Abstract
Introduction: Advances in technology have resulted in increasing survival rates for premature infants. Oxygen therapy is commonly used in neonatal units as part of respiratory support. The number of premature infants in our institution surviving with severe (stage ≥3) retinopathy of prematurity (ROP) prompted a review of oxygen therapy as a contributing factor. Prolonged exposure to high concentrations of oxygen may cause irreversible damage to the eyes of very-low-birth-weight preterm infants and is a potential cause of blindness.
Objective: We developed strategies to reduce incidence of severe ROP requiring laser surgery in premature infants.
Methods: We studied 37 preterm infants who were born at a gestational age of <32 weeks, with a birth weight of <1500 g, receiving supplemental oxygen, and had been admitted to our neonatal intensive care unit. Infants received oxygen via mechanical ventilator, nasal continuous positive airway pressure (CPAP), or intranasal (I/N) and titration of oxygen was based on each infant's measured oxygen saturation (Spo2). For each infant, we monitored the Spo2 trend, Spo2 alarm limit, and the percentage of time that the alarm limit was set incorrectly. We implemented a Spo2 targeting protocol and developed an algorithm for titrating fraction of inspired oxygen (Fio2).
Results: After phase 1 of implementation, the percentage of time that Spo2 readings were >95% was reduced to between 20% and 50%. However, our findings raised concern regarding the wide fluctuation of Spo2 readings because of inconsistency in Fio2 titration, which can contribute to deviation from the optimal target range. Accordingly, we developed an algorithm for titrating Fio2 aimed at maintaining each infant's Spo2 within the optimal target range. After phase 2 of implementation, the percentage of Spo2 readings >95% was markedly reduced to between 0% and 15%. The incidence of infants with severe ROP requiring laser surgery decreased from 5 to 1.
Conclusions: A change in clinical practice aimed at maintaining oxygen within the target range to avoid a high Spo2 was associated with a significant decrease in the incidence of both severe ROP and the need for laser surgery, thus reducing hospital costs and length of hospital stays for premature infants.
Introduction
Approximately 1500 babies, of which 240 are born prematurely, are delivered yearly at Singapore General Hospital. Advances in technology have resulted in increasing survival rates for premature infants. Oxygen is the commonly used therapy in neonatal units as part of respiratory support. Tin and Gupta1 reported that hypoxia or hyperoxia in infants results in diseases and disorders in both the short and long term, such as retinopathy of prematurity (ROP), bronchopulmonary dysplasia, growth delay, and neurodevelopmental delay.1 Pollan2 defined ROP as a disease of the retina that occurs after birth among premature infants who are born weighing <1500 g, when blood vessels in the vascular bed of the retina begin to grow abnormally. Very low birth weight (VLBW) is defined as a weight of <1500 g. ROP is a potential cause of blindness in children and accounts for up to 10% of cases of childhood blindness in developed countries.3 The goal for clinicians is to deliver adequate oxygen to the tissue without creating oxygen toxicity.
Pediatric ophthalmologists at our institution routinely perform eye examinations in preterm infants to detect ROP. Our team performed a retrospective review of premature infants with VLBW who were admitted to our neonatal intensive care unit in 2007 and found ROP in 11 of 55 (20%) infants. Of those infants screened, 36.4% had stage 1 disease, 9.1% had stage 2, 45.5% had stage 3, and 9.1% had stage 4. Of those 55 infants, 5 required laser treatment, 2 of whom developed blindness despite treatment. The increasing numbers of premature infants surviving with consequences of severe ROP (stage ≥3) prompted our review of oxygen therapy. According to Saugstad,4 therapy with a high concentration of oxygen is thought to be the major contributing factor to the development of ROP. Prolonged exposure to high concentrations of oxygen may cause irreversible damage to the eyes of preterm infants with VLBW.5 Hence, maintaining oxygen saturation (Spo2) in a range to avoid hyperoxia may result in improved ROP outcomes. Our team of neonatologists and nurses recognize the importance of managing oxygen delivery effectively to prevent progression to severe ROP or blindness.
