Abstract
3,5-Bis(2-fluorobenzylidene)-4-piperidone (EF24) is an anti-proliferative diphenyldifluoroketone analog of curcumin with more potent activity. The authors describe a liposome preparation of EF24 using a “drug-in-CD-in liposome” approach. An aqueous solution of EF24 and hydroxypropyl-β-cyclodextrin (HPβCD) inclusion complex (IC) was used to prepare EF24 liposomes. The liposome size was reduced by a combination of multiple freeze–thaw cycles. Co-encapsulation of glutathione inside the liposomes conferred them with the capability of labeling with imageable radionuclide Tc-99m. Phase solubility analysis of EF24-HPβCD mixture provided k1:1 value of 9.9 M−1. The enhanced aqueous solubility of EF24 (from 1.64 to 13.8 mg/mL) due to the presence of HPβCD helped in the liposome preparation. About 19% of the EF24 IC was encapsulated inside the liposomes (320.5 ± 2.6 nm) by dehydration–rehydration technique. With extrusion technique, the size of 177 ± 6.5 nm was obtained without any effect on encapsulation efficiency. The EF24-liposomes were evaluated for anti-proliferative activity in lung adenocarcinoma H441 and prostate cancer PC-3 cells. The EF24-liposomes demonstrated anti-proliferative activity superior to that of plain EF24 at 10 μM dose. When injected in rats, the Tc-99m-labeled EF24-liposomes cleared from blood with an α-t1/2 of 21.4 min and β-t1/2 of 397 min. Tissue radioactivity counting upon necropsy showed that the majority of clearance was due to the uptake in liver and spleen. The results suggest that using “drug-in-CD-in liposome” approach is a feasible strategy to formulate an effective parenteral preparation of EF24. In vitro studies show that the liposomal EF24 remains anti-proliferative, while presenting an opportunity to image its biodistribution.
Keywords: Curcumin, Hydroxypropyl-β-cyclodextrin, Liposomes, EF24, Inclusion complex, Nanomedicine
Introduction
Cancer accounts for nearly one-quarter of deaths in the United States, exceeded only by heart diseases. According to the American Cancer Society, it is estimated that about 1.5 million new cases of cancer will be diagnosed in 2009. Cancer death rates have been decreasing since 1990 in men, and since 1991 in women (American Cancer Society 2009). Much of the observed decline may be attributed to the enhanced understanding of cancer biology and the accompanying advances in therapeutic options. Chemotherapeutic drugs are the mainstay in managing patients diagnosed with any form of localized or metastasized cancer. For instance, meta-analysis of clinical data suggests that up to 85% of the patients with non-small cell lung cancer depend on systemic chemotherapy as part of the overall management (Akerley and Choy 1999). For certain patients, chemotherapy may be the only treatment used in an effort to cure, control, or relieve the symptoms of their cancer. In other patients, however, chemotherapy may be administered along with other therapies. The evidence suggests that the “curative intent” of most current chemotherapies is only able to provide palliative therapy. Therefore, development of better therapies is of contemporary interest.
3,5-Bis(2-fluorobenzylidene)-4-piperidone (EF24, Fig. 1a) is a synthetic analog of curcumin that possesses potent anti-proliferative activity against a number of cancer cell lines such as colon (Subramaniam et al. 2008), breast (Sun et al. 2006), and ovarian (Selvendiran et al. 2007). EF24 was first reported by a group of researchers in Emory University, Georgia (Adams et al. 2005). The authors recently depicted the crystal structure of EF24, and other related compounds (Lagisetty et al. 2009). Although more water soluble than curcumin, EF24 still has limited aqueous solubility. It has been reported that inadequate aqueous solubility of curcumin results in low bioavailability (Anand et al. 2007), and may also present a significant challenge in the development of parenteral formulation. Considering the immense potential of EF24 as an anti-cancer agent, a parenteral formulation for EF24 will be highly beneficial in preclinical and clinical trials.
Fig. 1.
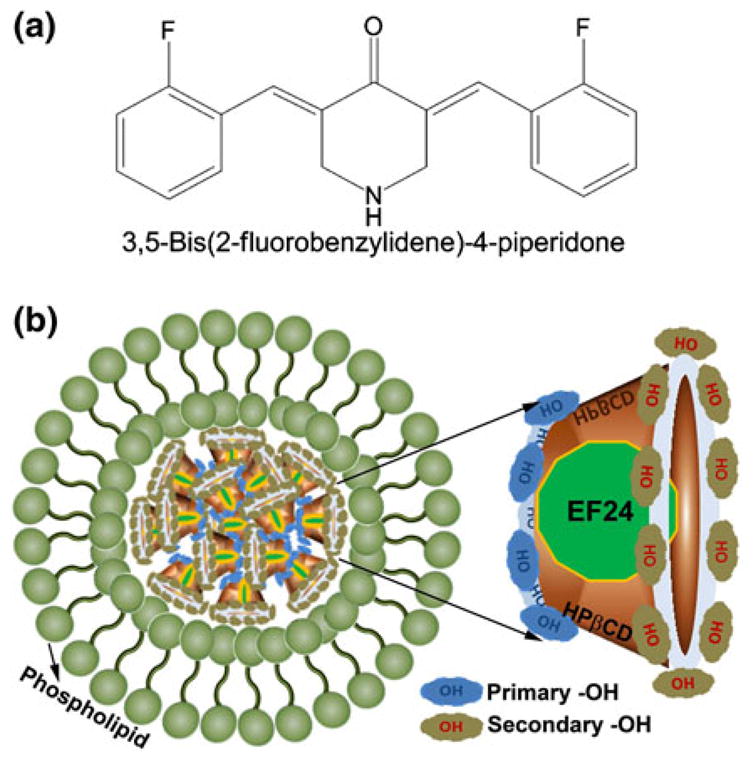
a Chemical structure of EF24 or 3,5-Bis(2-fluorobenzylidene)-4-piperidone, and b A schematic illustration of EF24 IC encapsulation inside the liposomes
The objective of this study was to develop and characterize a liposomal formulation of EF24. Attempts to encapsulate lipophilic drugs inside unilamellar liposomes generally end up poorly. Not only large amount of lipophilic material destabilizes the structural integrity of the liposomes, but the lipid bilayers also offer a very limited space for drug encapsulation. In order to overcome this problem, a “drug-in-HPβCD-in-liposome” approach as described by McCormick may be employed (Bekersky et al. 2002; McCormack and Gregoriadis 1998). The authors tested this strategy in this study and found that the enhanced solubility of EF24 in aqueous HPβCD solution helps in enhancement in EF24 encapsulation inside the liposomes. A schematic representation of such a liposome is shown in Fig. 1b. The authors also demonstrate that EF24-liposomes are effective as an anti-proliferative preparation in lung and prostate cancer cell lines. Finally, the authors provide preliminary data to show that the distribution of EF24-liposomes in the body can be non-invasively monitored because of the ability to radiolabel them with gamma ray emitting Tc-99m radionuclide.
Methods
Materials
The chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and/or various suppliers through VWR Scientific (West Chester, PA). The phospholipids were purchased from Lipoid (Ludwigshafen, Germany), Avanti Polar Lipids (Alabaster, AL), or NOF America Corporation (White Plains, NY). Cholesterol was obtained from Calbiochem (Gibbstown, NJ). Endotoxin-controlled cyclodextrins were purchased from CTD Inc (High Springs, FL).
