greater proportion of the variance for the 6-month sleep ratio, whereas the 18-month sleep ratio is mainly influenced by shared environmental influences making children in the same family more similar regardless of their genetic relatedness. All language measures show modest heritability and large shared environmental influences.
Table 5.
Bivariate genetic modeling results of sleep ratio at 6 and 18 months and language measures at 18, 30, and 60 months
| Parameter estimates |
|||
|---|---|---|---|
| Measure | A | C | E |
| Sleep | |||
| 6-month sleep ratio | 0.64 (0.56-0.71) | n.s. | 0.36 (0.29-0.44) |
| 18-month sleep ratio | n.s. | 0.58 (0.52-0.65) | 0.42(0.35-0.48) |
| Language | |||
| 18-month Language | 0.17 (0.07-0.28) | 0.68 (0.58-0.77) | 0.15 (0.12-0.19) |
| 30-month Language | 0.22 (0.06-0.44) | 0.45 (0.25-0.63) | 0.33 (0.26-0.42) |
| 60-month Language | 0.21 (0.02-0.41) | 0.51 (0.33-0.66) | 0.28 (0.22-0.35) |
| Bivariate models | RG | RC | RE |
| 6-month sleep ratio/18-month language | 0.32 (0.61-0.10) | n.s. | n.s. |
| 6-month sleep ratio/30-month language | 0.33 (1-0.10) | n.s. | n.s. |
| 18-month sleep ratio/60-month language | n.s. | 0.24 (0.41-0.06) | n.s. |
The top panel shows variance parameter estimates with 95% confidence intervals for sleep ratio and language measures. The bottom panel shows the covariance parameter estimates with 95% confidence intervals for significant sleep and language associations.
A, additive genetic parameter; C, shared environmental parameter; E, unique environmental parameter and error; RG, genetic correlation; RC, shared environmental correlation; RE, unique environmental correlation and error. Models include child sex and maternal language other than French or English as control variables.
The bottom part of the panel shows covariance estimates for the bivariate models of sleep ratios and language measures. Results indicate that the etiology of the association between sleep ratios and language skills differed depending on the developmental period. The association between the 6-month sleep ratio and both the 18- and 30-month language outcomes was explained by the same genetic factors (RGs = 0.32 and 0.33, respectively). By contrast, the association between the sleep ratio at 18 months and language at 60 months was explained by shared environmental influences affecting both sleep and language (RCs = 0.24).
DISCUSSION
The objectives of this study were two-fold. The first objective was to examine the contribution of sleep consolidation to language development between 18 and 60 months in a large sample of twins. This is the first study to address this question on a large population-based sample. The second objective was to assess the genetic and environmental etiology of sleep-language associations.
Regarding the first objective, results (1) replicate previous findings on smaller samples in terms of strength of association between sleep measures and language outcomes4,6,8,9; (2) show that the association is maintained in longitudinal analyses, and (3) that individual differences in sleep consolidation contribute to meaningful changes in language outcomes through time. In addition, the results are consistent with recent empirical evidence showing that day/night sleep duration ratios capture developmental changes better16 and are correlated more strongly with cognitive outcomes9 than total sleep durations during this developmental period. Indeed, the ratio of day/night consecutive sleeping durations was more strongly correlated to language outcomes than individual daytime or nighttime sleep durations in this study. The sleep ratios at both 6 and 18 months, but not 30 months, were modestly but significantly and consistently associated with all subsequent language outcomes and language progress between time-points. This was particularly true of children with transient language delays: 90% of them had average or better than average sleep ratios at both 6 and 18 months. This suggests that faster maturing sleep consolidation may be a protective factor for children with early language delays. By contrast, children with stable or late onset language delays by 5 years of age showed poorer sleep consolidation at both 6 and 18 months. Thus, although correlations between sleep consolidation and language outcomes were modest when looking at the overall sample, group comparisons show that early sleep consolidation is associated with clinically meaningful developmental outcomes of language delays. It may be that poor sleep consolidation is a risk factor for long-term language delays. Although previous studies had shown a link between various indices of sleep consolidation and language outcomes in early childhood,6–8,10 this is the first study to document the association between sleep consolidation and language development throughout the preschool period.