ROP is staged on a scale of 1 to 4, with stage 1 representing the least amount of damage and stage 4 representing partial retinal detachment or permanent blindness5 (Figure 1). “Plus” disease, which occurs when there is evidence of marked shunting of blood between the retinal arteries and veins, reflects extremely rapid disease progression, as described by Coe et al.5 Stage 3 with “plus” disease often requires immediate surgical intervention. In 2007, our institution encountered an increased incidence of severe ROP requiring laser surgery, stimulating us to develop strategies to reduce the incidence to zero.
Figure 1.
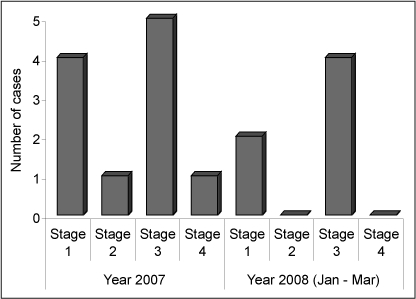
Distribution of retinopathy of prematurity (ROP) cases in our study according to disease stage.
Methods
Using a structured quality-improvement method, the Plan-Do-Check-Act cycle, we did the following: assessed the problems, conducted a literature review, conducted a staff assessment, developed a protocol and tracking tools, undertook education for all new and existing staff, and collected data to evaluate effectiveness.
Baseline Data Collection
We conducted a daily survey of Spo2 trends in our neonatal intensive care unit (NICU) during March 2008. We monitored the daily percentage of time that each infant's Spo2 values was >95%. Our baseline data showed that the incidence of hyperoxia ranged from 40% to 60% (Figure 2).
Figure 2.
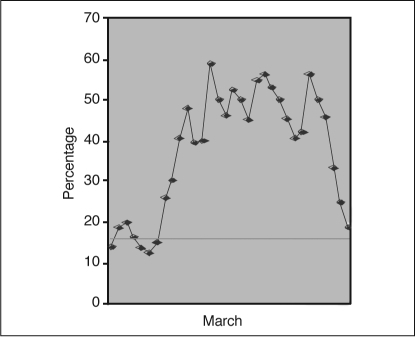
Percentage of time premature infants with oxygen saturation (Spο2) values >95% during March 2008. Horizontal line represents Mean.
Problem Analysis
During observation in our NICU, we identified the following root causes for hypoxia and hyperoxia (Figure 3):
Health care staff lacked proper orientation or guidelines for titrating fraction of inspired oxygen (Fio2).
The Spo2 target alarm limit was set improperly: The lower alarm limit, to provide an auditory alert regarding impending hypoxia, was set at 85%, but the upper alarm limit was commonly set at ≥98%.
Health care staff sometimes forgot to wean infants to a lower Fio2 after procedures or even after the Spo2 had returned to the target range.
Heavy workload resulted in nurses forgetting to wean infants to a lower Fio2, thus exposing infants to an unnecessarily high Fio2.
Critically ill infants with underlying medical conditions required a high Fio2 for respiratory support.
Figure 3.
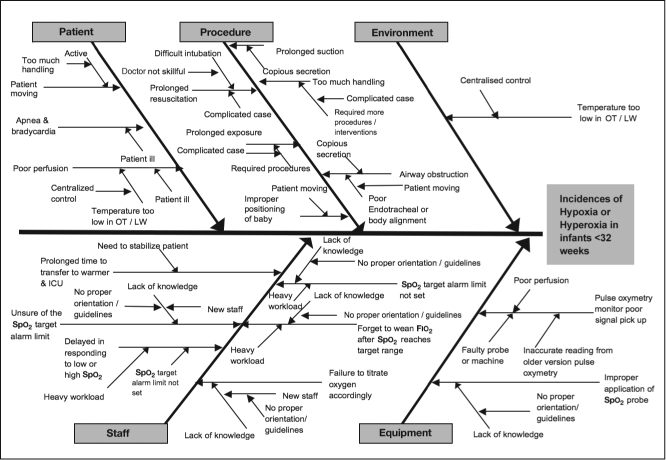
Cause and effect for hypoxia and hyperoxia in hospitalized preterm infants.
Fιο2 = fraction of inspired oxygen; ICU = intensive care unit; Spο2 = oxygen saturation.