Preformulation studies on 3,5-Bis(2-fluorobenzylidene)-4-piperidone (EF24)
EF24 was synthesized in >90% yield by the scheme reported elsewhere (Lagisetty et al. 2009). The synthesized compound was characterized by NMR and Mass Spectroscopy, and stored at 4 °C in dark. Octanol/water partition coefficient (CO/W) was determined by a standard method. In brief, about 2.5 mg of EF24 was dissolved in 5.5 mL of water-saturated octanol. The solution was allowed to equilibrate with 5.5 mL of octanol-rich water for 48 h on a multi-tube vortex mixer. The system was allowed to stand for 3 h, and the octanol and water phases were separated for spectrophotometric estimation of phase-solubilized drug. The spectrophotometric assay of EF24 was established by scanning an aqueous solution of EF24 between 250 and 700 nm wavelengths. The absorbance maximum at 330 nm was selected to obtain a Beer’s plot of absorbance against concentration. Non-interference of cyclodextrin in the assay of EF24 was also established.
Phase solubility analysis of EF24
Solubility studies were performed as described by Higuchi and Connors (1965). In brief, an excess of EF24 was added to various concentrations of HPβCD (0, 10, 20, 50, 100, 300, and 500 mM) in 1 mL of de-ionized water. The suspensions were shaken on a multiple tube vortex mixer at 25 °C for 48 h. The suspensions were allowed to settle for 6 h, and the supernatant was centrifuged at 14,000 rpm for 10 min. The supernatant was further clarified by passing through 0.22 μM cellulose acetate filters, and analyzed spectrophotometrically at 330 nm.
Assuming a 1:1 complex, the cyclodextrin complex of EF24 is depicted by Eq. 1, where k is the stability constant:
| (1) |
The total solubility (St) of EF24 in aqueous HPβCD is given by a linear equation 2.
| (2) |
where So is the intrinsic solubility of EF24. A plot of St versus [HPβCD]t provides a phase solubility profile of AL type that is characterized by a straight line with a slope [k*So/(1 + kSo)] and intercept Sint = S0. The stability constant k was calculated as
| (3) |
Formation of inclusion complex (IC)
IC of EF24 with HPβCD was obtained in the solution phase as reported previously for other hydrophobic drugs (Fatouros et al. 2001). In brief, EF24 (15 mg) was added to 2.5 mL of HPβCD solution (500 mg/mL). The mixture was continuously agitated on a shaker incubator at 25 °C for 72 h. After allowing coarse particulate matter to settle for 6 h, the dispersion was centrifuged at 14,000 rpm for 15 min to obtain a clear supernatant. The supernatant was passed through a 0.22 μm cellulose acetate sterile filter, and assayed for EF24.
Characterization of the IC
The HPβCD-EF24 IC was characterized by differential scanning calorimetry (DSC) and powder X-ray diffraction (XRD). DSC was performed using a Q1000 differential scanning calorimeter (TA Instruments, New Castle, DE) equipped with liquid nitrogen cooling. The instrument was routinely calibrated with four different standards (cyclopentane, biphenyl, indium, and tin) at 10 °C/min heating rate. EF24, HPβCD, and the IC were placed in aluminum DSC pans, and a drop of silicon oil was applied to the samples. The samples were scanned at a rate of 10 °C/min from −50 to 250 °C. For XRD of EF24, a Bruker AXS D8 Discover system (Bruker AXS, Madison, WI) with a 2D wire detector was used. Wide angle X-ray diffraction (WAXS) was carried out on EF24, HPβCD, and the IC at room temperature. Circularly symmetric WAXS patterns were collected in transmission on a Rigaku S-MAX 3000 system with a microfocusing copper tube source coupled with Confocal Max-Flux® optics. An image plate with a hole to allow passage of the main beam placed approximately 4 cm from the sample position was used to quantify X-ray intensity. Silver behenate was used to determine exactly the sample to detector position and used for the conversion from pixel position to scattering angle. X-ray intensities were scaled to improve presentation quality.
Preparation of EF24-liposomes
The dehydration rehydration liposomes were prepared using a method described earlier (Kirby and Gregoriadis 1984). In brief, a lipid composition of 1,2-disteroyl-sn-glycero-3-phosphatidylcholine: cholesterol: dimyristoylphosphatidyl glycerol (DSPC:CHO:DMPG as 50:50:5 mol%) was dissolved in a mixture of chloroform: methanol (2:1) and transferred to a round bottom flask. The solvent mixture was evaporated at 58 °C on R-210 rotavapor (Buchi Corporation, New Castle, DE) to obtain a thin film of lipids. Any residual traces of organic solvent were removed by keeping the film under high vacuum for 12 h. The phospholipid film was rehydrated with Hypure™ endotoxin-free cell culture gradewater (Hyclone, Logan, UT), maintaining the total phospholipid concentration to 12 mM (total lipid 2 g/dL). The resulting suspension of multilamellar vesicles was subjected to eight freeze–thaw (FT) cycles. An FT cycle consisted of snap-freezing the suspension in liquid nitrogen followed by immediate thawing in a 58 °C water bath. A sterile aqueous solution of EF24-HPβCD IC was added to the liposomal suspension to maintain various DSPC:EF24 M ratios (Table 1). The mixture was vortexed for 30 s and diluted with either sucrose solution or phosphate buffered saline (PBS, pH 7.4) to a lipid concentration of 2 mM. The sucrose amount was adjusted to either 3.5 or 7 g per gram of total lipid (Table 2). The mixture was distributed in sterile glass vials and lyophilized for 48 h in a Triad lyophilizer (Labconco, Kansas city, MO). The dried mass was rehydrated in a controlled manner typical of dehydration rehydration procedures (Kirby and Gregoriadis 1984). The thick suspension formed was allowed to stand at 25 °C for 30 min and further diluted several folds with PBS. The liposomes were separated from any un-entrapped material by ultracentrifugation at 35,000 rpm and 4 °C for 40 min. The liposomal pellet was washed thrice and reconstituted in sterile PBS for subsequent experiments.
Table 1.
Encapsulation efficiency of EF24 IC
| DSPC:EF24, M ratio | DSPC:HPβCD, M ratio | % Encapsulation (±SEM) |
|---|---|---|
| 14.19 | 1.84 | 6.88 ± 1.91 |
| 4.73 | 0.63 | 19.73 ± 2.90 |
| 2.50 | 0.32 | 1.93 ± 0.84 |
| 1.00 | 0.13 | 1.94 ± 0.58 |
Table 2.
Particle size and zeta potential of EF24-liposomes
| Sucrose (g/g of lipid) | Size, nm ± SEM % | Encapsulation ± SEM | ζ (mV) ± SEM |
|---|---|---|---|
| – | 448 ± 3.5 | 19.7 ± 2.9 | −80.9 ± 0.85 |
| 3.5 | 320.5 ± 2.6 | 18.6 ± 0.8 | −58.8 ± 2.85 |
| 7 | 287.5 ± 1.9 | 0.45 ± 0.6 | −75.5 ± 0.62 |
For experimental controls, a liposome preparation containing HPβCD solution without EF24 was identically prepared; the DSPC:HPβCD ratios of the control liposomes were matched with those of the EF24-liposomes. Strict aseptic conditions were maintained during the entire processing.
In order to get better control over the liposome size in the final preparation, as an alternative, the authors used extrusion technique to prepare EF24-liposomes. The extrusion method was essentially the same as has been published elsewhere (Awasthi et al. 1998). In brief, the thin film of lipid was hydrated with a solution of EF24 IC in sterile water for injection. The lipid suspension was extruded sequentially through membranes of 1 μm (8 times), 0.4 μm (5 times), and 0.2 μm (5 times) pore size in an extruder (Northern Lipids, Burnaby, Canada). The resultant liposomes were centrifuged in a Beckman ultracentrifuge at 45,000 rpm for 45 min to obtain a pellet. Supernatant liquid, containing extravesicular EF24 IC, was discarded. The liposome pellet was washed two times with PBS (pH 7.4). Finally, the liposomes were resuspended in PBS, characterized, and stored at 4 °C till further use.