Regarding the second objective, genetic analyses show (1) that the sleep ratio at 6 months is highly heritable, with genetic factors explaining 64% of the differences on this measure, whereas the ratio at 18 months was mainly due (58%) to shared environment factors, (2) that language outcomes are modestly heritable and mainly due to shared environment as previous studies have shown,35,36 and (3) that the mechanisms by which sleep is associated with language learning start with an early genetic mediation at 6 months with an increasing role of the family environment with age. It appears that genetic factors, possibly governing early brain maturation, were largely responsible for individual differences in sleep consolidation at 6 months and its association with subsequent language skills. This is consistent with intervention studies showing that the sleep consolidation of premature infants exposed to systematic manipulations in the early months did not mature faster.37 The maturation of the sleep-wake cycles in early life seems rather orchestrated by genetic predispositions. These could have a general effect on brain development. As infants actively process language during this period, with evidence of statistical learning of language properties in infants as young as 8 months,38 it is not surprising that genetically based maturation would play a role in individual differences in both sleep consolidation and language learning. Alternatively, these genes may play a more specific role in lower-order regulation processes, such as sleep/wake rhythms and parasympathetic activity, which in turn may have an organizing effect on the higher order processes involved in language processing and the consolidation of language information in memory.13
By 18 months of age, however, sleep consolidation shifts from being a mainly genetically driven process to an environmental one. Indeed, most infants fall asleep with little effort from caregivers during the first year, often during or at the end of feedings. The sleep routine with 18-month-old children appears quite different. Often children require some form of soothing to fall asleep, self or otherwise, and parental practices around night awakenings are known to have an impact on sleep duration.15 In addition, napping at this age may become a function of caregiving practices, which may create individual differences in sleep patterns that have little to do with initial genetic predispositions. Poor management of daytime and nighttime sleep routines due to lack of knowledge on the parents' part or modern-day family schedules may have more impact on the sleep consolidation process at this age.
The main limitations of this study stem from the usual draw-backs of large-scale longitudinal cohort studies in providing detailed and often time-consuming measures. Namely, the available sleep measures consisted of mother reported consecutive daytime and nighttime sleep bout durations in rounded hours. The use of rounded duration estimations may have limited the variance and increased measurement error of sleep measures more likely resulting in an underestimation of the association between sleep and language development i.e., an increase of type II error. Also, the reliance on a single informant, the mother, for measures in both twins may have increased overall familial aggregation. Results may need to be replicated with more objective sleep measures (actigraphy or polysomnography). Fortunately, many studies have shown high agreement between parental reports and actigraphic measures of sleep duration,39,40 and a recent study41 shows even stronger associations with child cognitive outcomes using parental reports, possibly because they reflect the overall sleep patterns better than single time-point objective measures. One should also keep in mind that twins may not be representative of the general population, mainly because of the larger proportion of premature infants in twin populations. However, there are no indications that the developmental sleep process is different in premature infants compared to full-term infants.37 Finally, the study remains correlational in nature, which means we can not conclude that earlier sleep consolidation fosters better language learning. It is possible that sleep-wake consolidation and language learning are associated because they both reflect the state of early neurodevelopmental integrity or other unmeasured processes.
Notwithstanding these limitations, the sample size and the control for confounding factors largely exceed that of previous studies. In addition, the larger developmental period covered by this study, with multiple assessments at crucial developmental periods between 6 and 60 months, suggests that early sleep consolidation has long-term associations with language development. More importantly, this is the first study to investigate genetic and environmental mechanisms at the basis of the association between sleep and language development. Results highlight major shifts in the underlying causes of this association during early childhood where there appears to be a complex developmental interplay between early genetic influences and an increasing organizing effect of the family environment by 18 months. This could be regarded as a window of opportunity for family-based interventions around early childhood sleep routines. Intervention studies are needed to determine if sleep consolidation can be improved by parental practices in the transition from infancy to toddlerhood, and if so, whether this has a direct effect on language learning and other high order regulatory processes.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The QNTS was supported by grants from the Canadian Institutes of Health Research, Social Sciences and Humanities Research Council of Canada, Canada Research Chair Program, Canadian Institute of Advanced Research, National Health Research and Development Program, Quebec Research Funds (FCAR, FQRSC, and FRSQ), Quebec Ministries of Health, Social Services and Families, Lucie and Andre Chagnon Foundation, Ste-Justine Hospital, University of Montreal, and Laval University.