We plotted these causes in a Pareto diagram (Figure 4) so that we could work to eradicate the “vital few” causes. As we looked at the causes in greater depth, we identified the lack of proper guidelines for titrating Fio2 and the lack of awareness of the adverse effects of high oxygen on premature infants as the most common reasons for infants having hypoxia or hyperoxia. Hence, we devised strategies to tackle it.
Figure 4.
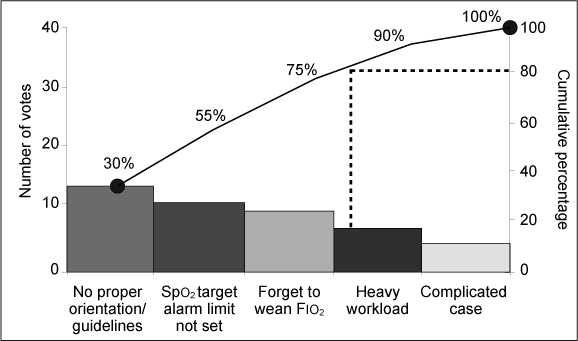
Root causes of hypoxia and hyperoxia in hospitalized preterm infants, based on the survey responses of the neonatology staff.
Fιο2 = fraction of inspired oxygen, Spο2 = oxygen saturation.
Study Participants
We prospectively monitored all infants born at a gestational age of <32 weeks and with birth weights of <1500g who received supplemental oxygen and were admitted to our 8-bed NICU between April and September 2008. These infants' Spo2 trends were monitored until they reach a corrected gestational age of 34 weeks. Our pediatric ophthalmologists examined their eyes according to the current guidelines of the Royal College of Ophthalmologists and classified retinopathy stages according to the International Classification of ROP.6 An initial eye screening was performed for infants with VLBW when they were 34 weeks old (corrected gestational age) or 4 to 6 weeks after birth, whichever occurred earlier. Thirty-seven premature infants were admitted during this period with gestational ages ranging from 24 to 30 weeks. Their birth weight varied from 530g to 1325g. For these infants, the mean duration of receiving oxygen was 45 days (range, 3 days to 182 days) and the mean length of NICU stay was 64 days (range, 17 days to 213 days). However, infants with major congenital anomalies, infants born in other hospitals, and infants deemed nonviable in the delivery room who received only palliative care until their demise were excluded from our study.
Infants were given supplemental oxygen via a mechanical ventilator, nasal continuous positive airway pressure, or intranasal oxygen. Oxygen titration was performed by making manual adjustments of the Fio2, to achieve the desired Spo2 target range for each infant. Their oxygen concentration requirements varied from 21% to 100%. Spo2 was continuously measured using a Philips HP Merlin multiparameter monitor and we recorded the Spo2 trend for each infant. Figure 5 is the algorithm that we followed for oxygen titration.
Figure 5.
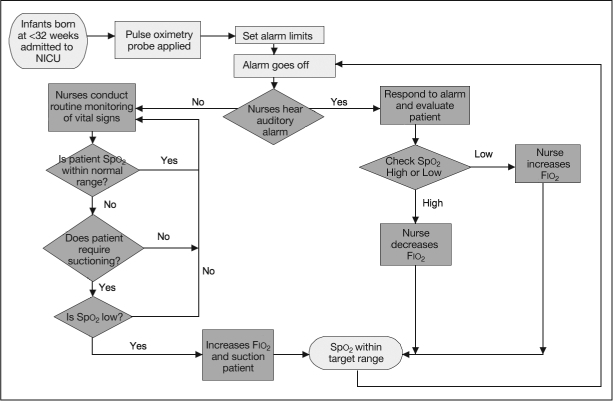
Algorithm for oxygen titration.
Fιο2 = fraction of inspired oxygen; NICU = neonatal intensive care unit; Spο2 = oxygen saturation.