Characterization of EF24 liposomes
Encapsulation efficiency of EF24-HPβCD IC inside the liposomes was determined by digesting an aliquot of liposome suspension in methanol and spectrophotometrically estimating EF24 at 330 nm after appropriate dilution with methanol. Control liposomes (liposomes identical to the EF24-liposomes in all respects but devoid of EF24) were also digested in methanol and used as a blank for estimation. The percent encapsulation efficiency (%EE) was calculated using the following formula:
Phospholipid concentration in the liposomes was determined by Stewart assay (Stewart 1980). The particle size of the liposomes was determined by photon correlation spectroscopy using a Brookhaven particle size analyzer equipped with Mas Option software. Zeta potential of preparations was measured using a Zeta PLUS Zeta potential analyzer (Brookhaven Instruments Corp, Holtsville, NY). For zeta potential, the liposomes (~40 μg of phospholipid) in 1.5 mL of 0.22-μm filtered de-ionized water were scanned at 25 °C for 10 runs, each run consisting of 20 cycles. Zeta potential values were obtained as millivolt ± standard error of mean.
The Transmission Electron Microscopy (TEM) was performed at the University of Oklahoma in Norman (OK). In brief, an ultradilute liposome suspension was stained with a solution consisting of 2.5% phosphotungstic acid and 2.5% trehalose (pH 7). A drop of liposome suspension was first applied to the copper grid, allowed to adsorb on the grid for 2 min, and then blotted with a filter paper. A drop of the stain was added to the wet grid and immediately blotted. TEM images were recorded on a Zeiss 10 electron microscope.
Stability of EF24-liposomes
The stability of EF24-liposomes was studied over 25 days by incubation of the preparations at 4, 25, and 37 °C. For these studies, the authors used EF24-liposomes prepared by extrusion process. At the end of the incubation period, the liposomes were monitored for particle size and EF24 leakage into extravesicular space. For serum challenge, EF24-liposomes were incubated for 24 h at 37 °C with equal volume with normal human serum. At the end of the incubation, the liposomes were diluted 100-fold with PBS and centrifuged at 14,000 rpm for 15 min. The supernatant was used to estimate leaked EF24.
In vitro efficacy of EF24-liposomes in cancer cells
Human lung adenocarcinoma NCI-H441 (ATCC # HTB-174) and prostate cancer PC-3 (ATCC # CRL1435) cell lines were obtained from American Type Culture Collection (Manassas, VA). H441 and PC-3 cells were maintained at 37 °C with 5% CO2 in McCoy’s 5A Medium (Invitrogen, Carlsbad, California), and RPMI 1640 Medium containing L-glutamine (GIBCO, Laboratories, Grand Island, NY), respectively. The media were supplemented with 5% heat-inactivated fetal bovine serum (FBS) and 50 μg/mL of gentamicin (GIBCO Laboratories, Grand Island, NY).
The cells were seeded in 96-well flat-bottom tissue culture plates at a density of 104 cells per well. The cells were allowed to adhere and grow overnight followed by treatment with EF24-liposomes equivalent to 0.1, 1, or 10 μM of EF24. The cells were allowed to incubate for 24, 48, or 72 h with the treatment. Inhibition of cell proliferation was determined as a decrease in hexosaminidase activity using p-nitrophenol-N-acetyl-beta-D-glucosaminide as a substrate (Landegren 1984). Anti-proliferative activity of EF24-liposomes was compared with that of plain EF24. Control liposomes containing identical amount of lipids and HPβCD were used as control for liposomal EF24. Plain EF24 was dissolved in cell culture medium containing about 0.08% DMSO. Medium containing 0.08% DMSO was used as a control for plain EF24.
Radiolabeling of EF24-liposomes
EF24-liposomes suitable for labeling by Tc-99m were formulated by co-encapsulating glutathione at the controlled rehydration step described above. For this purpose, the lyophilized dried mass was rehydrated with 100 mM glutathione solution (pH 6.3) without changing the further processing steps. EF24-liposomes thus prepared were labeled with Tc-99m essentially by the method described elsewhere (Awasthi et al. 2003; Phillips et al. 1992). In brief, EF24-liposomes (0.1 mL) were mixed with 1 mL of Tc-99m-hexamethyl propylene amine oxime (HMPAO). Tc-99m-HMPAO was obtained from OUHSC-Nuclear Pharmacy (Oklahoma City, OK). After 45 min of incubation at room temperature, the liposomes were passed through a PD-10 column (Amersham Pharmacia, Piscataway, NJ) to separate any radioactivity that was not associated with the liposomes. Fractions were collected by eluting the loaded liposome preparation with normal saline. Labeling efficiency was determined by measuring liposome-associated Tc-99m radioactivity before and after passing them through the column.
Biodistribution of EF24-liposomes
The distribution of Tc-99m-labeled EF24-liposomes was evaluated in normal Sprague–Dawley rats (n = 5). The animal experiments were performed according to the NIH Animal Use and Care Guidelines and were approved by the Institutional Animal Care Committee of the University of Oklahoma Health Sciences Center. The rat model with indwelling femoral artery catheter was prepared as described earlier (Awasthi et al. 2007). In brief, left femoral artery of male Sprague–Dawley rats (180–200 g) was cannulated with a polyethylene tube catheter, filled with heparin (1000 U/mL), and subcutaneously tunneled and secured at the nape. After closing the surgical area, the rats were given 2 days to recover from the procedure.
On the day of the experiment, the rats were anesthetized with isoflurane gas (2% in oxygen at 2 L/min) and a 25G butterfly was secured in the tail vein for the administration of radiolabeled preparation. About 250 μCi of Tc-99m-EF24 liposomes (0.2 mL, 3.7 mg phospholipid) was infused through the tail vein. Blood samples (50 μL) were withdrawn at various times through the arterial catheter for counting of blood-borne radioactivity. After 6 h of sampling, the animals were euthanized by an intraperitoneal overdose of a euthanasia solution (Euthasol). Various organs were excised, washed with saline, weighed, and appropriate tissue samples were counted in an automated gamma counter (Perkin-Elmer, Boston, MA). Total blood volume and muscle mass were estimated as 5.7 and 40% of body weight, respectively (Frank 1976; Petty 1982). A diluted sample of injected Tc-99m-EF24-liposomes served as a standard for comparison.
The percent of injected Tc-99m-EF24-liposome dose in various organs of the rats was used to generate a computer-simulated biodistribution visualization image. The simulation program was part of the image processing Invivoscope 1.41 software obtained with NanoSPECT imaging system (Bioscan Inc, Washington, DC).
Data analysis
The circulation kinetics data was presented as percent of the injected radioactivity. The accumulation of injected preparation in various organs at 6 h of biodistribution was also calculated as percent of injected radioactivity. All data were corrected for decay of Tc-99m radioactivity (T1/2 = 6 h) and background-subtracted. The half-life was calculated from the blood clearance data using a freeware Boomer (Version 3.3.3).
Results
The authors report a liposome preparation of a recently developed anticancer compound EF24 (Fig. 1a) using HPβCD as a solubilizing ligand for EF24. The assumption was that HPβCD will enhance aqueous solubility of EF24 whose KO/W was determined to be 2.85. EF24 was synthesized in our laboratory by the scheme reported elsewhere (Lagisetty et al. 2009). Since this compound is a new chemical entity, first the authors developed a spectrophotometric assay for EF24 in aqueous or water-miscible solvents. Aqueous EF24 showed an absorbance maximum at 330 nm that down-shifted to 315 nm in methanolic solution. At both wavelengths, EF24 followed Beer-Lambert law within a wide range of concentrations. The presence of HPβCD as an excipient was not found to interfere in EF24 determinations.