REFERENCES
- 1.Beckwith L, Parmelee AH., Jr EEG patterns of preterm infants, home environment, and later IQ. Child Dev. 1986;57:777–89. [PubMed] [Google Scholar]
- 2.Anders TF, Keener MA. Developmental course of nighttime sleep-wake patterns in full-term and premature infants during the first year of life: I. Sleep. 1985;8:173–92. doi: 10.1093/sleep/8.3.173. [DOI] [PubMed] [Google Scholar]
- 3.Borghese IF, Minard KL, Thoman EB. Sleep rhythmicity in premature infants: implications for developmental status. Sleep. 1995;18:523–30. doi: 10.1093/sleep/18.7.523. [DOI] [PubMed] [Google Scholar]
- 4.Gertner S, Greenbaum CW, Sadeh A, Dolfin Z, Sirota L, Ben-Nun Y. Sleep-wake patterns in preterm infants and 6 month's home environment: implications for early cognitive development. Early Hum Dev. 2002;68:93–102. doi: 10.1016/s0378-3782(02)00018-x. [DOI] [PubMed] [Google Scholar]
- 5.Dahl RE. The impact of inadequate sleep on children's daytime cognitive function. Semin Pediatr Neurol. 1996;3:44–50. doi: 10.1016/s1071-9091(96)80028-3. [DOI] [PubMed] [Google Scholar]
- 6.Dearing E, McCartney K, Marshall NL, Warner RM. Parental reports of children's sleep and wakefulness: longitudinal associations with cognitive and language outcomes. Infant Behav Dev. 2001;24:151–70. [Google Scholar]
- 7.Freudigman KA, Thoman EB. Infant sleep during the first postnatal day: An opportunity for assessment of vulnerability. Pediatrics. 1993;92:373–9. [PubMed] [Google Scholar]
- 8.Scher A. Infant sleep at 10 months of age as a window to cognitive development. Early Hum Dev. 2005;81:289–92. doi: 10.1016/j.earlhumdev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Bernier A, Carlson SM, Bordeleau S, Carrier J. Relations between physiological and cognitive regulatory systems: Infant Sleep regulation and subsequent executive functioning. Child Dev. 2010;81:1739–52. doi: 10.1111/j.1467-8624.2010.01507.x. [DOI] [PubMed] [Google Scholar]
- 10.Touchette É, Petit D, Séguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213–9. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev. 2006;10:323–37. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 13.Porges SW. Physiological regulation in high-risk infants: a model for assessment and potential intervention. Dev Psychopathol. 1996;8:29–42. [Google Scholar]
- 14.Decker MJ, Rye DB. Neonatal intermittent hypoxia impairs dopamine signaling and executive functioning. Sleep Breath. 2002;6:205–10. doi: 10.1007/s11325-002-0205-y. [DOI] [PubMed] [Google Scholar]
- 15.Touchette E, Petit D, Paquet J, et al. Factors associated with fragmented sleep at night across early childhood. Arch Pediatr Adolesc Med. 2005;159:242–9. doi: 10.1001/archpedi.159.3.242. [DOI] [PubMed] [Google Scholar]
- 16.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year old children. Sleep. 2005;28:1568–77. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- 17.Rivkees SA. Developing circadian rhythmicity in infants. Pediatrics. 2003;112:373–81. doi: 10.1542/peds.112.2.373. [DOI] [PubMed] [Google Scholar]
- 18.Touchette E, Mongrain V, Petit D, Tremblay RE, Montplaisir JY. Development of sleep-wake schedules during childhood and relationship with sleep duration. Arch Pediatr Adolesc Med. 2008;162:343–9. doi: 10.1001/archpedi.162.4.343. [DOI] [PubMed] [Google Scholar]
- 19.McGraw K, Hoffmann R, Harker C, Herman JH. Sleep rhythmicity in premature infants: implications for developmental status. Sleep. 1999;22:303–10. doi: 10.1093/sleep/22.3.303. [DOI] [PubMed] [Google Scholar]
- 20.Weissbluth M. Sleep rhythmicity in premature infants: implications for developmental status. Sleep. 1995;18:82–7. doi: 10.1093/sleep/18.7.523. [DOI] [PubMed] [Google Scholar]
- 21.Ingersoll EW, Thoman EB. Sleep/wake states of preterm infants: Stability, developmental change, diurnal variation, and relation with caregiving activity. Child Dev. 1999;70:1–10. doi: 10.1111/1467-8624.00001. [DOI] [PubMed] [Google Scholar]