Interventions
Phase 1
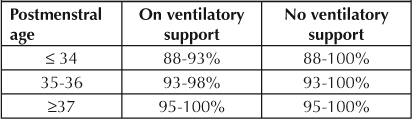
Before we made any changes in clinical practice, we had determined through our observation that 89% of the time, the lower alarm limit for Spo2 was set correctly. However, 82% of the time, upper alarm limits, which provide auditory alerts, were set at >98%; and 25% of the time, limits were set at 100%. Most staff did not realize that there is the potential for adverse vision outcomes when the Spo2 in premature infants is allowed to reach high levels for a prolonged period of time. Opinions vary among medical centers on the optimal Spo2 range for preterm infants. As reported in a study by Chow et al,7 the incidence of stage 3 or 4 ROP decreased from 12.5% to 2.5% when the Spo2 target for infants born at <32 weeks' gestation was kept between 85% and 93%. Therefore, in April 2008, we reviewed patients' chart data and created a visual reminder to be placed on each patient's clipboard that included an Spo2 target range chart (Table 1). Alarm limits were modified and set according to protocol.
Table 1.
Spo2 target range chart included a visual reminder for an infant's oxygen saturation level.
During orientation, new nurses were taught about the importance of adhering to the appropriate oxygen target range by using the visual reminder. This also helped create awareness of when to wean infants from oxygen support or to increase Fio2.
We monitored the Spo2 trend, Spo2 alarm limits, and the percentage of time that alarm limits were not set correctly. We conducted audits to ensure staff compliance. We also conducted education programs for all nurses to raise awareness regarding adverse vision outcomes and the importance of our Spo2 targeting protocol, so that we could avoid episodes of high Spo2. We recorded Spo2 values at 15-minute intervals, analyzed them, and presented the analysis monthly to all nurses to facilitate adherence to the protocol. We welcomed suggestions from ward nurses and from the medical team for improvements in the project.
Phase 2
We were concerned about the wide fluctuation of Spo2 readings because of inconsistency in titration of Fio2, which could result in deviation from the optimal target range. This led us to develop an algorithm for Fio2 titration (Figure 6) in June 2008, to manage high and low Spo2, with the aim of maintaining an Spo2 within the optimal target range of 88% to 93% for infants with VLBW.
Figure 6.
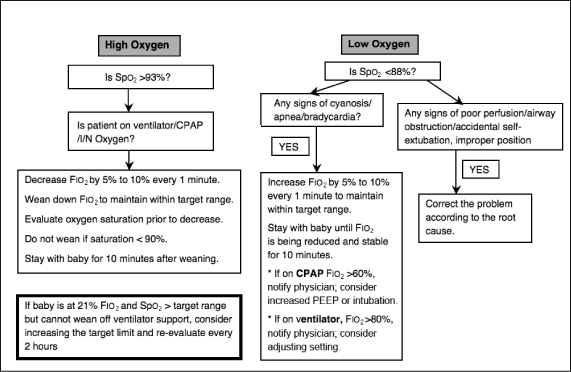
Algorithm for Fιο2 titration to manage the high and low oxygen saturation (Spο2) in premature infants.
CPAP = continuous positive airway pressure; Fιο2 = fraction of inspired oxygen; I/N = intranasal.
We frequently audited compliance with Spo2 target limits and guidelines for titrating Fio2 to ensure the sustainability of our protocol. We continue to monitor results. If we detect any deficiencies, we host a formal discussion to correct them.
Results
During the implementation of phase 1, we found that the lower alarm limits were usually set correctly. However, our team noted that some nurses increased upper alarm limits to 100%, particularly for infants who received ambient air or on lower concentrations of supplemental oxygen (at an Fio2 of 21% to 23%). Failure to adhere to protocol resulted in infants' having an Spo2 outside the acceptable target range because there were no auditory alerts regarding impending hyperoxia. Although nurses might have considered these infants' improved condition as less of a concern because the infants were receiving lower concentrations of supplemental oxygen, these infants were still premature and at risk for developing ROP. As reported by Clucas et al,8 adherence to alarm limits may improve targeting of oxygen levels, particularly for preterm infants. Hence, close monitoring allowed our team to quickly identify protocol breaches and ensure that alarm limits were returned to the acceptable range. After a few reminders, nurses were consistent in accurately setting upper and lower alarm limits according to the protocol, and more infants then had an Spo2 within the acceptable target range. One month after the initiation of the Spo2 protocol, nurses were tested to ascertain their understanding of the project goal and awareness of the adverse effects of oxygen on premature infants. Before our quality improvement project implementation, the percentage of Spo2 values that were higher than the target range was between 40% and 60% (Figure 7). After phase 1 of our intervention, the percentage decreased to between 20% and 50%.