Inclusion of EF24 inside HPβCD
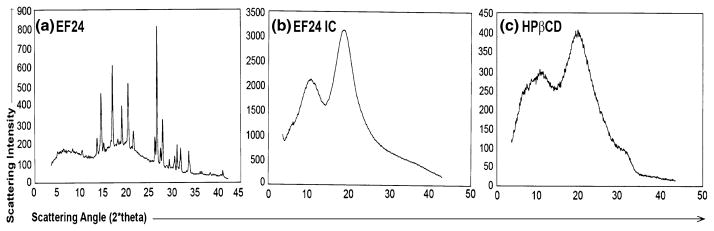
Figure 2 shows a typical AL type phase solubility diagram. Although, the linear fit has a high correlation value, a slight positive deviation in the initial concentrations is noticeable ( ). The apparent k value for the EF24-HPβCD complex was found to be 9.9 M−1. EF24 solubility in water increased remarkably from 1.64 mg/mL to 13.76 mg/mL after complexation. The EF24-HPβCD IC was characterized by DSC and powder XRD. The thermal curves for EF24 and the IC are shown in Fig. 3. EF24 showed multiple sharp endothermic peaks that were abolished by its inclusion inside the HPβCD cage. The X-ray diffraction pattern for the EF24 exhibited distinct sharp peaks at various diffraction angles of 2θ indicating the crystalline nature of the compound (Fig. 4a). The diffraction pattern for its IC exhibited only broad diffraction peak (Fig. 4b) which characterized the caging of the compound within the HPβCD molecules. The diffraction pattern of the IC was more or less similar to that of HPβCD (Fig. 4c), suggesting amorphization in otherwise crystalline nature of EF24.
Fig. 2.
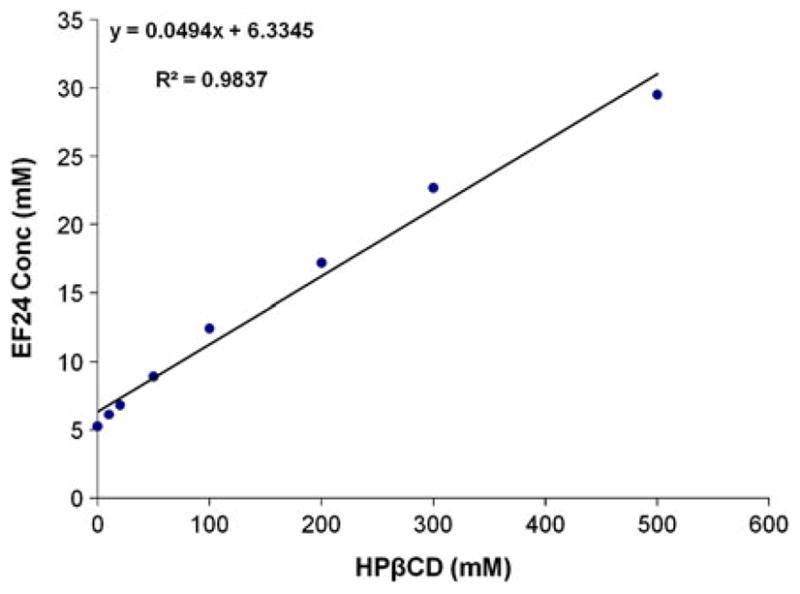
Phase solubility diagram of EF24 in HPβCD. The AL type curve indicated 1:1 stoichiometry between EF24 and HPβCD
Fig. 3.
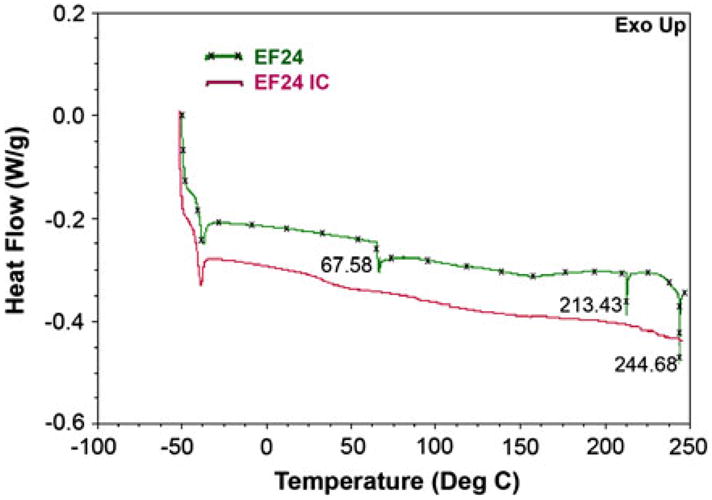
Differential scanning calorimetry of a EF24 and b the inclusion complex. The endothermic peaks corresponding to EF24 at 67.58, 213.43 and 244.68 °C disappeared after inclusion complex formation with HPβCD
Fig. 4.
Powder X-ray diffraction (XRD) of a crystalline EF24, b its inclusion complex with HPβCD, and c HPβCD. Sharp diffraction peaks of EF24 disappeared in XRD of the inclusion complex; rather a broad peak similar to that of plain HPβCD was observed
Liposome preparation
The IC was incorporated into liposomes using several DSPC to EF24 ratios. The %EE increased 2.86 folds when DSPC:EF24 ratio was decreased from 14.19 to 4.73. Any further decrease in DSPC:EF24 ratio resulted in reduced %EE (Table 1). The effect of the presence or absence of sucrose during freeze drying on the size of EF24-liposomes was studied. The size of the liposomes in the absence of sucrose was 448 ± 3.5 nm with a %EE of 19.73. When 3.5 g of sucrose per gram of total lipid was included during the freeze drying step, the size was significantly reduced to 320.5 ± 2.6 nm (p<0.001). The %EE was lower as well, but the change was not statistically significant. The size of the liposomes was further reduced to 287.5 ± 1.9 nm at 7 g of sucrose per gram of liposome, but the %EE fell significantly to 0.45 ± 0.58 (p<0.05). When extrusion technique was used to prepare EF24 liposomes, better control over particle size was obtained. After final extrusion through 0.2 μm pore size, the particle size was 177.6 ± 6.6 nm with polydispersity index of 0.127 ± 0.035.
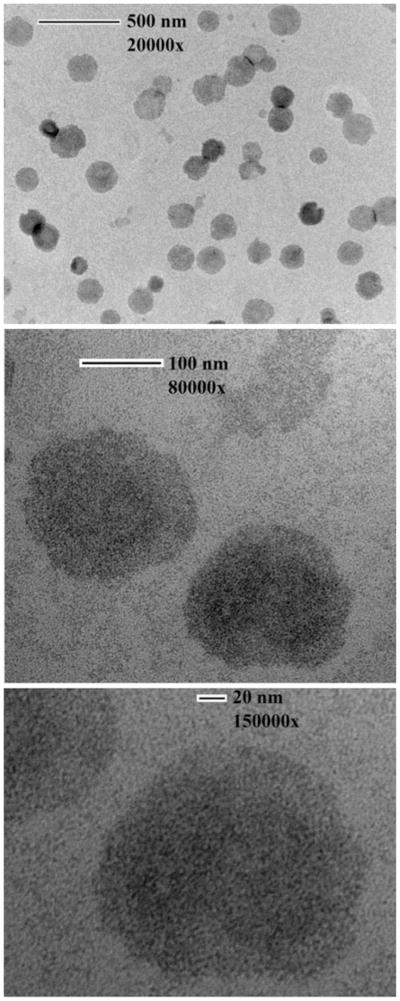
The zeta potential and particle size of the resultant liposomes are reported in Table 2. There was no clear correlation between the %EE, size, and zeta potential. The electron micrographs of the liposomes carrying ICs showed uniformly dispersed spherical structure of liposomes (Fig. 5). An aliquot of the glutathione-containing EF24-liposomes was labeled with Tc-99m. The liposomes were labeled with 64% efficiency. The radiolabeled liposomes essentially eluted in the void volume of the gel exclusion column, and there was clear distinction of the labeled liposomes from the free radioactivity peak (Fig. 6).
