Abstract
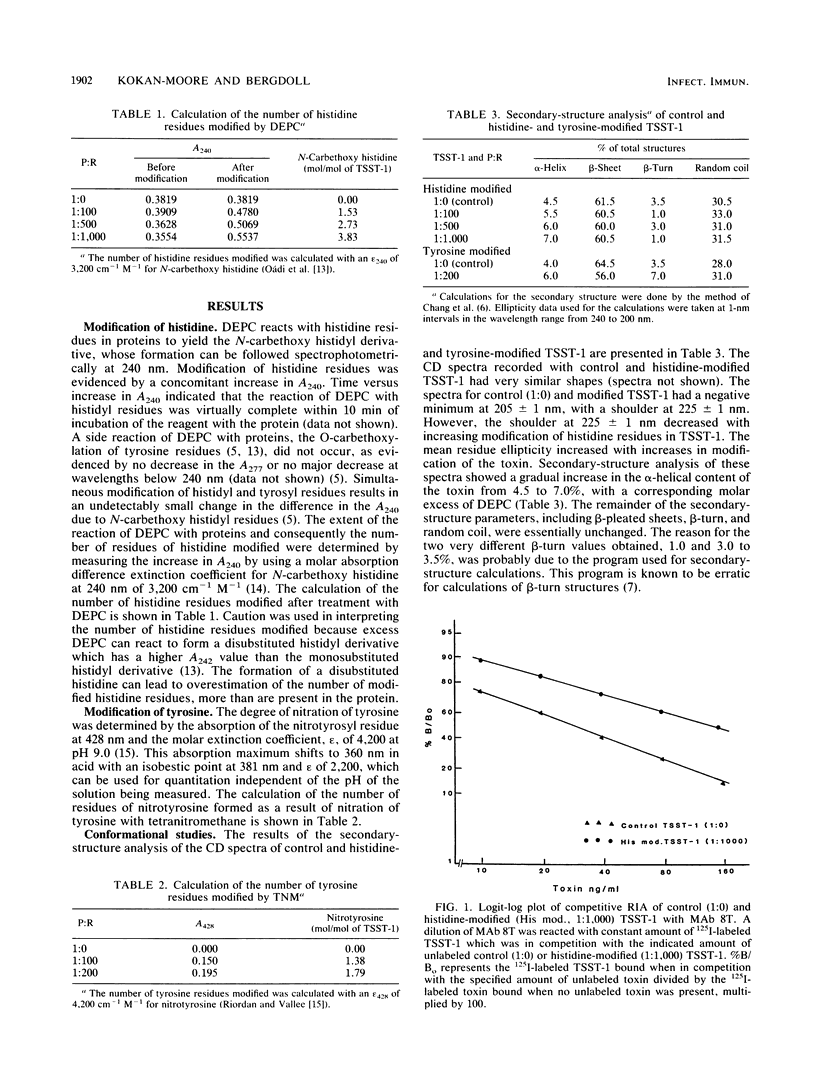
Modification of three or four of the five histidine residues in the toxic shock syndrome toxin 1 (TSST-1) with diethylpyrocarbonate did not inhibit the precipitin reaction of the modified TSST-1 with polyvalent antisera to the toxin. Monoclonal antibody 7T did not react with the modified TSST-1, but monoclonal antibody 8T did react with the toxin. Up to 50% of the mitogenic reaction of TSST-1 was inhibited by the histidine modification. Modification of one or two of the nine tyrosine residues in TSST-1 did not inhibit the precipitin reaction with polyclonal antisera to the toxin but did inhibit 85% of the mitogenic reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergdoll M. S., Crass B. A., Reiser R. F., Robbins R. N., Davis J. P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981 May 9;1(8228):1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Kreiswirth B. N., Kornblum J. S., Novick R. P., Schlievert P. M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986 Nov 25;261(33):15783–15786. [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Novick R. P., Schlievert P. M. Localization of biologic functions of toxic shock syndrome toxin-1 by use of monoclonal antibodies and cyanogen bromide-generated toxin fragments. J Immunol. 1986 Dec 1;137(11):3572–3576. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Walsh K. A., Neurath H. Evidence of an essential histidine residue in thermolysin. Biochemistry. 1974 Jan 1;13(1):205–210. doi: 10.1021/bi00698a030. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Edwin C. Structure-function analysis of toxic shock syndrome toxin 1. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S137–S139. doi: 10.1093/clinids/11.supplement_1.s137. [DOI] [PubMed] [Google Scholar]
- Freed R. C., Evenson M. L., Reiser R. F., Bergdoll M. S. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl Environ Microbiol. 1982 Dec;44(6):1349–1355. doi: 10.1128/aem.44.6.1349-1355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokan-Moore N. P., Bergdoll M. S. Determination of biologically active region in toxic shock syndrome toxin 1. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S125–S129. [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Miller B. A., Reiser R. F., Bergdoll M. S. Detection of staphylococcal enterotoxins A, B, C, D, and E in foods by radioimnunoassay, using staphyloccal cells containing protein A as immunoadsorbent. Appl Environ Microbiol. 1978 Sep;36(3):421–426. doi: 10.1128/aem.36.3.421-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Khoe G. P., Bergdoll M. S. Purification and some physicochemical properties of toxic-shock toxin. Biochemistry. 1983 Aug 2;22(16):3907–3912. doi: 10.1021/bi00285a028. [DOI] [PubMed] [Google Scholar]
- Robbins R., Gould S., Bergdoll M. Detecting the enterotoxigenicity of Staphylococcus aureus strains. Appl Microbiol. 1974 Dec;28(6):946–950. doi: 10.1128/am.28.6.946-950.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Singh B. R., Kokan-Moore N. P., Bergdoll M. S. Molecular topography of toxic shock syndrome toxin 1 as revealed by spectroscopic studies. Biochemistry. 1988 Nov 29;27(24):8730–8735. doi: 10.1021/bi00424a007. [DOI] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Bergdoll M. S. Inactivation of staphylococcal enterotoxin A by chemical modification. Biochem Biophys Res Commun. 1982 Mar 15;105(1):121–126. doi: 10.1016/s0006-291x(82)80019-3. [DOI] [PubMed] [Google Scholar]