- 22.Dale PS, Price TS, Bishop DVM, Plomin R. Outcomes of early language delay: I. Predicting persistent and transient language difficulties at 3 and 4 years. J Speech Lang Hear Res. 2003;46:544–60. doi: 10.1044/1092-4388(2003/044). [DOI] [PubMed] [Google Scholar]
- 23.Bishop DVM, Price TS, Dale PS, Plomin R. Outcomes of early language delay: II. etiology of transient and persistent language difficulties. J Speech Lang Hear Res. 2003;46:561–75. doi: 10.1044/1092-4388(2003/045). [DOI] [PubMed] [Google Scholar]
- 24.Goldsmith H. A zygosity questionnaire for young twins: A research note. Behav Genet. 1991;21:257–69. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- 25.Forget-Dubois N, Russe D, Turecki G, et al. Diagnosing zygosity in infant twins: Physical similarity, genotyping, and chorionicity. Twin Res. 2003;6:479–85. doi: 10.1375/136905203322686464. [DOI] [PubMed] [Google Scholar]
- 26.Fenson L, Pethick S, Renda C, Cox J, Dale P, Reznick JS. Short-form versions of the MacArthur Communicative Development Inventories. Appl Psycholinguist. 2000;21:95–116. [Google Scholar]
- 27.Dunn LM, Theriault-Walen CM. Manuel pour les formes A et B. Toronto: PSYCAN; 1993. Échelle de vocabulaire en images Peabody- adaptation française du Peabody pictures vocabulary test - revised. [Google Scholar]
- 28.Bates JE, Freeland CAB, Lounsbury ML. Measurement of infant difficultness. Child Dev. 1979;50:794–803. [PubMed] [Google Scholar]
- 29.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Boivin M, Pérusse D, Dionne G, et al. The genetic-environmental etiology of parents' perceptions and self-assessed behaviours toward their 5-month-old infants in a large twin and singleton sample. J Child Psychol Psychiatry. 2005;46:612–30. doi: 10.1111/j.1469-7610.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- 31.Arbuckle JL. Amos 5 [Computer Software] Chicago: Smallwaters; 2003. [Google Scholar]
- 32.McCartney K, Burchinal M, Bub KL. Best practices in quantitative methods for developmentalists. Monogr Soc Res Child Dev. 2006;71:1–145. doi: 10.1111/j.1540-5834.2006.07103001.x. [DOI] [PubMed] [Google Scholar]
- 33.Graham J, Olchowski A, Gilreath T. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–13. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- 34.Neale M. The Mx statistical package. [cited; Available from: http://www.vcu.edu/mx/
- 35.Byrne B, Olson RK, Samuelsson S, et al. Genetic and environmental influences on early literacy. J Res Read. 2006;29:33–49. [Google Scholar]
- 36.Dionne G, Dale P. S, Boivin M, Plomin R. Genetic evidence for bidirectional effects of early lexical and grammatical development. Child Dev. 2003;74:394–412. doi: 10.1111/1467-8624.7402005. [DOI] [PubMed] [Google Scholar]
- 37.Ariagno RL, Thoman EB, Boeddiker MA, et al. Developmental care does not alter sleep and development of premature infants. Pediatrics. 1997:100e9. doi: 10.1542/peds.100.6.e9. [DOI] [PubMed] [Google Scholar]
- 38.Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–8. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 39.Sadeh A. Assessment of intervention for infant night waking: Parental reports and activity-based home monitoring. J Consult Clin Psychol. 1994;62:63–8. doi: 10.1037//0022-006x.62.1.63. [DOI] [PubMed] [Google Scholar]
- 40.Sekine M, Chen X, Himanishi S, Wang H, Kagamimori S. The validity of sleeping hours of healthy young children as reported by their parents. J Epidemiol. 2002;12:237–42. doi: 10.2188/jea.12.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geiger A, Achermann P, Jenni OG. Association between sleep duration and intelligence scores in healthy children. Developmental Psychol. 2010;46:949–54. doi: 10.1037/a0019679. [DOI] [PubMed] [Google Scholar]