Fig. 5.
Transmission electron micrographs of liposomes encapsulating EF24-HPβCD IC
Fig. 6.
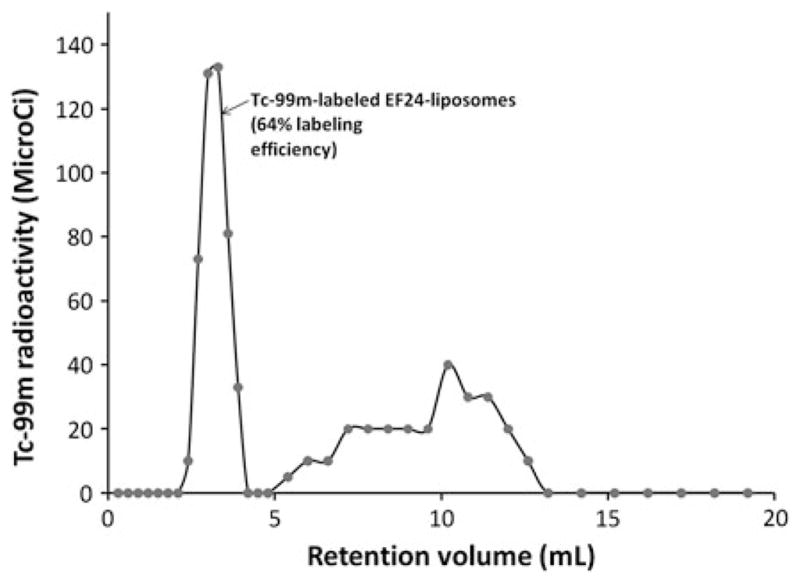
Elution of Tc-99m-labeled EF24-liposomes from a PD-10 column. The liposomes are collected in the void volume, whereas the free radioactivities (Tc-99m-TcO4− and Tc-99m-HMPAO) elute separately as broad peak
Stability of EF24-liposomes
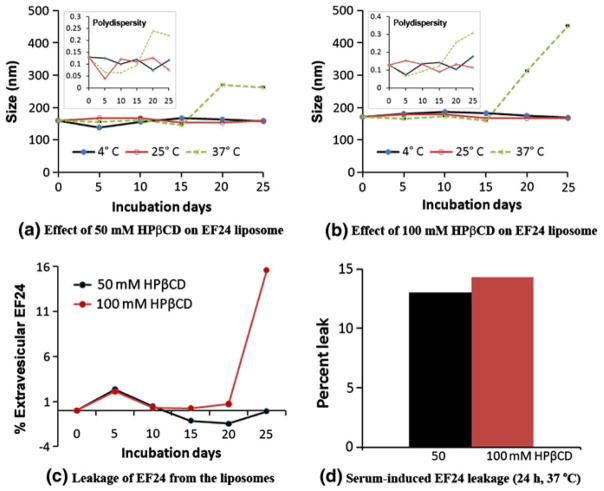
The authors studied in vitro stability of EF24-liposomes over 25 days by incubating them at different temperatures. The change in particle size and the leakage of EF24 into the extravesicular space were assessed. Because the authors used 0.2-μm extruded liposomes for the stability study, the initial size of these EF24-liposomes was about 177 nm. HPβCD significantly increased the liposomes size after 20 days of incubation at 37 °C (Fig. 7). The effect was more pronounced when 100 mM HPβCD was used to make EF24 IC (Fig. 7b). However, no change in particle size was observed even with 100 mM HPβCD when the liposomes were kept at 4 and 25 °C. The effect of HPβCD on the time-dependent change in liposome size was corroborated by the results from leakage study. The use of 100 mM HPβCD in EF24 IC resulted in a determinable leakage of EF24 after 25 days of incubation, but 50 mM HPβCD had no impact (Fig. 7c). When challenged with serum for 24 h at 37 °C, about 14% of encapsulated EF24 was released into the extravesicular space (Fig. 7d).
Fig. 7.
Time- and temperature-dependent stability of EF24-liposomes. Aliquots of EF24-liposomes were incubated at 4, 25, and 37 °C for 25 days. Samples were taken out on various days, and the liposome size was determined. Simultaneously extravesicular EF24 was spectrophotometrically determined. For serum challenge, EF24-liposomes were incubated with equal volume of human serum for 24 h at 37 °C. Insets in a and b show the changes in polydispersity index of EF24-liposomes with respect to time
In vitro efficacy of EF24-liposomes in cultured cancer cells
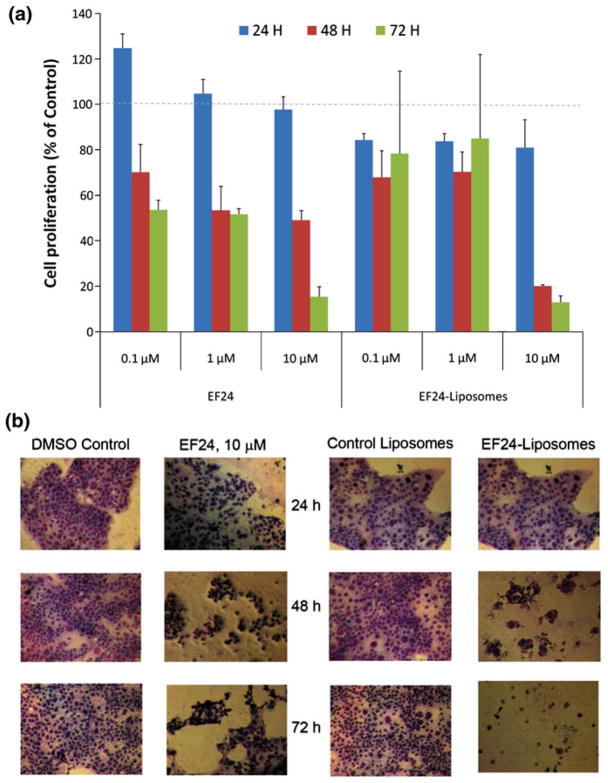
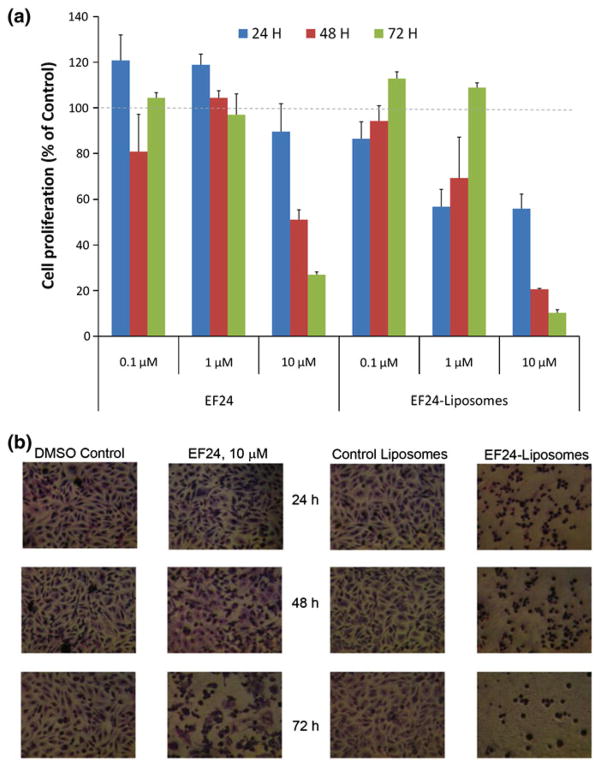
Efficacy of liposomal EF24 was compared against that of plain EF24 in H441 lung adenocarcinoma (Fig. 8) and PC-3 prostate cancer (Fig. 9) cells. Inhibition of cell proliferation was measured by the decrease in hexosaminidase activity in response to treatments. In general, it was observed that liposomal EF24 exceeded the anti-proliferative activity to plain EF24 (p<0.05). In fact, in both the cell lines, the efficacy of liposomal EF24 was superior to that of its plain counterpart at 10 μM (Figs. 8a and 9a). A significantly higher cell death was visible in the light microscopic pictures after the treatment (Figs. 8b and 9b). The control liposomes did not have any inherent affect on cell proliferation.