Figure 7.
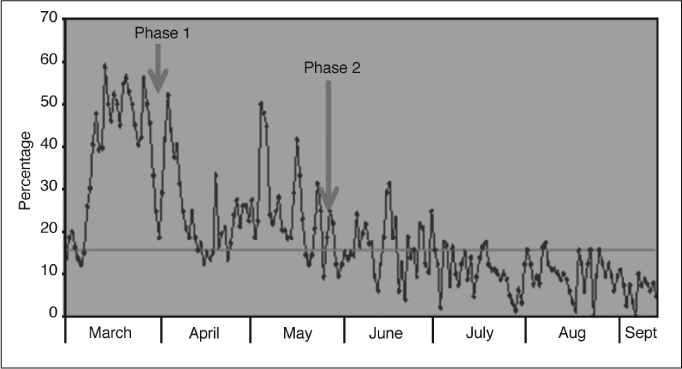
Percentage of time premature infants' oxygen saturation (Spο2) values that were higher than target range before, during, and after implementation (from March to September 2008). Horizontal line represents Mean.
During phase 2 of our implementation, we found that despite careful adherence to the Spo2 protocol and correct setting of alarm limits, there were still wide fluctuations of Spo2 levels, which contributed to deviation from the optimal target range. Irregular, shallow breathing and frequent apneic episodes, which may contribute to acute hypoxic episodes, are commonly seen in premature or very sick infants. We observed that because our nurses were concerned that these infants might experience oxygen deprivation and its negative effects on neurodevelopment, they aggressively increased Fio2 in response to hypoxic episodes, which sometimes resulted in rebound hyperoxia. The nurses did not clearly understand how much manual adjustment of the Fio2 was required to achieve and maintain the desired Spo2 target range, so we developed an algorithm for Fio2 titration. Because gradual oxygen titration is recommended, we reminded staff to adjust the Fio2 in small increments of 5% to 10% to avoid overshooting the target range. We aimed to bring Spo2 levels closer to the optimal target range for an acceptable percentage of time and avoid persistent or fluctuating hypoxia or hyperoxia. Repeated episodes of alternating of hypoxia or hyperoxia can cause significant alterations in vascular tone in immature infants, according to a study by Ford et al.9 Fluctuations in blood oxygen tension have been found to be predictive of ROP.10 Since phase 2 of our intervention, we have seen sustainable improvement in maintaining Spo2 within the target range: The percentage of high Spo2 levels was markedly reduced to between 0 and 15% (Figure 7).
Discussion
We implemented our new strategies between April and September 2008. Before this intervention (January 2007 to March 2008), five infants had severe ROP requiring laser surgery. After the intervention, the number of infants with severe ROP requiring laser surgery was reduced to 1 (Figure 8). That infant had an extremely low birth weight of 530 g when born at a gestational age of 24 weeks and severe respiratory problems requiring high-frequency ventilation. The infant had multiple medical conditions secondary to prematurity and developed stage 3 ROP despite treatment. A study by Vyas et al11 showed that smaller, more immature, and sicker infants are more likely than their more robust counterparts to develop ROP. We found no significant differences in mortality rates before versus after implementation of our protocol, and there was no increase in mortality rate after implementation.
Figure 8.
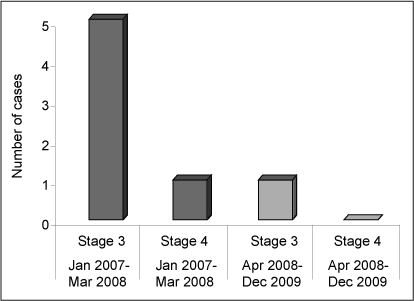
Comparison of the number of premature infants with severe retinopathy of prematurity (stage 3 or 4) requiring laser surgery before and after a two-phase intervention.