Fig. 8.
In vitro anti-proliferative activity of EF24-liposomes in lung adenocarcinoma H441 cells. a Hexosaminidase activity after 24, 48, and 72 h of treatment with plain EF24 or EF24-liposomes. b Light micrographs of H441 cells (×2,560) after treatments
Fig. 9.
In vitro antiproliferative activity of EF24-liposomes in prostate cancer PC-3 cells. a Hexosaminidase activity after 24, 48, and 72 h of treatment with plain EF24 or EF24-liposomes. b Light micrographs of PC-3 cells (×2,560) after treatments
Biodistribution of Tc-99m-labeled EF24-liposomes
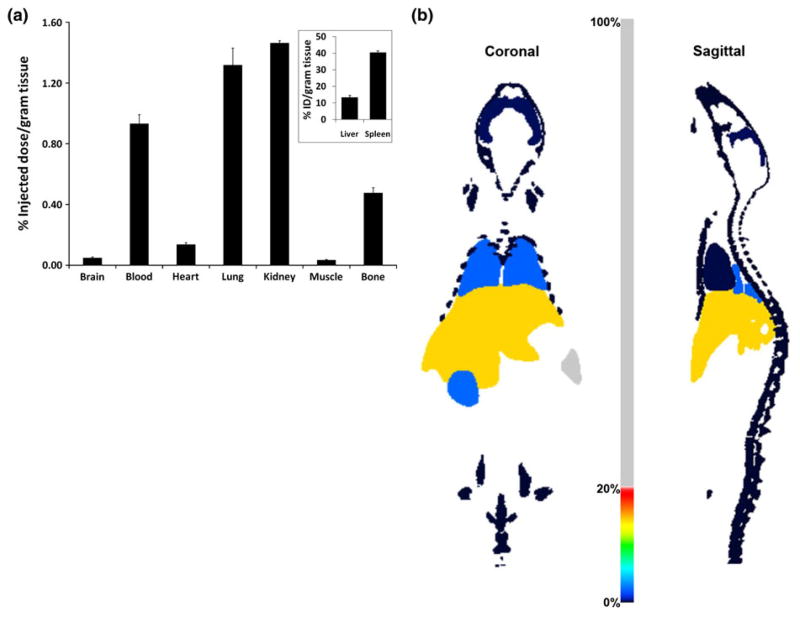
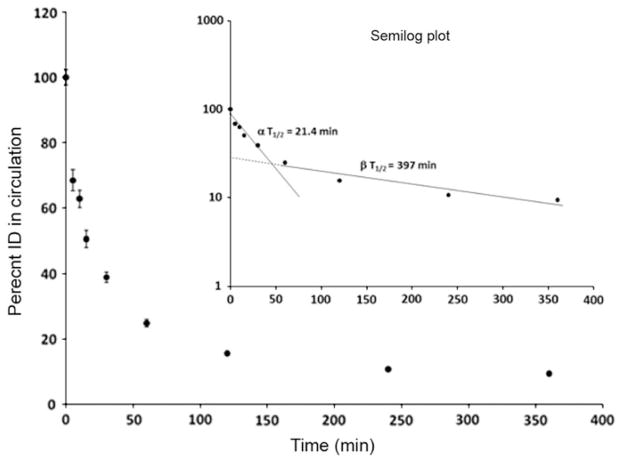
The Tc-99m-EF24-liposomes were evaluated for biodistribution and circulation half-life in normal Sprague–Dawley rats. As expected, the organs of reticuloendothelial system—liver and spleen—accumulated the majority of liposomes (Fig. 10). About 10% of injected dose was still in circulation at 6 h when necropsy was performed. Other organs accumulated negligible amount of liposomes. The observation that there was very little excretion of radioactivity through kidney attests to the stability of radiolabel within the liposomes. The biodistribution data were visualized as simulated, computer-generated image (Fig. 10b). The circulation profile shows that the clearance of liposomes from blood followed bi-exponential pattern with an α half-life of 21.4 min followed by a β half-life of 6.6 h (Fig. 11). It is apparent that the clearance of EF24-liposomes from blood within the first 30 min of injection was rapid and accounted for more than 60% of the total clearance; after 30 min, the clearance was relatively gradual.
Fig. 10.
Biodistribution of Tc-99m-labeled EF24-liposomes in Sprague–Dawley rats. a Percent of injected dose per gram of excised tissue. The inset shows select tissues/organs where the accumulation percent was higher than that allowed by the y-axis scale in the main plots. b Percent of injected dose in various organs visualized by biodistribution visualization utility in the image processing Invivoscope 1.41. As shown, the color-scale in middle ranges from 0 to 100%
Fig. 11.
Circulation kinetics of Tc-99m-EF24-liposomes in rats. This data was used to calculate half-life of circulation of the injected preparation
Discussion
EF24 is a synthetic analog of curcumin which is being investigated as a potent anticancer agent (Adams et al. 2005). Though the exact mechanism of EF24’s action is unclear and may vary with the cell context, it has been shown to suppress cell proliferation and angiogenesis by downregulating various cancer promoting genes, such as COX-2, IL-8, and VEGF (Subramaniam et al. 2008). It has also been found to induce G2/M cell cycle arrest and apoptosis in cisplatin-resistant human cancer cells (Selvendiran et al. 2007). A recent study suggests that EF24 suppresses NF-kB signaling by directly inhibiting IkB kinase (Kasinski et al. 2008). Most in vitro biochemical assays with EF24 may be satisfactorily performed with dimethylsulfoxide (DMSO) as a solvent, but in vivo studies require EF24 in an appropriate dosage form. While the potency of a new chemical entity may often be a function of its lipophilicity, formulation of these drugs into a soluble or easily administrable dosage form becomes technically challenging (Loftsson et al. 2005a, b). Technically, EF24 is very slightly soluble in water (1.6 mg/mL), and poses a significant challenge from the formulation and bioavailability viewpoint. For instance, its congener curcumin has been found to be minimally absorbed even at an oral dose of several grams (Sharma et al. 2001). At the same time, its insolubility in aqueous solvents precludes solution dosage forms for intravenous administration. In order to enable parenteral EF24 administration, the authors investigated the possibility of encapsulating it inside the liposomes.
Our initial attempts of preparing EF24 liposome using conventional techniques resulted in poor encapsulation. For instance, the conventional thin film hydration method provided only 0.4% encapsulation which increased to 1% when a reverse phase evaporation method was used for liposome preparation. Apparently, lipid compartment of the liposomes was not able to host EF24, and the aqueous solubility of EF24 was not large enough for its accommodation inside the aqueous core. The liposome encapsulation of poorly water soluble drugs is governed by a variety of factors like physicochemical properties of drugs, lipid composition, affinity between drug molecules and lipid film and method of preparation (Walde and Ichikawa 2001). To address this problem, the authors encapsulated EF24-HPβCD ICs inside the liposomes. This strategy has been successfully demonstrated for other drugs with poor water solubility, such as prednisolone (Fatouros et al. 2001), and has been reviewed (Challa et al. 2005; McCormack and Gregoriadis 1994). Cyclodextrins are highly water-soluble cyclic oligosaccharides with a lipophilic internal cavity that serves as a host to lipophilic drugs (Brewster and Loftsson 2007). Our initial experiments with β-CD were only moderately successful with only 4-fold increase in the solubility of EF24. HPβCD, which has higher water solubility compared to β-CD (Gould and Scott 2005; Loftsson et al. 2005a, b), resulted in about 8-fold enhancement of EF24 in aqueous phase. When EF24-HPβCD IC was used to prepare liposomes, EF24 could be encapsulated in practically useful amounts. The enhancement in encapsulation efficiency could be attributed to the encapsulation of more soluble IC into the aqueous liposomal core which provides a relatively larger volume than the lipid bilayers.
The choice of liposomes in preference to plain IC as intravenous preparation is ascribed to several reasons. While the IC themselves may be useful as drug delivery vehicle, rapid renal elimination of IC (Frijlink et al. 1990; McCormack and Gregoriadis 1996) limits their utility as compared to liposomes. Liposomes can be made to circulate for a prolonged time (Awasthi et al. 2003) to allow their continued accumulation in tumor because of the enhanced permeability and retention phenomenon. They can be actively targeted by conjugating the surface with appropriate ligand. Lastly, the authors showed that the liposomes could be labeled with Tc-99m for non-invasive tracking in the body by gamma camera imaging. Although not performed for EF24-liposomes, imaging has been used on several occasions in the previous drug development research by the group including one of the authors of this study—Awasthi (Awasthi et al. 2003, 2004a, b).
Considering that the liposome preparation method would need optimization to control particle size within a close range, the authors used extrusion of lipid suspension through the polycarbonate filters of well-defined pore sizes up to 200 nm. The resultant liposomes have consistently shown a unidisperse particle size of 177 nm. These liposomes prepared by extrusion method were used in the stability study reported here. In the presence of 50 mM HPβCD, the liposomes maintained their integrity and there were insignificant changes in size of the liposomes over a period of 25 days in storage. At higher 100 mM HPβCD and over prolonged storage at 37 °C, the unoccupied cyclodextrin host might have destabilized the liposomes by extracting lipids from the bilayers (Piel et al. 2007). Storage temperature has significant impact on the eventual stability of EF24 liposomes. Apparently, the choice of high-melting saturated phospholipid DSPC and the presence of cholesterol help in preserving the integrity of EF24 liposomes with 50 mM HPβCD (Anderson and Omri 2004; Bhardwaj and Burgess 2010).
Implicitly, EF24 liposomes could be kept at 4–8 °C for at least 30 days; however, an accelerated stability study would be required to accurately predict the storage stability and conditions.
Our in vitro cell proliferation studies suggest that liposomes are significantly more effective than plain EF24. The reason for the enhanced efficacy is not clear, but better cross-membrane availability of EF24 in liposome package may be one explanation. A more relevant evaluation of liposomal EF24 performance would be in vivo because liposomes are optimal for drug availability in tumor when excretory mechanisms in the body are present. Nevertheless, our studies established two important things—first, that the control liposomes did not have any effect on the cancer cells, and second, that the liposome encapsulation retains anti-proliferative activity of encapsulated EF24.
The biodistribution of EF24 liposomes was investigated by labeling them with Tc-99m radionuclide. This technique has been widely used to non-invasively visualize liposome distribution in live animals (Awasthi et al. 2003; Goins et al. 1993; Laverman et al. 2003). From the results, it is clear that majority of liposomes were taken up by liver and spleen (Fig. 10). Such a disposition is typical of liposomes that do not carry stealth-promoting ingredients (Awasthi et al. 2003, 2004a, b). The uptake of liposomes by the reticuloendothelial system is saturable and is non-Michaelis–Menton phenomenon (Kume et al. 1991). The pharmacokinetics of EF24-liposomes in rats showed that even though the liposomes did not carry stealth coating of poly(ethylene glycol) or PEG, substantial amounts were present in circulation at the time of necropsy (>10% at 6 h). Once encapsulated, the encapsulated drugs follow the kinetics of liposome distribution. In general, circulation T1/2 of conventional liposomes decreases with increasing size, negative charge density, and fluidity of the bilayer. Past experience with liposome circulation kinetics suggests that a circulation T1/2 of 12–20 h in rats or mice translates into 40–60 h in humans (Woodle et al. 1995). On the same scale, a 6.6 h β T1/2 of EF24-liposomes in rat would translate into a T1/2 of about a day (22 h) in humans. The circulation persistence of EF24-liposomes can be further augmented by decreasing the particle size and/or incorporating PEG-lipid in the lipid composition. The results of this study corroborate very closely with our past observations on comparative circulation persistence of non-PEGylated and PEGylated liposomes (Awasthi et al. 2003, 2004a, b). It has been previously demonstrated by our group that in order to maximize encapsulation space and pay-load, the size of around 275–300 nm is optimal for enhanced circulation persistence of PEGylated liposomes (Awasthi et al. 2003). Besides decelerated renal excretion and improved stability toward proteolysis, the PEG modification is believed to reduce immunogenicity. However, it has been shown that even PEGylated liposomes demonstrate accelerated blood clearance upon multiple injections (Ishida and Kiwada 2008; Laverman et al. 2001). This phenomenon has been attributed to the induction of IgM-mediated immune response (Ishida et al. 2007) and/or complement system (Ishida et al. 2002; Szebeni 1998; Szebeni et al. 2000).
Conclusions
In this article, the development of EF24-liposomes was described. These colloidal carriers were found to have anti-proliferative action in H441 and PC-3 cell lines. The preparation could be radiolabeled with Tc-99m radionuclide for convenient imaging of its accumulation in cancer tissue. This will satisfy the growing demand among investigators to non-invasively determine drug distribution in visual, temporal, and quantitative format. As indicated above, the tested liposome formulation has short circulation half-life. It might be important to further improve the circulation kinetics of these liposomes for superior in vivo performance. This limitation will need reconciliation in animal models by formulating stealth EF24-liposomes. Moreover, the in vivo stability of the reported formulation of EF24 liposomes needs to be addressed in future.
Acknowledgments
The authors acknowledge the help of Ms. Prachi Vilekar (Graduate Student) in cell culture studies. The radiolabeling and biodistribution study was performed in the Small Animal Imaging Facility of the College of Pharmacy. This study was made possible by the financial support under the National Cancer Institute grant 1R03 CA143614-01.