The cost of providing care for premature babies in NICUs is high. Patients who need prolonged hospital stays because of complications and who require laser surgery will incur additional costs. In our institution, the cost of laser surgery was about $746, and medications and miscellaneous related treatments could add as much as $474 more.
The average daily cost for a NICU stay was $260, and the average length of stay in a NICU after laser surgery was five days. Avoiding laser surgery alone would be a cost savings of approximately $7398 per infant. Hence, our intervention has reduced the progression of ROP among our patient population, protects vision, prevents blindness, avoids surgery, reduces the length of hospital stays, and reduces health care costs.
Conclusion
The implementation of a change in clinical practice aimed at avoiding a high Spo2 was associated with a significant decrease in the incidence of severe ROP and in the need for laser surgery, thus reducing length of hospital stays and health care costs. If oxygen delivery is managed effectively, premature infants will not be exposed to the risk of hypoxia and hyperoxia and can thus conserve their energy for optimal growth and neurologic development.
Because our interventions produced marked benefits, we shared the importance of Spo2 targeting outcomes with other institutions, both locally and internationally. We found that raising awareness among nurses and other allied clinicians, along with conducting frequent audits to ensure compliance with Spo2 target ranges and guidelines for Fio2 titration, can decrease episodes of hyperoxia in premature infants. Severe ROP can be prevented by implementing simple improvements in caregiving procedures, but only with a strong commitment from all clinicians and nurses involved.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
References
- Tin W, Gupta S. Optimum oxygen therapy in pre-term babies. Arch Dis Child Fetal Neonatal Ed. 2007 Mar;92(2):F143–7. doi: 10.1136/adc.2005.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollan C. Retinopathy of prematurity: an eye toward better outcomes. Neonatal Netw. 2009 Mar–Apr;28(2):93–101. doi: 10.1891/0730-0832.28.2.93. [DOI] [PubMed] [Google Scholar]
- Goggin M, O'Keefe M. Childhood blindness in the Republic of Ireland: a national survey. Br J Ophthalmol. 1991 Jul;75(7):425–9. doi: 10.1136/bjo.75.7.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad OD. Is oxygen more toxic than currently believed? Pediatrics. 2001 Nov;108(5):1203–5. doi: 10.1542/peds.108.5.1203. [DOI] [PubMed] [Google Scholar]
- Coe K, Butler M, Reavis N, et al. Special Premie Oxygen Targeting (SPOT): a program to decrease the incidence of blindness in infants with retinopathy of prematurity. J Nurs Care Qual. 2006 Jul–Sep;21(3):230–5. doi: 10.1097/00001786-200607000-00007. [DOI] [PubMed] [Google Scholar]
- An international classification of retinopathy of prematurity. Prepared by an international committee. Br J Opthalmol. 1984 Oct;68(10):690–7. doi: 10.1136/bjo.68.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LC, Wright KW, Sola A, CSMC Oxygen Administration Study Group Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003 Feb;111(2):339–45. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- Clucas L, Doyle LW, Dawson J, Donath S, Davis PG. Compliance with alarm limits for pulse oximetry in very preterm infants. Pediatrics. 2007 Jun;119(6):1056–60. doi: 10.1542/peds.2006-3099. [DOI] [PubMed] [Google Scholar]
- Ford SP, Leick-Rude MK, Meinert KA, et al. Overcoming barriers to oxygen saturation targeting. Pediatrics. 2006 Nov;118(Suppl 2):S117–86. doi: 10.1542/peds.2006-0913P. [DOI] [PubMed] [Google Scholar]
- Urschitz MS, Horn W, Seyfang A, et al. Automatic control of the inspired oxygen fraction in preterm infants: a randomized crossover trial. Am J Respir Crit Care Med. 2004 Nov 15;170(10):1095–100. doi: 10.1164/rccm.200407-929OC. [DOI] [PubMed] [Google Scholar]
- Vyas J, Field D, Draper ES, et al. Severe retinopathy of prematurity and its association with different rates of survival in infants of less than 1251 g birth weight. Arch Dis Child Fetal Neonatal Ed. 2000 Mar;82(2):F145–9. doi: 10.1136/fn.82.2.F145. [DOI] [PMC free article] [PubMed] [Google Scholar]