Contributor Information
H. Agashe, Department of Pharmaceutical Sciences, University of Oklahoma Health Sciences Center, 1110 N. Stonewall Avenue, Oklahoma City, OK 73117, USA
P. Lagisetty, Department of Pharmaceutical Sciences, University of Oklahoma Health Sciences Center, 1110 N. Stonewall Avenue, Oklahoma City, OK 73117, USA
K. Sahoo, Department of Pharmaceutical Sciences, University of Oklahoma Health Sciences Center, 1110 N. Stonewall Avenue, Oklahoma City, OK 73117, USA
D. Bourne, Department of Pharmaceutical Sciences, University of Oklahoma Health Sciences Center, 1110 N. Stonewall Avenue, Oklahoma City, OK 73117, USA
B. Grady, School of Chemical, Biological and Materials Engineering, 100 East Boyd, Norman, OK 73019, USA
V. Awasthi, Email: vawasthi@ouhsc.edu, Department of Pharmaceutical Sciences, University of Oklahoma Health Sciences Center, 1110 N. Stonewall Avenue, Oklahoma City, OK 73117, USA
References
- Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- Akerley W, Choy H. Single-agent paclitaxel and radiation for non-small cell lung cancer. Semin Radiat Oncol. 1999;9:85–89. [PubMed] [Google Scholar]
- American Cancer Society. Cancer Statistics 2009 Presentation. American Cancer Society; 2009. http://www.cancer.org/docroot/PRO/content/PRO_1_1_Cancer_Statistics_2009_Presentation.asp. [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Anderson M, Omri A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004;11:33–39. doi: 10.1080/10717540490265243. [DOI] [PubMed] [Google Scholar]
- Awasthi V, Goins B, Klipper R, Loredo R, Korvick D, Phillips WT. Imaging experimental osteomyelitis using radiolabeled liposomes. J Nucl Med. 1998;39:1089–1094. [PubMed] [Google Scholar]
- Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253:121–132. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: effect of postinserted poly(ethylene glycol)-distearoylphosphatidylethanolamine on distribution and circulation kinetics. J Pharmacol Exp Ther. 2004a;309:241–248. doi: 10.1124/jpet.103.060228. [DOI] [PubMed] [Google Scholar]
- Awasthi VD, Garcia D, Klipper R, Phillips WT, Goins BA. Kinetics of liposome-encapsulated hemoglobin after 25% hypovolemic exchange transfusion. Int J Pharm. 2004b;283:53–62. doi: 10.1016/j.ijpharm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol. 2007;103:28–38. doi: 10.1152/japplphysiol.00136.2006. [DOI] [PubMed] [Google Scholar]
- Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002;46:828–833. doi: 10.1128/AAC.46.3.828-833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Burgess DJ. Physicochemical properties of extruded and non-extruded liposomes containing the hydrophobic drug dexamethasone. Int J Pharm. 2010;388:181–189. doi: 10.1016/j.ijpharm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6:E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros DG, Hatzidimitriou K, Antimisiaris SG. Liposomes encapsulating prednisolone and prednisolone-cyclodextrin complexes: comparison of membrane integrity and drug release. Eur J Pharm Sci. 2001;13:287–296. doi: 10.1016/s0928-0987(01)00114-2. [DOI] [PubMed] [Google Scholar]
- Frank DW. Physiological data of laboratory animals. In: Melby ECJ, editor. Physiological data of laboratory animals. CRC Press; Boca Raton, FL: 1976. pp. 23–64. [Google Scholar]
- Frijlink HW, Visser J, Hefting NR, Oosting R, Meijer DK, Lerk CF. The pharmacokinetics of beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin in the rat. Pharm Res. 1990;7:1248–1252. doi: 10.1023/a:1015929720063. [DOI] [PubMed] [Google Scholar]
- Goins B, Klipper R, Rudolph AS, Cliff RO, Blumhardt R, Phillips WT. Biodistribution and imaging studies of technetium-99m-labeled liposomes in rats with focal infection. J Nucl Med. 1993;34:2160–2168. [PubMed] [Google Scholar]
- Gould S, Scott RC. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem Toxicol. 2005;43:1451–1459. doi: 10.1016/j.fct.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Connors K. Phase solubility techniques. Adv Anal Chem Instrum. 1965;4:127–212. [Google Scholar]
- Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon induced by administration of PEGylated liposome. Yakugaku Zasshi. 2008;128:233–243. doi: 10.1248/yakushi.128.233. [DOI] [PubMed] [Google Scholar]
- Ishida T, Harashima H, Kiwada H. Liposome clearance. Biosci Rep. 2002;22:197–224. doi: 10.1023/a:1020134521778. [DOI] [PubMed] [Google Scholar]
- Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, Wang CY, Shoji M, Sun A, Snyder JP, Liotta D, Fu H. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene) piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby C, Gregoriadis G. Dehydration–rehydration vesicles (DRV): a new method for high yield drug entrapment in liposomes. Biotechnology. 1984:979–984. [Google Scholar]
- Kume Y, Maeda F, Harashima H, Kiwada H. Saturable, non-Michaelis-Menten uptake of liposomes by the reticuloendothelial system. J Pharm Pharmacol. 1991;43:162–166. doi: 10.1111/j.2042-7158.1991.tb06658.x. [DOI] [PubMed] [Google Scholar]
- Lagisetty P, Powell DR, Awasthi V. Synthesis and structural determination of 3,5-bis(2-fluorobenzylidene)-4-piperidone analogs of curcumin. J Mol Str. 2009;936:23–28. [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984;67:379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- Laverman P, Boerman OC, Oyen WJG, Corstens FHM, Storm G. In vivo applications of PEG liposomes: unexpected observations. Critical reviews in therapeutic drug carrier systems. 2001;18:551–566. [PubMed] [Google Scholar]
- Laverman P, Boerman OC, Storm G. Radiolabeling of liposomes for scintigraphic imaging. Methods Enzymol. 2003;373:234–248. doi: 10.1016/S0076-6879(03)73015-8. [DOI] [PubMed] [Google Scholar]
- Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005a;302:18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Loftsson T, Jarho P, Masson M, Jarvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005b;2:335–351. doi: 10.1517/17425247.2.1.335. [DOI] [PubMed] [Google Scholar]
- McCormack B, Gregoriadis G. Entrapment of cyclodextrin-drug complexes into liposomes: potential advantages in drug delivery. J Drug Target. 1994;2:449–454. doi: 10.3109/10611869408996821. [DOI] [PubMed] [Google Scholar]
- McCormack B, Gregoriadis G. Comparative studies of the fate of free and liposome-entrapped hydroxypropyl-beta-cyclodextrin/drug complexes after intravenous injection into rats: implications in drug delivery. Biochim Biophys Acta. 1996;1291:237–244. doi: 10.1016/s0304-4165(96)00096-7. [DOI] [PubMed] [Google Scholar]
- McCormack B, Gregoriadis G. Drugs-in-cyclodextrinsin-liposomes: an approach to controlling the fate of water insoluble drugs in vivo. Int J Pharm. 1998;162:59–69. [Google Scholar]
- Petty C. Research techniques in the rats. Charles C. Thomas; Springfield, IL (USA): 1982. [Google Scholar]
- Phillips WT, Rudolph AS, Goins B, Timmons JH, Klipper R, Blumhardt R. A simple method for producing a technetium-99m-labeled liposome which is stable in vivo. Nucl Med Biol. 1992;19:539–547. doi: 10.1016/0883-2897(92)90149-s. [DOI] [PubMed] [Google Scholar]
- Piel G, Piette M, Barillaro V, Castagne D, Evrard B, Delattre L. Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int J Pharm. 2007;338:35–42. doi: 10.1016/j.ijpharm.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, Hideg K, Kuppusamy P. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–28618. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, Ramanujam RP, Hideg K, Dieckgraefe BK, Houchen CW, Anant S. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68:1962–1969. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- Sun A, Shoji M, Lu YJ, Liotta DC, Snyder JP. Synthesis of EF24-tripeptide chloromethyl ketone: a novel curcumin-related anticancer drug delivery system. J Med Chem. 2006;49:3153–3158. doi: 10.1021/jm051141k. [DOI] [PubMed] [Google Scholar]
- Szebeni J. The interaction of liposomes with the complement system. Crit Rev Ther Drug Carrier Syst. 1998;15:57–88. [PubMed] [Google Scholar]
- Szebeni J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M, Stahl GL, Bunger R, Alving CR. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am J Physiol. 2000;279:H1319–H1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- Walde P, Ichikawa S. Enzymes inside lipid vesicles: preparation, reactivity and applications. Biomol Eng. 2001;18:143–177. doi: 10.1016/s1389-0344(01)00088-0. [DOI] [PubMed] [Google Scholar]
- Woodle MC, Newman MS, Working PK. Biological properties of sterically stabilized liposomes. In: Lasic DD, Martin F, editors. Stealth liposomes. CRC Press; Boca Raton: 1995. pp. 103–117. [Google Scholar]